Open Access Article
Open Access ArticleAn overview of recent progress in dental applications of zinc oxide nanoparticles
Hedaiat Moradpoora,
Mohsen Safaei *b,
Hamid Reza Mozaffaric,
Roohollah Sharifid,
Mohammad Moslem Imanie,
Amin Golshahe and
Negin Bashardoustf
*b,
Hamid Reza Mozaffaric,
Roohollah Sharifid,
Mohammad Moslem Imanie,
Amin Golshahe and
Negin Bashardoustf
aDepartment of Prosthodontics, School of Dentistry, Kermanshah University of Medical Sciences, Kermanshah, Iran
bAdvanced Dental Sciences Research Center, School of Dentistry, Kermanshah University of Medical Sciences, Kermanshah, Iran. E-mail: mohsen_safaei@yahoo.com; safaei@kums.ac.ir
cDepartment of Oral and Maxillofacial Medicine, School of Dentistry, Kermanshah University of Medical Sciences, Kermanshah, Iran
dDepartment of Endodontics, School of Dentistry, Kermanshah University of Medical Sciences, Kermanshah, Iran
eDepartment of Orthodontics, School of Dentistry, Kermanshah University of Medical Sciences, Kermanshah, Iran
fStudents Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran
First published on 15th June 2021
Abstract
Nanotechnology is an emerging field of science, engineering, and technology concerning the materials in nanoscale dimensions. Several materials are used in dentistry, which can be modified by applying nanotechnology. Nanotechnology has various applications in dentistry to achieve reliable treatment outcomes. The most common nanometals used in dental materials are gold, silver, copper oxide, magnesium oxide, iron oxide, cerium oxide, aluminum oxide, titanium dioxide, and zinc oxide (ZnO). ZnO nanoparticles (NPs), with their unparalleled properties such as high selectivity, enhanced cytotoxicity, biocompatibility, and easy synthesis as important materials were utilized in the field of dentistry. With this background, the present review aimed to discuss the current progress and gain an insight into applications of ZnO NPs in nanodentistry, including restorative, endodontic, implantology, periodontal, prosthodontics, and orthodontics fields.
1. Introduction
Nanotechnology is a rapidly expanding field with the potential to diagnose and cure diseases.1 Nanotechnology can be applied to dentistry, known as nanodentistry or nanodontics, to improve prevention, diagnosis, and therapy of oral and dental diseases.2,3 Many different types of materials are used in dentistry and nanotechnology has significant potential to improve their properties.4 Several nanostructures can be utilized in dentistry, including nanofibers, dendrimers, nanopores, nanoshells, nanorods, nanoparticles, dental nanorobots, nanorobotic dentifrice, nanosolutions, and nanoneedles.2Nanoparticles (NPs) are an excellent candidate for nanodentistry and can be made by ceramics, polymers, and metals.5 Nanometals have been extensively studied, and many review papers concerning metal NPs-based medical science have been published.6 Various materials in dentistry were modified by incorporating metal NPs such as gold, silver, platinum, palladium, nickel, copper, zirconium, aluminum, titanium, chromium, beryllium, boron, and zinc.7 Among various metals, zinc has attracted considerable attention in medicine, owing to its antibacterial effects. Oxide and sulfide materials have also been produced. ZnO NPs are a newer type of promising candidate and are used extensively owing to its high safety and useful physicochemical advantages. In addition, ZnO NPs, due to their good biocompatibility, high stability, low-cost and, low-toxicity, have shown promising potential in biomedical applications.8
Zinc oxide nanoparticles (ZnO NPS) have been widely investigated over the past two decades because of their superior antibacterial, antifungal, electrical, chemical and optical properties. ZnO NPs can easily be synthesized by numerous techniques. Therefore, ZnO has a potential biocompatibility over many other metal oxides and has explored many pronounced applications in current antiviral, antimicrobial, biomedical, and environmental areas.
Interestingly, recent studies have revealed that ZnO NPs have cytotoxic effects bactericidal cells, while at the same concentration; ZnO NPs have non-toxic effects on human cells. ZnO NPs shown higher toxic effects on bacterial cells than other metal oxide NPs such as TiO2, due to their ion-shedding ability.
As a result, ZnO NPs are rapidly gaining in popularity as a good candidate for nanodentistry, and several research groups have begun to explore the role of NPs in the field of nanodentistry. The present review aims to gain an insight into the applications of ZnO NPs in dentistry.
2. Nanomedicine and nanodentistry
Nanotechnology is the most widely accepted technologies in the 21st century.9 The term of “nano” is derived from the Greek word meaning “dwarf”.9,10 For the first time, Richard Feynman introduced the term of nanotechnology and after fifteen years in 1974, Norio Taniguchi defined the concept of nanomedicine as “nanotechnology mainly consists of the processing of separation, consolidation, and deformation of materials by one atom or one molecule”.9 Nanotechnology employs structures measured in the nanoscale range (1–100 nm).10 Materials with the nanoscale range have unique physicochemical properties that markedly differ from the bulk materials.11 The fabrication of nanomaterial structures can be classified into two groups: top-down approach and bottom-up approach.10Nanotechnology is able to change the nanoscience theory to effective applications and to improve the quality of life with novel properties such as large surface area to volume ratio, uniform particle size, tunable optical, magnetic and electronic properties, in addition to biocompatibility, bioconjugation and easy surface functionalization.9,12
Many types of nano-sized structures have been used in nanotechnology, including NPs, nanofibers, nanorods, nanowires, nanobelts, nanotubes, nanoribbons, quantum dots, and hollow spheres.13 Nanotechnology has exceptional capability of yielding groundbreaking results in approximately every branches of science such as physics, chemistry, engineering, electronic, material science, biology and medicine.9 Nanotechnology has attracted considerable attention in recent years owing to its wide application in nanomedicine.14
Nanomedicine, as the specific application of nanotechnology in the health system, is a new field for better prevention, diagnostics, and therapy of diseases.7,13 It is the incorporation of the knowledge of molecular biology, pharmaceutics, material science, information technology and medicine. Nanotechnology provides a good opportunity to have a better understanding of disease mechanisms.15 Many numerous kinds of nanomaterials, such as lipids, liposomes, polymers, metals, oxides, silica, and carbon-based NPs, have been developed in recent decades.16 However, EMA and FDA for use in humans have approved some of nanomaterials. According to the reports only 175 nanostructured products were approved to be used in medicine by regulatory agencies and generally made of polymeric or inorganic materials with metal and liposomal. Application of nanotechnology is still expanding in medical science.
The recently developed interest in nanotechnology has provided new insights into the application of NPs in dentistry. Nanoparticles are used in many different approaches of dentistry owing to high surface volume, biocompatibility, bioactivity, and good mechanical properties. Nanotherapeutics offers the possibility to control biofilms by utilizing the properties of NPs like ZnO-NPs. However, the emergence of nanotechnology has opened new avenues for the use of NPs.17
3. Nanoparticles in dentistry
Nanoparticles, as novel dental materials, have unrivaled physicochemical and biological properties, making them suitable to overcome complications associated with traditional dental treatments through the therapeutic intervention.18–21 Preventive therapy, curative therapy and tissues regenerative therapy are triple purposes in dental therapeutic treatments.22 Nano-products are widely used in numerous dental applications in order to improve the quality of products such as endodontic, periodontics, orthodontics, restorative and adhesive dentistry, implant dentistry, acrylic resins, tissue engineering, and oral cancer.21,23 Nanoparticles are usually incorporated into dental materials-related restorative materials, cements/sealants, adhesives bonding, and prosthesis bases systems.22 NPs used in dentistry can be made of silica, carbon-based NPs, different polymers, solid lipids, hydroxyapatite, hydrogel, dendrimers and metal/metal-oxide.24 It has been found that NPs are present in approximately 3500 dental materials.25 Over the past decade, NPs applied in dentistry mostly include noble metals, such as platinum, gold, silver and metal oxide NPs, including iron oxide, zinc oxide, titania, and zirconia owing to their broad-spectrum bactericidal properties.25,26 For these reasons, many recent studies have focused on the properties of NPs.14 Nanoparticles can be divided into three main categories of dentistry as follows (Fig. 1).3.1. Antibacterial nanoparticles
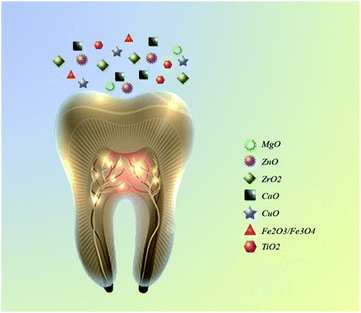
Nanoparticles can act as an efficient antibacterial agent and is widely accepted in biomedicine.17,23 The superior bactericidal activity of NPs with antibacterial activities is attributed to their electrostatic attraction between positively charged NPs and have the potential to reduce or eliminate the evolution of more resistant bacteria, since their mechanism of action is directly in contact with the bacterial cell wall and simultaneously targets multiple biomolecules.25 The small size of NPs improves not only their antimicrobial action with minimal adverse effects, including hypersensitivity and allergic reactions, but also their mechanical properties.17 Many studies investigated the antibacterial effect of NPs combined with a wide range of dental materials.18,26–29 Fig. 2 summarizes the most common metal oxides NPs used in dentistry. These NPs are less toxic and have a higher antibacterial activity in comparison to other metal oxide nanoparticles such as AuO, AgO/AgO2, etc. The uses of AuO, AgO/AgO2 NPs in dentistry have been severely limited and have not yet been fully studied because of the difficult or expensive to obtain or synthesize. The metal oxides NPs such as ZnO, ZrO2, MgO, TiO2 have useful applications in reducing the biofilm formation. The use of these NPs as orthodontic adhesives to control the oral biofilm and decrease the demineralization around the brackets has attracted much attention.29 The implantation failure can be caused by the plaque accumulation in the oral cavity during the early stage.10Therefore, the use of NPs can significantly reduce the risk of implantation failure. Numerous researches have investigated the effect of using antibacterial NPs in composite resins and glass ionomers. They reported that addition of NPs remarkably boosted the mechanical and antibacterial activity.10,29
Research in recent years has exhibited that addition of antimicrobial NPs plays a crucial role in the prevention of primary and persistent endodontic infections after treating or recolonizing the filled canal system.30 These facts have prompted a renewed interest in the use of various antibacterial NPs to improve their potency and applications in dentistry.29
3.2. Reinforcement nanoparticles
Regardless of which material is used as the filling agent, numerous research efforts have focused on the restorative material efficiency and properties in the past decade.31 Silica, quartz and radiopaque silicate particles based on the oxides of barium, strontium, zinc, aluminum, and zirconium are a range of conventional composite generally used as the restorative material.9 Nanotechnology can provide dental resin-based composites with different particle size, ranging from supra-micron to nanosized with unique characteristics.31 Nanoparticles can be utilized at the site of dental defects, owing to their mechanical properties, high surface volume, biocompatibility and bioactivity.17 Addition of NPs into various restorative and adhesive systems is the main strategy to enhance the mechanical properties and the compressive strength of the restorative material.17 Nanosized particles were added to the filler to fill the space between larger filler particles, and to reduce the resin content of resin-based composites.21 Restorative materials are often broken due to the biofilm accumulation, secondary caries, and bulk fracture.7 Addition of certain NPs into orthodontic adhesives/cements or acrylic can improve the mechanical properties, and the hardness of composites decreases the polymerization shrinkage and provides the composites with smooth surface and high optical properties.3,9 In general, researchers have found that adding the Ag, Cu, ZnO, Zr, TiO2, ZrO2, and SiO2 NPs to the base materials of composite resins, cavity varnishes, and glass ionomer cement (GICs) may produce more useful results.7 Modification of the dentin adhesive agent with nanofillers increases the bond strength to the dentin surface and the mechanical properties of the adhesive layer due to the increasing viscosity of adhesive, thereby forming a thicker adhesive layer capable of preventing fluid movement from dentin tubules.22 The incorporation of nano-glass particles into conventional GICs can improve the compression strength and elastic modulus, and reduce the setting time. In this regard, restorative dentistry in the last few years focused on the use of nanomaterials hoping that contemporary nanofillers will improve the overall mechanical properties of fillers.3.3. Therapeutics nanoparticles
Nanotherapeutics is a fundamental issue in developing effective NPs to control biofilms.21 Nanoparticles can be utilized as nanocarriers with antimicrobial therapeutic actions.17,32 Furthermore, they can be used to prevent and cure oral diseases like oral cancer and act as osteogenic agents owing to their biocompatibility.24 Recent studies have showed promising results in the regeneration of the periodontal apparatus, including stimulation of periodontal ligament cells. Furthermore, the recent development in the use of NPs for dental tissues regenerative applications and denture bases has shown acceptable outcomes.18 For replacement dentures, addition of NPs to polymers for tissue conditioners can decrease the chance of denture stomatitis.17 Bacteria have been never thoroughly eliminated from the root canals, but reduction of the bacterial population in the root canal has been the key to successful dental treatment. Consequently, NPs have been successfully used against root canal infective organisms to reduce microleakage in canal spaces.11 The researchers concluded that NPs of Au, Ag, Pt, and Pd could be utilized for imaging techniques as contrast enhancers for oral diagnosis such as detection of oral cancer and identification of infectious pathogens.7,18 Certainly, recent studies have revealed that ZnO NPs have cytotoxic effects toward cancerous cells, while at the same concentration; ZnO NPs have negligible effects on normal cells, leading to speculation that they can be used in cancer treatment.33–36 Thus, it is obvious that an alteration in zinc levels in cancer cells can reason a deleterious effect. The selective localization of ZnO NPs towards cancer cells due to boosted permeability, electrostatic interaction, retention effect and selective cytotoxicity through increased oxygen species present in cancer cells show that ZnO NPs can selectively target and kill cancer cells, making them a promising anticancer agent.33–36Nanoparticles as dental implant coating materials are widely used in dentistry to improve wear resistance and bone graft.14 Many different NPs, including Ag, ZrO2, and TiO2 were coated on the surfaces of dental implants. Nanoparticles were able to create a novel method to treat many dental diseases through incorporation with dental materials.22
4. Properties, method of synthesis and antimicrobial activity of ZnO nanoparticles
ZnO has a potential biocompatibility over many other metal oxides and has explored many pronounced applications in antimicrobial and biomedical areas. The physical and chemical properties of ZnO nanoparticles are influenced both by their shape and size (Table 1). Over the past decades, a great number of efforts have been devoted in nanoscience to the control of ZnO NPs with controllable and adjustable shapes. Different physical, chemical, and biological (green chemistry) methods used to produce uniform ZnO nanostructures can be found in the literature.37,38| Synthesized method | Applications | Findings | Ref. |
|---|---|---|---|
| ZnO NPs synthesized with (Zn(CH3COO)2·2H2O) | – Evaluating the effect of shape and size on their antimicrobial and photo catalytic activity | – Methods of synthesis of ZnO NPs could controlling their shape, size | 51 |
| – The value of Eg with concentration of Zn(CH3COO)2·2H2O can be attributed to the change in the shape/size of the ZnO NPs | |||
| – The shapes of the synthesized NPs varied with the concentration of the originator Zn(CH3COO)2·2H2O | |||
| A one-pot, organometallic method for synthesis of ZnO NPs | – Evaluating the influence of the different parameters on the size and shape of ZnO NPs | – Miscibility with water, coordinating ability, and evaporation rate of solvent appear to be important for the control of the growth of particles | 52 |
| – An increase in the length of the alkyl chain results in an increase of the shape anisotropy | |||
| – Concentration, time, and temperature showed an important impact on shape and size | |||
| ZnO NPs from solution by the precipitation method containing LiOH, NH4OH, NaOH | – Investigation of the ionic template effect on the size and shape of ZnO NPs | – The results showed that the presence of K2SO4, KNO3 or LiNO3 led to the formation of slightly smaller ZnO NPs | 53 |
| – The ZnO NPs were free from impurities | |||
| ZnO NPs prepared using a simple polyol synthesis | – Controlling the shapes and sizes of ZnO NPs | – Increasing the amount of water added to the precursor solution enlarged the aspect ratio of the rod-shaped particles and increased the particle size of the equiaxial particles | 54 |
| ZnO NPs prepared using the chemical precipitation method | – Controlling the morphology of ZnO NPs | – A moderate ammonia concentration was beneficial in limiting the particle growth | 55 |
| – The particle size of calcined ZnO powders were very sensitive to the calcination time | |||
| – The average size of the particles of ZnO obtained using the flow injection technique was approximately 20 nm while the crystallite size was 10 ± 15 nm | |||
| ZnO NPs prepared using a modified polyol process | – Characterization and optical properties of ZnO NPs with controlled size and morphology | – The increasing of the alkaline ratio results in a great change of the elaborated particles morphology that evolved from irregular and anisotropic forms to spherical one | 56 |
| Synthesis of ZnO nano particles by precipitation techniques | – Evaluating the effect of temperature on the morphology of ZnO nanoparticles: a Comparative study | – The particles synthesized from sulphate precursors showed very nicely organized rods and grains morphology arranged like flowers before calcinations but the morphology changed after calcination at 400 °C to only flakes type. However, the morphology did not alter even after calcinations for the nano particles synthesized from other two precursors such as zinc acetate and zinc nitrate | 57 |
| ZnO NPs synthesized using Laurus nobilis L. leaves aqueous extract and two different zinc salts | – Controlling the morphology of ZnO NPs | – The precursors have played a vital role in surface morphology and structure of ZNPs | 58 |
| ZnO NPs synthesized via a solvothermal method | – Characterization of narrow size distribution of ZnO NPs | – A narrow size distribution can be obtained for ZnO NPs prepared by a solvo thermal method using TEA as a polymerization agent | 59 |
| Microwave assisted synthesis of ZnO nanoparticles | – Evaluating the effect of precursor reagents, temperature, irradiation time, microwave radiation power, and additives addition on the final morphology of ZnO NPs | – All the mentioned variables influenced to some extent the shape and/or size of the synthetized nanoparticles | 60 |
ZnO NPs can be prepared by several methods, resulting in nanostructures of different shapes such as solvent-based ultrasonic irradiation,39 hydrothermal,40 microemulsion,41 physical vapor deposition,42 arc plasma,43 thermal evaporation,44 solvothermal,45 microwave synthesis,46 wet chemical,47 solgel,48 and green methods like plant extracts.49,50
Various size and shape particles of ZnO were obtained via altering reaction conditions such as the concentration of either additive, the molar ratio of the starting materials, the pH of the reactants and the temperatures. Generally, the synergistic effects of kinetic or thermodynamic aspects are considered as key roles in determining the shape formation of inorganic NPs.
Traditional nucleation and growth kinetics in colloidal synthetic method have been used to control the size of nanoparticles. As well as, precursor concentrations, the precursor to surfactant ratio, and a reduction temperature by choosing a solvent are all regarded as important factors to control the shape of nanoparticles. Moreover, it was found that the size of nanoparticles could be controlled by the reaction time.
Size, shape, dissolution, surface charges, aggregation, and concentration are the key factors in determining the toxicity and antibacterial effects of ZnO NPs.61,62 The antibacterial properties increase with increasing the surface area.38
All these characteristics depended on the preparation method of ZnO NPs.63 Various nanostructures of ZnO, such as nanorods, nanopowders, nanotubes, nano/micro flowers microspheres, quantum dots, thin films/NPs, and capped NPs, have been developed, which can be used for antimicrobial application.61
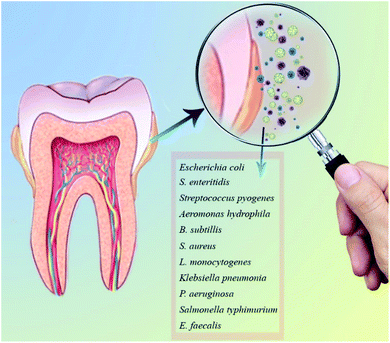
ZnO NPs are commonly used as an additive in medicine and industry owing to its unique thermal, optical, mechanical, chemical, piezoelectric properties and electric conductivity.64,65 It is biocompatible, biosafe, and nontoxic with considerable bactericidal properties for the broad range of bacteria (Gram-positive and Gram negative) and fungi, including A. hydrophila, B. subtillis, E. coli, E. faecalis, S. pyogenes, S. typhimurium, K. pneumonia, S. aureus, S. enteritidis, L. monocytogenes, and P. aeruginosa, etc. (Fig. 3).61
The antibacterial activity of ZnO NPs has been studied in recent years.44 Tayel et al. evaluated the antibacterial potentiality of ZnO NPs and conventional ZnO powder was evaluated against nine bacterial strains, including E. coli, E. cloacae, P. fluorescens, P. aeruginosa, S. typhimurium, S. enteritidis and S. aureus.67 They found that the NPs form of ZnO had more efficient antibacterial action than those of the powder form against all examined strains.67 Chang et al. revealed that the prepared ZnO films enhanced the antibacterial activity of the Ti-based implant against Streptococcus mutans.66 Furthermore, the antibiofilm behavior of teeth surfaces coated with ZnO and CuO NPs was examined against Streptococcus mutans.68 The ZnO and CuO NPs coated teeth surfaces showed a remarkable decrease in biofilm formation by 85% and 70%, respectively, compared to the uncoated tooth.68 Khan et al. reported that incorporation of ZnO NPs or Ag NPs into composites showed considerable antibacterial activity against Lactobacillus and S. mutans, and the effect of ZnO NPs on S. mutans was higher than that of Ag NPs.69 Wang et al. found that ZnO NPs had excellent antibacterial activity against P. gingivalis and A. naeslundii, and ZnO NPs had low cell cytotoxicity in vitro.70 Mirhosseini et al. evaluated the antimicrobial activity of different concentrations and sizes of ZnO NPs against E. faecalis, C. albicans, and S. mutans, L. fermentum. They observed that the antimicrobial action of ZnO NPs increased with decreasing the particle size, and C. albicans, E. faecalis, and S. mutans had the highest sensitivity to ZnO size changes than to other bacteria.71 Antibacterial activity of different size of ZnO NPs against S. sobrinus and S. mutans via microdilution technique was assisted by Bakhori et al., and the results showed that ZnO NPs with size distributions of 21 nm exhibited higher inhibition on both bacteria compared to ZnO NPs with size distributions of 52 nm.72
5. Application of ZnO nanoparticles in dentistry
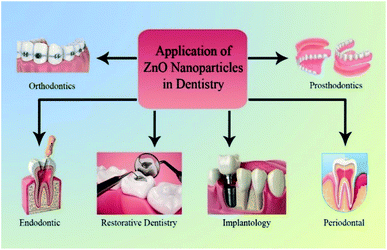
Depending on application of NPs, the use of ZnO NPs in dentistry can be categorized as follows: restorative dentistry, endodontic, implantology, periodontal, prosthodontics, and orthodontics fields (Fig. 4).5.1. Restorative dentistry
Dental caries disease is a polymicrobial biofilm disease that according to the WHO, nearly 100% of adults worldwide suffer from a toothache due to tooth cavity.73 Amalgam, composite, gold, ceramic and glass ionomer are the commonly used materials for dental restorative.74 In the last decades, incorporation of metallic NPs into dental restorative materials resulted in improved dental restorative materials properties.73 Therefore, many researchers have used various inorganic metal oxide nanofillers, such as TiO2, Al2O3, SiO2, AlN, BN, ZnO, and etc. in dental materials to enhance the antibacterial activity, mechanical properties and physical properties.73,75 The proper dental restorative materials with good mechanical properties should have better adhesion between filler and hard dental tissues.76 In this regard, ZnO NPs are developed and mostly used to decrease the activities of caries-related bacteria owing to their various potential properties such as electrical, optical, piezoelectric, electromagnetic shielding, thermal, mechanical, sensing, and biomedical (non-toxic and eco-friendly) properties.73 Numerous studies have demonstrated the potency of ZnO NPs as reinforcements, to elevate the mechanical and physical properties and to enhance the antimicrobial properties (Table 2).77,78| Formulations NPs | Applications | Findings | Ref. |
|---|---|---|---|
| ZnO NPs incorporated into resin composite | – Evaluating the effect of ZnO NPs incorporated into resin composite for the potential one-step treatment of caries lesion | – The loading of ZnO NPs on the demineralized dental surface and their infiltration power were significantly improved when ZnO NPs were carried by the resin | 88 |
| SYNT–ZnO NPs | – Evaluation of the compressive strength and radiopacity of calcium silicate cement containing ZnO NPs | – Incorporation of ZnO NPs increased compressive strength and radiopacity | 96 |
| ZnO/AgNPs in a composite resin | – Application of Ag doped ZnO NPs in a composite resin | – The nanospheres of ZnO/Ag lead to a better biofilm inhibition | 83 |
| – The nanospheres of ZnO/Ag could be a good option as a new restorative material | |||
| Polymerized acrylic resins-ZnO NPs | – Evaluation of incorporating zinc oxide NPsto autopolymerized acrylic resins on their flexural strength | – The incorporation of zinc oxide NPs has a significant effect on the flexural strength of auto-polymerized acrylic resins | 84 |
| ZnO NP–glass ionomer cement | – Evaluation of properties of glass ionomer cement reinforced with ZnO NP | – Addition of ZnO NPs to type IIa GIC led to a marginal increase in mechanical properties | 93 |
| GIC–ZnO NP | – Evaluation of the antibacterial activity of zinc oxide NPs incorporated into selfcured glass ionomer cement (GIC) and light-cured resin-reinforced GIC on Streptococcus mutans biofilm | – A low concentration of NPs does not improve the antibacterial properties of the GIC | 94 |
| ZnO NP–PMMA resin | – Evaluation of the antifungal activity of ZnO NPs against Candida albicans | – Addition of ZnO NPs to PMMA resin increased antifungal activity | 90 |
| PC–ZrO2–ZnO NPs | – Evaluating the antibiofilm activity against Enterococcus faecalis and radiopacity, compressive strength of Portland cement (PC) added to zirconium oxide (ZrO2), with ZnO NPs | – The addition of nanoparticulated ZnO decreased the compressive strength of PC | 97 |
| – All materials presented higher radiopacity and antibiofilm activity than pure PC | |||
| – The presence of ZnO (5% or 10%) significantly decreased the compressive strength of the materials | |||
| ZnO NP–glass ionomer cement | – Evaluating antibacterial and mechanical properties of ZnO NP to the conventional glass ionomer cement | – GIC incorporated with 3% w/w concentration of size ZnO NP was the best alternative to conventional GIC for restorative purposes which provides greater antibacterial property | 92 |
| – Incorporation of ZnO NPs has no significant difference over the mechanical properties of set glass ionomer cement | |||
| Flowable resin composites containing ZnO NPs | – Evaluating the physical–mechanical properties and antibacterial activity of resin composites containing ZnO NPs against Streptococcus mutans | – The ZnO containing resins show significantly lower depth of cure, and higher bond strength | 87 |
| – The flexural strength and compressive modulus remain unchanged by incorporation of NPs | |||
| – Compressive strength and flexural modulus significantly increase | |||
| – Antibacterial activity significantly increases | |||
| Amalgam–Al2O3–ZnO NP | – Hardness improvement of dental amalgam using Al2O3–ZnO NP | – Al2O3 NPs as filler improved hardness of dental amalgam material | 95 |
| – The hardness of Silverfil increased as the percentage of ZnO loading increased | |||
| ZnO and ZnO:Ag nano sealers | – Evaluation of microleakage and antibacterial properties of prepared ZnO and ZnO:Ag nano sealer | – Ag doped ZnO nanopowders for using as sealer exhibit better microleakage and antibacterial properties comparing to common sealer | 98 |
| ZnO/MgO nanocomposite in zinc polycarboxylate dental cement | – Characterization of NPs and nanocomposite of ZnO and MgO for zinc polycarboxylate dental cement preparation | – The zinc polycarboxylate dental cements obtained by synthesized nano-scale powders revealed excellent mechanical strength | 89 |
| – Zinc polycarboxylate dental cement had higher strength than conventional Harvard and Adhesor zinc polycarboxylate cements | |||
| – Mechanical strength of dental cements was dependent on composition size scale | |||
| ZnO-NP-containing composites | – Evaluation of the antibacterial effectiveness of ZnO-NPs against Streptococcus sobrinus | – An 80% reduction in bacterial counts was observed with 10% ZnO-NP-containing composites | 82 |
Composite is a commonly used material for the restorations of the tooth.79,80 According to the existing literature, addition of ZnO NPs into commercial resin composite can modify resin composite for better biofilm inhibition.73,81–83
The influence of incorporating ZnO NPs into auto-polymerized acrylic resins on flexural strength was investigated by Kati, and after addition of ZnO NPs, a significant increase in the flexural strength was observed.84
Similar results were obtained by Al-Shammari for heat-polymerized acrylic resin.85 Wang et al. studied the effect of cellulose nanocrystal/zinc oxide nanohybrids on the reinforcing and antibacterial properties of dental resin composites.
They reported that the resin composites with 2 wt% incorporated cellulose nanocrystal/zinc oxide nanohybrids significantly increased the amount compressive strength and flexural modulus, and decreased the mechanical properties compared to resin composites with no cellulose nanocrystal/zinc oxide nanohybrids.86 Tavassoli Hojati et al. contributed to the antibacterial action of resin composite containing ZnO NPs against Streptococcus mutans and examined their physical and mechanical properties.87 They found that the flexural strength and compressive modulus remained unchanged by incorporation of ZnO NPs, while the compressive strength and flexural modulus were remarkably increased. In addition, they reported that incorporation of ZnO NPs into a flowable resin composite would affect physical and mechanical properties, bond strength, and antimicrobial activity.87 Resin composites containing ZnO NPs emerged as a good option for the potential one-step treatment of caries lesion.88
Various techniques have been employed to prepare the nanocomposite of ZnO. For example, ZnO/MgO nanocomposite was successfully synthesized based on the sonochemical method by Karimi et al.89 Addition of ZnO NPs is one of the most important approaches to achieve antibacterial activity in resin-based restorative materials (Cierech, Wojnarowicz et al. 2016). ZnO NPs with sizes smaller than 100 nm were successfully incorporated into the polymethyl methacrylate resin material to assist the antifungal activity against Candida albicans.90 Glass ionomer cement (GIC) materials have been widely used in dentistry as restorative materials due to the ability to modify their physical properties by changing the chemical formulation or the powder/liquid ratio.91 Several attempts have been made to improve poor mechanical properties, such as low fracture strength, toughness and wear of GIC materials by addition of agents.91 Vanajassun et al. observed the significant increase in the antibacterial properties of the set GIC containing ZnO NPs.92 Agarwal et al. proved that incorporation of ZnO NPs into type IIa GIC led to a marginal increase in mechanical properties.93 Garcia et al. investigated the antibacterial activity of ZnO NPs incorporated into selfcured GIC as a restorative material on the Streptococcus mutans biofilm.94 They reported that no change showed in cell morphology in relation to the type of GIC, maturation time, and NPs concentration, and addition of ZnO NPs at concentrations of 1% and 2% by weight to GICs did not improve their antimicrobial activity against S. mutans.94 Since a long time ago, dental amalgam is one of the mostly used materials in restorative dentistry. It is favorable owing to high strength, easy application, and low cost. ZnO is naturally white, and its incorporation into dental amalgam might fade the metallic color of the conventional amalgam out. Based on the results obtained by Yahya et al. the hardness of dental amalgam was increased with incorporation of ZnO NPs.95
5.2. Endodontic
Endodontic therapy has been suggested since 1930s as a method for diagnose, prevent, and treat pulp diseases and their sequelae, including extraction of pulp tissue, cleaning of root canal, and the root canal filling. Endodontic treatment is one of the most common procedures to eliminate endodontic infection.99–101 Complete elimination of dental infection is impossible, since endodontic infections are of polymicrobial nature with various bacteria and microorganisms such as E. faecalis, S. mutans and S. anginosus, F. nucleatum, and S. aureus (about 150 types).102,103 The root canal space should be filled with root-end filling materials after removing the infections and shaping root canals.99,104,105An perfect root canal filling materials should easily fill the canals, easily adhere to the walls, not shrink, and should be harmless to the periapical tissue and permanent tooth germ.99 Moreover, biocompatibility and antimicrobial properties are two key factors in choosing the best of them.104,106 Recently, many materials have been used as the root canal filling, including methacrylate resins, epoxy resins, polydimethylsiloxane, calcium hydroxides, silicone, and ZnO.107 Since 1930s, ZnO has been most widely used as a root canal filling material.108
It has been reported that nanosize materials play a significant role in the regenerative endodontic field and disinfection of the root canal space. It is known that ZnO is nearly insoluble in water due to high polarity; therefore, nanostructures of ZnO might be helpful to overcome this limitation. ZnO NPs can have selective toxicity to bacteria and antibacterial effects on numerous Gram positive/negative bacterial strains.104 Furthermore, ZnO NPs are known as one of the important antibacterial NPs with minimal effects on human cells. The antibacterial properties of ZnO NPs depend on their concentration, consequently, higher levels, resulting in the maximum antibacterial effect. In addition, smaller NPs have more antibacterial properties when in contact with an aqueous medium.100,104 Some studies showed positive results in the incorporation of ZnO NPs into the root canal sealer, such as using chitosan–ZnO NPs in endodontic disinfection and ZnO–graphite-type carbon NPs in endodontic regeneration.34,104
Table 3 summarizes the number of![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) studies examining the use of ZnO NPs in root canal infections. Antibacterial properties of ZnO NPs against Enterococcus faecalis biofilm to disintegrate the biofilms within the root canal system have attracted considerable attention during recent years.109
studies examining the use of ZnO NPs in root canal infections. Antibacterial properties of ZnO NPs against Enterococcus faecalis biofilm to disintegrate the biofilms within the root canal system have attracted considerable attention during recent years.109
| Formulations NPs | Applications | Findings | Ref. |
|---|---|---|---|
| ZnO NPs–Gt microparticles | – Evaluation of the antibacterial effect and biocompatibility of zinc oxide (ZnO) NPs and graphite-type carbon (Gt) microparticles as endodontic materials | – ZnO improved wound healing by enhancing tissue regeneration | 113 |
| – ZnO nano-powder can be used as a potential material in the regenerative endodontic field | |||
| – ZnO and Gt powders have an efficient antibacterial activity | |||
| ZnO NPs | – Enhance the antibacterial activity | – ZnO NPs sealer had higher antimicrobial properties compared to AH26 | 114 |
| ZnO NPs–Gt microparticles | – Evaluation of the antibacterial effect and biocompatibility of zinc oxide (ZnO) NPs and graphite-type carbon (Gt) microparticles as endodontic materials | – ZnO improved wound healing by enhancing tissue regeneration | 104 |
| – ZnO nano-powder can be used as a potential material in the regenerative endodontic field | |||
| – ZnO and Gt powders have an efficient antibacterial activity | |||
| ZnO pulp canal sealer–ZnO/Ag NPs | – Measuring the depth of tubular penetration of zinc oxide based pulp canal sealer when mixed with zinc oxide NPs | – Incorporation of NPs improved the flow properties of the endodontic sealer materials | 115 |
| ZnO NPs-AH26/ZOE sealer | – Evaluating the sealing ability | – The synthesized ZnO NPs sealers were suitable in root canal therapy to prevent leakage | 116 |
| Pure ZnO and ZnO:Ag NPs–gelatin | – Preparing suitable sealer to decrease the microleakage of the root canals as well as having good antibacterial property | – The synthesized pure ZnO and ZnO:Ag nanopowders exhibit better microleakage and antibacterial properties in comparison with ZOE and AH26 sealers | 112 |
| ZnO NPs | – Evaluation of ZnO NPs compared to NaOCL/EDTA treatment in eliminating E. faecalis form infected root canals | – The ZnO NP were significantly more effective at killing bacteria in the dentinal tubules than the NaOCl/EDTA treatment | 111 |
Shrestha et al. demonstrated that Chitosan and ZnO NPs had strong antibacterial activity against E. faecalis.110 Paul contributed to the antimicrobial properties of ZnO NPs against E. faecalis penetrating into dentinal tubules.89 They found that NPs could eliminate the planktonic E. faecalis and reduce the biofilm thickness.111 Samiei et al. contributed to ZnO NPs, a mixture of ZnO and CS NPs (CS/ZnO-NPs), and ZnO NPs with multilayered coating of CS (CS-layer-ZnO-NPs). They reported that the root canal surface treated with cationic antibacterial NPs, including ZnO-NP, CS/ZnO-NP, or CS-layer-ZnO-NP could reduce the adherence of E. faecalis to the NPs-treated dentin.34 According to Shayani Rad et al. the micro leakage and antibacterial properties of ZnO nanopowders, silver–ZnO nanopowders, zinc oxide eugenol (ZOE) and epoxy resin sealer (AH26) were compared to each other.112
ZnO and ZnO/Ag nanopowders showed better microleakage and antibacterial properties than ZOE and AH26 sealers. In addition, the nano-ZnO sealer showed higher antimicrobial properties than two common endodontic sealers AH26 (resin-based) and Pulpdent (ZOE-based) did against E. faecalis, E. coli, C. albicans, S. mutans, and S. aureus.112 Although, incorporation of ZnO NPs improved the properties of the endodontic sealer materials and could be considered a potential material in regenerative endodontic field, further validation studies are needed to verify their safety regarding prolonged exposure.
5.3. Oral implantology
Orthopedic or dental implants have been successfully employed to replace dental elements.117 Orthopedic and dental implant failures are one of the most serious complications after surgery that occur due to oral biofilms consisting mainly of Streptococcus spp. accumulated on implants.118,119NPs play a key role in the development of dental implants.120 NPs can be used to improve the bone incorporation of dental implants and soft tissue integration.95 Implants can either be coated or impregnated with metallic NPs as efficient antimicrobial agents to prevent dental implant failure.121 Chemical modifications on implant surfaces have been performed in order to minimize effects of preventing the dental implant failure.122
Recent studies have indicated that the use of ZnO NPs as suitable candidates for coating of dental implants due to their unique properties has attracted considerable attention.123 Titanium based materials have been extensively employed for dental implants owing to their ability.117
Abdul Kareem et al. coated ZnO, hydroxyapatite, and a combination of (ZnO + HA) NPs onto Ti discs, and contributed to the antibiofilm activity of NPs. They found that the non-viable microorganisms concentrations were higher on ZnO NPs and composite (ZnO NPs + HA NPs) coated surfaces compared to HA NPs coated and uncoated titanium.98 Trino et al. performed the functionalization of chemical modifications on commercial pure titanium with four different bifunctional organic molecules by HA NPs to minimize effects of corrosion in dental implant. The ZnO functionalized samples improved the anti-corrosive properties.122 Previous studies indicated that coating of dental implant with ZnO NPs could improve the physical properties consisting the bone incorporation of dental implants, the osteogenic potential of implants, and the fixation of implants.117,120,124
Mixtures of ZnO NPs and nano hydroxyapatite have been reported to modify the surface of glass substrates as innovative coating materials to decrease the bacterial adhesion and support the osteoblast growth onto the surface of glass substrates for dental implants by the electrohydrodynamic atomization method to deposit mixtures. The results revealed that ZnO NPs and ZnO/nano-hydroxyapatite coated substrates had considerable antimicrobial activity and implant-bone bonding characteristics. In addition, the substrate exclusively coated with ZnO NPs showed more efficiency than the one with composite surface coatings.123
Goel et al. studied the microstructural, wetting, biological, and topological properties of co-sputtered titania–ZnO nanocomposite thin films to enhance the bonding of metallic fixture with bone and reported that both S. aureus and E. coli growth were markedly inhibited.124 The results are similar to the findings of Kulshrestha et al. who demonstrated the potential of graphene/ZnO nanocomposite as a coating for dental implants against the cariogenic properties of S. mutans.118 Modified titanium implant materials, by building a hybrid system composed of N-halamine and ZnO NPs on titanium, showed a superior antibacterial action against P. aeruginosa, E. coli and S. aureus without using antibiotics.125 Poly(lactic-co-glycolic acid)/Ag/ZnO nanorods coating on the surface of Ti metallic implants using a hydrothermal method and subsequent spin-coating of mixtures of poly(lactic-co-glycolic acid) and Ag NPs have been shown to exhibit excellent self-antibacterial potential against both S. aureus and E. coli, good cytocompatibility and good biocompatibility.126 Therefore, researchers have attempted to modify dental implants with ZnO NPs as multifunctional inorganic NPs to improve osteogenesis inducing ability to inhibit bacterial colonization.119,127 Table 4 summarizes studies in the field of application of ZnO NPs in oral implantology.
| Formulations NPs | Applications | Findings | Ref. |
|---|---|---|---|
| ZnO NPs | – Evaluate the antibacterial activity of ZnO NPs against isolates from internal cavity of dental implant | – ZnO NPs were effective against bacteria isolated from internal cavity of dental implant | 128 |
| ZnO NPs–Ti films on Si substrates | – Evaluate the antibacterial and biocompatible nanocomposite for dental implant applications | – The strongly inhibit the growth of both Staphylococcus aureus and Escherichia coli observed | 124 |
| – The superior adhesion and proliferation ability on the nC–titania–zinc-oxide coated substrates as compared to uncoated ones observed | |||
| ZnO NPs deposited on Ti | – Evaluate the corrosion resistance of ZnO NPs deposited on commercial pure titanium implants | – ZnO functionalized samples have improved anti-corrosive properties | 122 |
| PEEK/ZnO composites | Enhancing the mechanical performance of poly(ether ether ketone)/ZnO nanocomposites to provide promising biomaterials for orthopedic implants | – PEEK/ZnO composites were good candidates for orthopedic materials and trauma implants | 127 |
| PSA–ZnO–SiO2–DMH NPs on Ti implant | – Enhancing antibacterial ability of Ti implants against both Pseudomonas aeruginosa (P. au), Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) | – Novel surface system provided a promising self-antibacterial bioplatform for metallic implants without using antibiotics | 103 |
| ZnO on carbon nanotubes/chitosan modified Ti | Biofunctionalization of carbon nanotubes/chitosan hybrids on Ti implants deposited ZnO nanostructures | – CNTs can strengthen the antibacterial activity against E. coli and S. aureus by 8% and 39%, respectively | 129 |
| – CS can improve the cytocompatibility of CNTs and ZnO | |||
| ZnO NPs-hydroxyapatite coated onto Ti discs | – Determine the antibiofilm activity of nanoparticulate coated titanium (Ti) dental implant as coating materials | – Coating Ti dental implant surfaces with ZnO NPs to provide an antimicrobial function | 120 |
| ZnO NPs and nanohydroxyapatite onto the surface of glass substrates | – ZnO NPs material for dental implants to inhibit bacterial adhesion and promote osteoblast growth | – 100% ZnO NPs and 75% ZnO NPs/25% nanohydroxyapatite composite coated substrates have significant antimicrobial activity | 123 |
| – ZnO NPs can, on its own, provide an optimal coating for future bone implants that are both antimicrobial and biocompatible | |||
| Graphene/ZnO nanocomposite | – The potential of graphene/ZnO nanocomposite (GZNC) film protects dental implant surfaces against the cariogenic properties of Streptococcus mutans | – Significant reduction in biofilm in the presence GZNC | 118 |
| – The anti-biofilm behavior of artificial acrylic teeth surfaces coated with GZNC | – The potential of GZNC as an effective coating agent for dental implants by efficiently inhibiting S. mutans biofilms | ||
| Coated implants with ZnO and WO3 NPs | – Evaluate the antibacterial activity against Staphylococcus aureus, Escherichia coli, Staphylococcus epidermidis and Pseudomonas aeruginosa | – ZnO was more bactericidal than WO3 | 121 |
5.4. Periodontal
Periodontal disease has increased in the last two decades as a prevalent bacterial-mediated inflammatory disease.130 It is one of the major public health problems caused by local accumulation of bacteria and their metabolic products in the region between the teeth and the gums and consequently leads to destructions at tooth-supporting tissues, including gums, alveolar bone, periodontal ligament and cementum.131–133Nanodentistry can provide useful solutions to treat periodontal disease.134 Therefore, many nanoscale systems have been expanded to treat periodontal disease efficiently.133 Metal oxide NPs as antimicrobial agents have excellent potential for periodontal therapy to improve its therapeutic efficacy. ZnO NPs properties exhibit good features among other NPs, including strong antimicrobial activity.135 Since 2010, much research has been conducted on the application of the ZnO NPs treatment in periodontal disease (Table 5).
| Formulations NPs | Applications | Findings | Ref. |
|---|---|---|---|
| PCL loaded with OTC–ZnO NPs | – Develop the PCL–OTC–ZnO nanofibers for use in the treatment of periodontal diseases | – PCL–OTC–ZnO nanofibers were non-cytotoxic | 133 |
| – The PCL–OTCz nanofibers developed have great potential as a drug delivery system for the PD treatment | |||
| – PCL–OTCz exhibited excellent activity against a mixed bacterial culture | |||
| PCL–ZnO NPs | – Evaluation of the osteoconductivity and antibacterial properties | – The engineered membrane exerts both osteoconductive and antibacterial properties | 140 |
| – Demonstrating its great potential for periodontal tissue engineering | |||
| Sealer incorporated with Ag–ZnO NPs | – Evaluation of the antimicrobial efficacy of ZnO based sealer incorporated with silver and zinc oxide NPs compared with simple zinc oxide sealer against Enterococcus faecalis | – After three weeks from obturation, silver NPs had a superior antibacterial effect, but after one week of obturation there was no significant difference between the groups | 141 |
| ZnO NPs | – Estimate the effect of ZnO NPs on salivary AST activity in chronic periodontitis patients | – Increasing salivary activity in presence of ZnO NPs | 135 |
| ALP, AST–ZnO NPs | – Determine of kinetic study of ALP, AST and peroxidase in presence and absence of ZnO NPs in saliva of chronic periodontitis patients | – The activity of salivary AST was increased in presence of ZnO NPs | 142 |
| – The inhibited effect of ZnO NPs on both salivary ALP and peroxidase activities was confirmed | |||
| PCL/gelatin–ZnO NPs | – The synthesis of bioactive scaffolds for periodontal regeneration and \development of a cell-friendly disinfection strategy through the fabrication of drug delivery systems | – PCL/GEL-based membranes containing a low content of ZnO NPs can potentially function as a biologically safe antimicrobial membrane for guided tissue/bone regeneration with satisfactory cytocompatibility | 139 |
| ZnO NPs–gelatin–PLC membrane | – Evaluate the antimicrobial capacity and cytocompatibility of ZnO-loaded membranes for periodontal regeneration | – The mechanical properties of the membranes were reduced upon ZnO incorporation, except for PCL-based membranes containing ZnO at the 30 wt% concentration | 130 |
| – All ZnO-containing membranes displayed antibacterial activity | |||
| – All membranes synthesized demonstrated satisfactory cytocompatibility, although the presence of 30 wt% ZnO led to decreased viability |
Tissue regeneration is highly influenced by the lack of infection, and therefore, incorporation of antimicrobial agents such as ZnO NPs into guided tissue/bone generation membranes (GTR/GBR) can be utilized as a good barrier to prevent Porphyromonas gingivalis colonization, which is commonly involved in periodontitis.130,136
Münchow et al. successfully incorporated ZnO NPs and gelatin into PCL-based electrospun membranes.130 They investigated the materials characterization, antibacterial activity and cytocompatibility of these membranes in periodontal tissue regeneration. They reported that all ZnO-containing membranes demonstrated satisfactory cytocompatibility and antibacterial activity, but the mechanical properties of ZnO-containing membranes were decreased, except for membranes containing ZnO at the 30 wt% concentration.130 In addition, they revealed that the presence of 30 wt% ZnO led to decreased viability. However, another study suggested that the cytotoxicity of ZnO NPs on fibroblasts influenced by concentration and duration of exposure and ZnO NPs had good toxic effects on both of the primary fibroblastic cells at concentrations of 50–100 lg mL−1.137
Dias et al. studied the addition of two antibacterial agents, oxytetracycline hydrochloride (OTC) and ZnO to polycaprolactone (PCL) nanofibers to treat periodontal diseases.133 They found that polycaprolactone (PCL) nanofibers loaded with OTC and PCL loaded with OTC–ZNO displayed good antibacterial activity against a mixed bacterial culture, and PCL–OTCz nanofibers showed considerable potential as a drug delivery system to treat periodontal diseases.133
A serum albumin microsphere containing minocycline and ZnO NPs has been prepared and incorporated into a Carbopol 940VR hydrogel to investigate the ability of gingival tissue self-repairing. They reported that the hydrogel exhibited excellent physicochemical properties and better antibacterial activity when concentration of ZnO NPs was over 0.2 mg mL−1.138 Bottino developed bioactive poly(ε-caprolactone) (PCL) and PCL/gelatin (PCL/GEL) scaffolds loaded with distinct concentrations of ZnO NPs for periodontal regeneration. Finally, the potential of all ZnO-containing membranes as antibacterial agents was reported. They showed that the antibacterial activity was increased with increasing the ZnO content.139 Nasajpour et al. developed poly(caprolactone) (PCL) composite membrane containing ZnO NPs to increase the osteoconductive ability, antibacterial activity, and flexibility for periodontal tissue regeneration.140 In the last decade, increased research was conducted on the application of NPs in periodontal applications.
5.5. Prosthodontics
In prosthodontics, both dental prosthesis and denture appear to have a significant impact on improving the quality of oral health care.143 In the past few years, the trends have shifted toward denture base materials in the field of prosthodontics. In general, three main types of denture materials, including metal alloys, ceramics, and plastics have been regarded to produce complete dentures.144Polymethyl methacrylate (PMMA), also called “acrylic glass”, is one of the most widely used materials in dentistry for the fabrication of complete and partial removable dentures owing to its biocompatibility, ease of processing, and passable mechanical properties.145–148 It has been used as an essential part of prosthodontics practice since 1937.143 The thermal and mechanical properties of the PMMA composites were improved with the filler of ZnO NPs.149
The major drawback of this material is easy accumulation of denture plaque on its surface due to properties such as surface roughness and apparent porosity.145,148 Denture stomatitis is one of the most common clinical problems for complete denture wearers that are derived mainly from Candida albicans.145,147 Many researches have been developed on NPs to modify the chemical composition of PMMA.148 Moreover, NPs can increase the PMMA surface tension when they are implanted in a matrix of PMMA.150
Incorporation of NPs, such as zinc oxide, titanium dioxide, and barium sulfate, into resin is an efficient procedure to reduce Candida adhesion to repaired PMMA denture bases and removable prosthesis.145,151 Anaraki et al. investigated the antifungal ability of ZnO NPs in PMMA against C. albicans and Ag NPs in PMMA.147 Ag and Zno NPs could significantly decrease the population of C. Albicans, but Ag NPs showed higher antifungal activity than ZnO NPs.147 Cierech et al. examined the antifungal ability of both nanocomposites PMMA–ZnO-NPs and PMMA-coated ZnO NPs. They found that such a modification considerably improved the activity of the nanocomposite, and antifungal properties were increased with increasing the concentration of ZnO NPs.148 According to Cierech et al. zinc oxide released from the denture to the oral environment would induce antifungal effects on microorganisms without exerting cytotoxic effects on the host cells.81 Cierech et al. demonstrated that the increased hydrophilicity and hardness with absorbability within the normal range can explain the reduced microorganisms' growth on the denture base.151
Tabrez Khan et al. demonstrated the effect of ZnO and CuO NPs on oral cavity bacteria and assessed the inhibition of biofilm formation on different matrices such as glass, polystyrene plates, acrylic dentures. The CuO and ZnO NPs showed considerable inhibitory activity on oral bacteria and biofilm formation.47 Biocompatibility and antibacterial activity of a hybrid biomaterials based on self-polymerizing resin used in prosthodontics were improved by modifying the resin with zinc oxide/chitosan and Ag/zinc oxide/chitosan composite NPs.144
The applications of ZnO NPs in the modification of dentures or of implant surfaces, and consequently the continuous contact of the organism with these NPs might have a potential impact on human health. Pokrowiecki et al. investigated the stability and behaviour of ZnO and Ag nanoparticle (NP) compounds in saliva based when used against of oral Gram-positive and Gram-negative bacteria. They found that all NPs suspensions displayed significant destabilisation and high destabilisation over the 24 h of the analyses. The agglomeration processes of NPs in human saliva can be reversible.152
Impregnation of metallic NPs such as ZnO NPs into PMMA can improve the physical, mechanical, microbicidal and antifungal properties of the resin.147 The previous research exhibited that incorporation of ZnO NPs at 2.0 to 2.5% by weight into silicone could improve the mechanical properties, including hardness, tensile and strength.153
Popović synthesized a new composite material containing PMMA and ZnO NPs to identify its properties and behavior of modified PMMA. The ZnO NPs in the matrix of PMMA led to an increase of density owing to the reduced presence of residual monomers.150 Charoenkijkajorn and Sanohkan indicated that addition of 0.5 to 2.0% of ZnO NPs remarkable decreased the color change of the silicone elastomer, and addition of 1.5% of ZnO NPs could improve the color stability of silicone prosthesis.153
Moreover, Cierech et al. investigated the final ZnO NPs concentration on cytotoxicity and ZnO NPs released from PMMA–ZnO nanocomposites and the ZnO NPs layer produced on pure PMMA before being used as an alternative material for denture bases.81 Summary of studies examining use of ZnO NPs in prosthodontics applications are shown in Table 6.
| Formulations NPs | Applications | Findings | Ref. |
|---|---|---|---|
| Nanocomposites PMMA–ZnO NPs | – Antifungal properties for denture stomatitis treatment and prevention | – Antifungal properties increase with increasing concentration of ZnO-NPs | 148 |
| MDX4-4210 silicone prostheses–ZnO NPs | – Evaluation of color change of MDX4–4210 facial silicone elastomer | – Incorporation of 1.5% of nano zinc oxide can improve the color stability of silicone prosthesis | 153 |
| CuO and ZnO NPs | – The effect of ZnO- and CuO-NPs on oral cavity bacteria on acrylic dentures | – The strong bactericidal action of CuO and ZnO-NPs | 69 |
| – Significant reduction in the oral bacterial | |||
| PMMA–ZnO nanocomposites | – Evaluation of cytotoxicity and release of PMMA–ZnO nanocomposites designed for denture bases | – Significant antifungal effect on microorganisms without exerting a cytotoxic effect | 81 |
| PMMA–Ag/ZnO NPs | – Evaluation of antifungal effects of Ag/ZnO NPs in acrylic resin | – Ag/ZnO NPs could significantly decrease population of C. albicans | 147 |
| PMMA–ZnO NPs | – Evaluation of the flexural strength of PMMA–ZnO NPs | – The addition of ZnO NPs in all concentrations increased the flexural strength of PMMA–ZnO NPs | 154 |
| ZnO NPs-denture base material | – Evaluation of thermal diffusivity of ZnO NPs on flexible denture base material | – ZnO NPs improve the thermal diffusivity of flexible denture base material | 155 |
| PMMA–ZrO2/ZnO NPs | – The influence of ZrO2/ZnO NPs on properties of PMMA denture base | – The addition of ZrO2/ZnO NPs increase thermal conductivity, completion strength and a decrease roughness | 156 |
| PMMA–ZnO NPs | – Investigate the antifungal activity of ZnO NPs against C. albicans for the production of denture bases | – The first successful attempt to produce PMMA resin for bases of dentures modified with ZnO NPs | 46 |
| Tissue conditioner–ZnO–Ag NPs | – Evaluate the antibacterial and antifungal properties of a tissue conditioner used in complete dentures | – Inhibition of bacterial proliferation | 157 |
| PMMA–metal oxides nanomaterials | – Prevention of Candida colonies on PMMA denture base by altering the surface and incorporations of NPs | – Significant influence of nanocomposites PMMA–ZnO-NPs on C. albicans solution | 158 |
| – The effectiveness of sputtering of ZnO NPs on the PMMA | |||
| PMMA/ZnO NPs | – Reinforcement of PMMA denture base resin with ZnO NPs | – The addition of ZnO NPs improve the mechanical and thermal properties of denture base materials | 159 |
| – Improve in flexural strength |
Therefore, many attempts have been made to modify the chemical composition of denture resins by NPs in order to treat and prevent denture stomatitis in patients vulnerable to this disease.
5.6. Orthodontics
Nanoparticles can be incorporated into various aspects of orthodontics to enhance the quality of treatment.160 Excellent properties of NPs make them applicable as nano-coated archwires.161 orthodontic adhesives,162 orthodontic brackets.161 Nanoparticles can be well coated on to the surfaces of orthodontic appliances in order to reduce microbial adhesion and enamel demineralization.161,162 Both orthodontic attachments and bonding materials such as the resin orthodontic brackets for bonding may provide a platform for microbial plaque to accumulate, and consequently incidence of white spot lesions, enamel demineralization and tooth decay that are an unacceptable in results of orthodontic treatment.163,164Addition of antimicrobial agents to orthodontic material can minimize enamel demineralization.165 Antimicrobial NPs like ZnO NPs are used mostly in orthodontic applications, including nano-adhesives, composites and GICs.161 Table 7 summarizes studies examining the use of ZnO NPs in orthodontic applications.
| Formulations NPs | Applications | Findings | Ref. |
|---|---|---|---|
| Light cure glass ionomer cement containing ZnO | – The effect of addition of zinc oxide on the shear bond strength of the bonding material | – The shear bond strength decreases as the concentration of ZnO increases | 175 |
| – The antimicrobial effect of zinc oxide when incorporated into an orthodontic bonding material against S. mutans | – The antimicrobial effect of zinc oxide lasts at least for 1 month | ||
| – Zinc oxide powder when added to GIC produces antimicrobial effect, which increases as the concentration of zinc oxide is increased | |||
| Transbond ™ XT adhesive containing ZnO NPs | – The antibacterial properties of orthodontic adhesive primer against S. mutans after adding the three different types of NPs (Ag, ZnO, or TiO2) | – The incorporation of these NPs (Ag, ZnO, and TiO2) into Transbond ™ XT adhesive primer helps to enhance the antibacterial properties of primer against the S. mutans | 165 |
| Orthodontic adhesive incorporating cCur/ZnONPs | – Evaluate the antimicrobial properties of an orthodontic adhesive incorporating cationic curcumin doped zinc oxide NPs (cCur/ZnONPs) against cariogenic bacteria including S. mutans, S. sobrinus, and L. acidophilus | – Adhesive with 7.5% wt. cCur/ZnONPs showed the highest concentration of cCur/ZnONPs and shear bond strength value | 166 |
| – The photo-activated 7.5% wt cCur/ZnONPs can serve as an orthodontic adhesive additive to control the cariogenic multispecies biofilm | |||
| Stainless steel orthodontic wire and bracket coated with ZnO NPs | – Evaluate frictional forces by coating orthodontic wires and porcelain brackets with zinc oxide NPs | – The frictional force coated wire and bracket (3.07 ± 0.4 N) was the highest | 170 |
| – Coating of porcelain bracket surfaces with ZnO NPs can decrease friction in the sliding technique | |||
| Adhesives incorporated with Ag, ZnO, and TiO2 NPs | – Investigate the influence of silver (Ag), zinc oxide (ZnO), and titanium dioxide (TiO2) NPs on shear bond strength | – Incorporation of various NPs into adhesive materials in minimal amounts may decrease SBS and may lead to the failure of bracket or adhesive | 162 |
| NiTi orthodontic wires coated with ZnO NPs | – Friction-reducing and antibacterial coating with zinc oxide (ZnO) NPs on nickel-titanium (NiTi) wire | – Stable and well-adhered ZnO coating on the NiTi wires was obtained | 171 |
| – The coated wires presented up to 21% reduction in the frictional forces and antibacterial activity against Streptococcus mutans | |||
| – ZnO nanocoating significantly improved the surface quality of NiTi wires | |||
| Brackets coated with ZnO, CuO NPs | – Comparison of antibacterial effects of ZnO and CuO NPs coated brackets against Streptococcus mutans | – The growth of S. mutans was significantly reduced by ZnO NPs in comparison with the control group | 168 |
| – CuO and ZnO–CuO NPs coated brackets have better antimicrobial effect on S. mutans than ZnO coated brackets | |||
| RMGICs–ZnO NPs | – Evaluate the shear bond strength of resin-modified glassionomer cements (RMGICs) modified by nano-zinc oxide (NZnO) and nano hydroxyapatite (NHA) in comparison with composite resins | – Adding 2% NZnO and 5% NHA particles to RMGICs had no negative effect on their SBS in comparison with composite resin | 174 |
| – RMGICs can be as effective as composite resins for bonding of metal brackets to enamel surfaces | |||
| Dental composite incorporated with ZnO–chitosan NPs | – Antibacterial effectiveness of adding ZnO and chitosan NPs used in dental composite for orthodontic against Streptococcus mutans, Streptococcus sanguis and Lactobacillus acidophilus grown | – A mixture of ZnO-NPs and CS-NPs has induced an antibacterial activity in resin composite; especially in 10% weight concentrations | 164 |
| Polymer adhesives containing the ZnO:Eu3+ NPs | – Examine photoluminescent and mechanical properties of polymerized adhesive containing the ZnO:Eu3+ NPs | – Safer and complete removal of orthodontic adhesives after orthodontic treatments | 172 |
| – Feasible incorporation of Eu3+-doped ZnO NPs into orthodontic adhesives | |||
| Nano-ZnO incorporated titania composite | – Evaluate the anticorrosive and antibacterial applications | – The incorporation of nano-ZnO in the titania coating can improve the corrosion resistance and antibacterial activity of the coating due to the synergistic actions of both titania and ZnO, with special reference to orthodontic applications | 167 |
Mirhashemiet et al. examined the inhibition of L. acidophilus, S. sanguis and S. mutans biofilms using ZnO NPs and CS NPs blended with orthodontic composite, which showed increased antibacterial activity in the resin composite, especially in 10% weight concentrations.164
The antimicrobial activity of an orthodontic adhesive incorporating cationic curcumin (Cur) doped ZnO NPs was evaluated by Pourhajibagher et al. They reported that Cur/ZnO NPs could be used as an orthodontic adhesive to control the cariogenic multispecies biofilm, and to reduce their metabolic activity.166 Shibli et al. reported high corrosion resistance and antibacterial activity were reported for ZnO NPs incorporated nanotitania coating.167 In addition, the ability of ZnO NPs to reduce demineralization and enhance remineralization has been proven.164
Ramazanzadeh et al. investigated the antibacterial effects of coated brackets with ZnO and CuO NPs on S. mutans to decrease the risk of caries around the orthodontic brackets during the treatment.168 The ZnO–CuO NPs coated brackets exhibited better antimicrobial effect on S. mutans than ZnO coated brackets,169 and after two hours the number of bacteria were reduced to zero in ZnO–CuO NPs coated brackets. Nowadays, there is an increased trend to use ceramic brackets instead of steel brackets, but these brackets generate more friction resistance than steel brackets.170
Numerous studies have been conducted to coat stainless steel wires with ZnO NPs to determine the friction between wires and orthodontic brackets.162,170,171
Behroozian et al. reported that the coating of porcelain bracket surfaces with ZnO NPs could reduce friction in the sliding technique, and they did not recommend wire coating combined with bracket coating due to its effect on friction.170 Kachoei et al. investigated the antibacterial characteristics of orthodontic wires and brackets when coated with ZnO NPs. They found that the frictional forces of coated wires were decreased, and their antibacterial activity against S. mutans was increased.171
Nano-adhesives are used for bonding orthodontic applications with several advantages such as longer shelf life, higher dentin and enamel bond strength, higher stress absorption and durable marginal seal.161 Khatria et al. evaluated Eu3+ doped ZnO NPs as an orthodontic adhesive to make them visible for complete removal after orthodontic treatment.172 Recently, it has been shown that bacterial adhesion to chitosan (CS) and ZnO NPs is less than that of conventional composites. Studies in the literature established that addition of titanium dioxide, zinc oxide, and silver NPs to adhesive materials in minimal amounts might decrease shear bond strength and lead to the failure of bracket or adhesive.162
The use of GICs as orthodontic bonding agents has attracted researchers' attention.148 Jatania et al. concluded that improved antibacterial properties were seen in ZnO NPs added to an orthodontic bonding material. They showed that the shear bond strength was decreased when the concentration of ZnO was increased.173 However, other studies have reported that ZnO NPs improve the physical properties and flexural strength of GICs, since these particles bind to the polyacrylic liquid of GICs.174 ZnO NPs can be widely used in orthodontic applications for better treatment, including improved strength of utilized materials, and reduced microorganisms.
6. Conclusion
Nanotechnology and its role in caries therapy are an integral part of ongoing from efficacy research. Nanomaterials used in dental materials are more efficient than conventional materials. As shown above, ZnO NPs are biocompatible, biosafe, and nontoxic metal oxide NPs and act as a strong antibacterial agent against a broad range of bacteria (Gram-positive and Gram-negative) and fungi. Several studies have demonstrated that ZnO NPs possess an inhibitory effect against A. hydrophila, B. subtillis, E. faecalis, E. coli, S. typhimurium, S. Aureus, S. enteritidis, S. pyogenes, L. monocytogenes, K. pneumonia, and P. aeruginosa. Generally, size, shape, dissolution, surface charges, aggregation, and concentration are the key factors playing a crucial role in the synthesis of ZnO NPs. The antibacterial properties increase with increasing the surface area. Still many problems related to targeted delivery and toxicity need to be solved. Moreover, further studies are needed to investigate the ZnO NPs release and long-term properties of new ZnO NPs containing dental materials. According to studies, the dispersion of ZnO NPs in resin and composites can increase their flexural strength, decrease the shear bond strength, and decrease the compressive strength. Thus, it is important to find a balance between antimicrobial capability and mechanical properties, and more in vivo experiments are required in this regard.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by grants (990517) from the Kermanshah University of Medical Sciences.References
- R. Rezaei, M. Safaei, H. R. Mozaffari, H. Moradpoor, S. Karami, A. Golshah, B. Salimi and H. Karami, Open Access Maced. J. Med. Sci., 2019, 7, 1884–1890 CrossRef PubMed.
- A. Das and I. Nasim, J. Adv. Pharm. Educ. Res., 2017, 7, 43–45 CAS.
- J. Sharan, S. Singh, S. V. Lale, M. Mishra, V. Koul and O. P. Kharbanda, J. Nanosci. Nanotechnol., 2017, 17, 2235–2255 CrossRef CAS.
- A. Bharghava, J. Cheung, M. M. Eshaghian-Wilner, W. Lee and K. Ravicz, IJNST, 2016, 5, 100–101 Search PubMed.
- R. S. Chaughule, Dental applications of nanotechnology, Springer, 2018, p. 277, DOI:10.1007/978-3-319-97634-1.
- S. Chen, R. Yuan, Y. Chai and F. Hu, Microchim. Acta, 2013, 180, 15–32 CrossRef CAS.
- R. Agnihotri, S. Gaur and S. Albin, Biol. Trace Elem. Res., 2019, 1–19 Search PubMed.
- H. M. Xiong, Adv. Mater., 2013, 25, 5329–5335 CrossRef CAS.
- A. Besinis, T. De Peralta, C. J. Tredwin and R. D. Handy, ACS Nano, 2015, 9, 2255–2289 CrossRef CAS PubMed.
- W. Song and S. Ge, Molecules, 2019, 24, 1033 CrossRef CAS.
- J. M. Corrêa, M. Mori, H. L. Sanches, A. D. d. Cruz, E. Poiate and I. A. V. P. Poiate, Int. J. Biomater., 2015, 2015, 485275 Search PubMed.
- M. Taran, M. Safaei, N. Karimi and A. Almasi, Biointerface Res. Appl. Chem., 2021, 11, 7860–7870 CAS.
- S. Kargozar and M. Mozafari, Mater. Today: Proc., 2018, 5, 15492–15500 CAS.
- M. Safaei, M. Taran, L. Jamshidy, M. M. Imani, H. R. Mozaffari, R. Sharifi, A. Golshah and H. Moradpoor, Int. J. Biol. Macromol., 2020, 158, 477–485 CrossRef CAS PubMed.
- R. N. Krishna, R. Gayathri and V. Priya, Int. J. Pharma Sci. Res., 2017, 9, 24 CAS.
- T. S. Lopes, G. G. Alves, M. R. Pereira, J. M. Granjeiro and P. E. C. Leite, J. Cell. Biochem., 2019, 120, 16370–16378 CrossRef CAS PubMed.
- B. A. Ranjeet, J. P. Chaitanya, B. Prachi, C. V. Tanay, P. Rohit, J. Naveen, G. Bapi, K. Sukant and K. Prashant, Drug Discovery Today, 2019, 24, 85–98 CrossRef.
- H. Lboutounne, Int. J. Dent. Oral Health, 2017, 3, 145–157 Search PubMed.
- R. A. Bapat, T. V. Chaubal, C. P. Joshi, P. R. Bapat, H. Choudhury, M. Pandey, B. Gorain and P. Kesharwani, Mater. Sci. Eng., C, 2018, 91, 881–898 CrossRef CAS PubMed.
- G. C. Padovani, V. P. Feitosa, S. Sauro, F. R. Tay, G. Durán, A. J. Paula and N. Durán, Trends Biotechnol., 2015, 33, 621–636 CrossRef CAS PubMed.
- G. Schmalz, R. Hickel, K. L. van Landuyt and F. X. Reichl, Dent. Mater., 2017, 33, 1298–1314 CrossRef CAS PubMed.
- M. Biondi, A. Borzacchiello, L. Mayol and L. Ambrosio, Gels, 2015, 1, 162–178 CrossRef PubMed.
- L. Rastogi, A. J. Kora and J. Arunachalam, Mater. Sci. Eng., C, 2012, 32, 1571–1577 CrossRef CAS PubMed.
- E. Bílková, A. Imramovský, V. Buchta and M. Sedlák, Int. J. Pharm., 2010, 386, 1–5 CrossRef PubMed.
- Y. N. Slavin, J. Asnis, U. O. Häfeli and H. Bach, J. Nanobiotechnol., 2017, 15, 1–20 CrossRef PubMed.
- E. Ferrando-Magraner, C. Bellot-Arcís, V. Paredes-Gallardo, J. M. Almerich-Silla, V. García-Sanz and M. Fernández-Alonso, Medicina, 2020, 56, 55 CrossRef PubMed.
- F. Shafiei, A. Ashnagar, M. Ghavami-Lahiji, F. Najafi and S. M. Amin Marashi, J. Dent. Biomater., 2018, 5, 510–519 CAS.
- P. Chandra Mouli, S. Manoj Kumar and S. Parthiban, Int. J. Biol. Med. Res., 2012, 3, 1550–1553 Search PubMed.
- A. Borzabadi-Farahani, E. Borzabadi and E. Lynch, Biomater. Invest. Dent., 2014, 72, 413–417 CAS.
- M. Samiei, A. Farjami, S. M. Dizaj and F. Lotfipour, Mater. Sci. Eng., C, 2016, 58, 1269–1278 CrossRef CAS PubMed.
- P. N. Shah and S. Kapoor, Int. J. Pharm. Sci. Invent., 2017, 6, 1–6 CAS.
- M. Safaei, M. Taran, M. M. Imani, H. Moradpoor, F. Rezaei, L. Jamshidy and R. Rezaei, Pol. J. Chem. Technol., 2019, 21, 116–122 CrossRef CAS.
- J. Wang, S. Gao, S. Wang, Z. Xu and L. Wei, Int. J. Nanomed., 2018, 13, 3441 CrossRef CAS PubMed.
- A. Ali, A. R. Phull and M. Zia, Nanotechnol. Rev., 2018, 7, 413–441 CrossRef CAS.
- S. Verma and S. P. Singh, MRS Commun., 2019, 9, 1227–1234 CrossRef CAS.
- N. Wiesmann, W. Tremel and J. Brieger, J. Mater. Chem. B, 2020, 8, 4973–4989 RSC.
- A. Król, P. Pomastowski, K. Rafińska, V. Railean-Plugaru and B. Buszewski, Adv. Colloid Interface Sci., 2017, 249, 37–52 CrossRef PubMed.
- E. Demir, A. Creus and R. Marcos, J. Toxicol. Environ. Health, Part A, 2014, 77, 1292–1303 CrossRef CAS PubMed.
- Y. M. Mohamed and Y. A. Attia, Appl. Organomet. Chem., 2020, 34, 5758 CrossRef.
- D. B. Bharti and A. V. Bharati, Luminescence, 2017, 32, 317–320 CrossRef CAS PubMed.
- K. Pemartin, C. Solans, G. Vidal-Lopez and M. Sanchez-Dominguez, Chem. Lett., 2012, 41, 1032–1034 CrossRef CAS.
- K. Richter, A. Birkner and A. V. Mudring, Angew. Chem., 2010, 49, 2431–2435 CrossRef CAS PubMed.
- K. Senthilkumar, O. Senthilkumar, S. Morito, T. Ohba and Y. Fujita, J. Nanopart. Res., 2012, 14, 1–9 CrossRef.
- Q. Li, Y. Chen, L. Luo, L. Wang, Y. Yu and L. Zhai, J. Alloys Compd., 2013, 560, 156–160 CrossRef CAS.
- X. Bai, L. Li, H. Liu, L. Tan, T. Liu and X. Meng, ACS Appl. Mater. Interfaces, 2015, 7, 1308–1317 CrossRef CAS PubMed.
- J. Wojnarowicz, T. Chudoba, S. Gierlotka and W. Lojkowski, Nanomaterials, 2018, 8, 343 CrossRef PubMed.
- N. G. Shimpi, S. Jain, N. Karmakar, A. Shah, D. C. Kothari and S. Mishra, Appl. Surf. Sci., 2016, 390, 17–24 CrossRef CAS.
- Y. T. Chung, M. M. Ba-Abbad, A. W. Mohammad, N. H. Hairom and A. Benamor, Mater. Des., 2015, 87, 780–787 CrossRef CAS.
- H. Agarwal, S. V. Kumar and S. Rajeshkumar, Resour.-Effic. Technol., 2017, 3, 406–413 Search PubMed.
- S. Ahmed, S. A. Chaudhry and S. Ikram, J. Photochem. Photobiol., B, 2017, 166, 272–284 CrossRef CAS PubMed.
- S. Sharma, K. Kumar, N. Thakur, S. Chauhan and M. S. Chauhan, Bull. Mater. Sci., 2020, 43, 1–10 CrossRef.
- M. L. Kahn, M. Monge, V. Colliere, F. Senocq, A. Maisonnat and B. Chaudret, Adv. Funct. Mater., 2005, 15, 458–468 CrossRef CAS.
- K. Nejati, Z. Rezvani and R. Pakizevand, Int. Nano Lett., 2011, 1, 75–81 CAS.
- S. Lee, S. Jeong, D. Kim, S. Hwang, M. Jeon and J. Moon, Superlattices Microstruct., 2008, 43, 330–339 CrossRef CAS.
- L. Wang and M. Muhammed, J. Mater. Chem., 1999, 9, 2871–2878 RSC.
- A. Dakhlaoui, M. Jendoubi, L. S. Smiri, A. Kanaev and N. Jouini, J. Cryst. Growth, 2009, 311, 3989–3996 CrossRef CAS.
- V. V. Gopal and S. Kamila, Appl. Nanosci., 2017, 7, 75–82 CrossRef.
- S. Fakhari, M. Jamzad and H. Kabiri Fard, Green Chem. Lett. Rev., 2019, 12, 19–24 CrossRef CAS.
- A. K. Zak, R. Razali, W. H. Abd Majid and M. Darroudi, Int. J. Nanomed., 2011, 6, 1399 Search PubMed.
- G. P. Barreto, G. Morales and M. L. Quintanilla, J. Mater., 2013, 478681 Search PubMed.
- R. Kumar, A. Umar, G. Kumar and H. S. Nalwa, Ceram. Int., 2017, 43, 3940–3961 CrossRef CAS.
- A. K. Srivastav, M. Kumar, N. G. Ansari, A. K. Jain, J. Shankar, N. Arjaria, P. Jagdale and D. Singh, Hum. Exp. Toxicol., 2016, 35, 1286–1304 CrossRef CAS PubMed.
- K. S. Siddiqi, A. ur Rahman and A. Husen, Nanoscale Res. Lett., 2018, 13, 1–13 CrossRef PubMed.
- A. Sirelkhatim, S. Mahmud, A. Seeni, N. H. Kaus, L. C. Ann, S. K. Bakhori, H. Hasan and D. Mohamad, Nano-Micro Lett., 2015, 7, 219–242 CrossRef CAS PubMed.
- S. P. K. Malhotra and T. Mandal, Scirea J. Chem., 2016, 1, 67–89 Search PubMed.
- Y. Y. Chang, C. H. Lai, J. T. Hsu, C. H. Tang, W. C. Liao and H. L. Huang, Clin. Oral Investig., 2012, 16, 95–100 CrossRef PubMed.
- A. A. Tayel, W. F. El-Tras, S. Moussa, A. F. El-Baz, H. Mahrous, M. F. Salem and L. Brimer, J. Food Saf., 2011, 31, 211–218 CrossRef CAS.
- M. Eshed, J. Lellouche, S. Matalon, A. Gedanken and E. Banin, Langmuir, 2012, 28, 12288–12295 CrossRef CAS PubMed.
- S. T. Khan, M. Ahamed, A. Al-Khedhairy and J. Musarrat, Mater. Lett., 2013, 97, 67–70 CrossRef.
- J. Wang, L. Du, Y. Fu, P. Jiang, X. Wang and J. Zhang, BMC Oral Health, 2019, 19, 1–11 CrossRef PubMed.
- F. Mirhosseini, M. Amiri, A. Daneshkazemi, H. Zandi and Z. S. Javadi, Front. Dent., 2019, 16, 105 Search PubMed.
- S. K. M. Bakhori, S. Mahmud, C. A. Ling, A. H. Sirelkhatim, H. Hasan, D. Mohamad, S. A. Masudi, A. Seeni and R. Abd Rahman, Mater. Sci. Eng., C, 2017, 78, 868–877 CrossRef PubMed.
- A. A. Balhaddad, A. A. Kansara, D. Hidan, M. D. Weir, H. H. Xu and M. A. S. Melo, Bioact. Mater., 2019, 4, 43–55 CrossRef PubMed.
- U. Lohbauer, Materials, 2010, 3, 76–96 CrossRef CAS.
- S. Mahna, H. Singh, S. Tomar, D. Bhagat, A. Patnaik and S. R. Kumar, Nanotechnology, 2019, 8, 90–99 CAS.
- A. S. Budi, A. H. Permana, H. Nasbey and Y. Fujii, J. Tech. Soc. Sci., 2017, 1, 47–55 Search PubMed.
- E. Eid, A. Fouda and E. S. M. Duraia, Mater. Sci. Eng., A, 2016, 657, 104–114 CrossRef CAS.
- B. N. Raju, K. Ramji and V. Prasad, Mater. Today: Proc., 2015, 2, 2817–2825 Search PubMed.
- L. Dara, D. Buchi, S. R. Mantena, M. Varma and V. Chandrappa, Int. J. Dent. Mater., 2019, 1, 48–54 CrossRef.
- B. Pratap, R. K. Gupta, L. Denis and D. Goswami, Mater. Today: Proc., 2020, 21, 1563–1565 CAS.
- M. Cierech, J. Wojnarowicz, A. Kolenda, A. Krawczyk-Balska, E. Prochwicz, B. Woźniak, W. Łojkowski and E. Mierzwińska-Nastalska, Nanomaterials, 2019, 9, 1318 CrossRef CAS PubMed.
- B. Aydin Sevinç and L. Hanley, J. Biomed. Mater. Res., Part B, 2010, 94, 22–31 Search PubMed.
- H. B. Dias, M. I. B. Bernardi, V. S. Marangoni, A. C. de Abreu Bernardi, A. N. de Souza Rastelli and A. C. Hernandes, Mater. Sci. Eng., C, 2019, 96, 391–401 CrossRef CAS PubMed.
- F. Abd Kati, J. Oral. Res., 2019, 8, 37–41 CrossRef.
- F. A. K. Al-Shammari, Mustansiriya Dent. J., 2016, 13, 77–84 Search PubMed.
- Y. Wang, H. Hua, W. Li, R. Wang, X. Jiang and M. Zhu, J. Dent., 2019, 80, 23–29 CrossRef CAS PubMed.
- S. T. Hojati, H. Alaghemand, F. Hamze, F. A. Babaki, R. Rajab-Nia, M. B. Rezvani, M. Kaviani and M. Atai, Dent. Mater., 2013, 29, 495–505 CrossRef PubMed.
- N. Angel Villegas, M. J. Silvero Compagnucci, M. M. Sainz Ajá, A. Sainz, D. M. Rocca, M. C. Becerra, G. Fabián Molina and S. D. Palma, J. Healthc. Eng., 2019, 2019, 6367919 Search PubMed.
- M. A. Karimi, M. Mazloum Ardakani, R. Asadiniya and S. Haghdar Roozbahani, Int. Nano Lett., 2011, 1, 43–51 CAS.
- M. Cierech, J. Wojnarowicz, D. Szmigiel, B. Bączkowski, A. M. Grudniak, K. I. Wolska, W. Łojkowski and E. Mierzwińska-Nastalska, Acta. Bioeng. Biomech., 2016, 18, 31–41 Search PubMed.
- D. Kim, H. Abo-Mosallam, H. Y. Lee, J. H. Lee, H. W. Kim and H. H. Lee, J. Appl. Oral Sci., 2015, 23, 369–375 CrossRef CAS PubMed.
- P. P. Vanajassun, M. Nivedhitha, N. Nishad and D. Soman, Adv. Hum. Biol., 2014, 4, 31 Search PubMed.
- P. Agarwal, R. Nayak, P. N. Upadhya, K. Ginjupalli and L. Gupta, Mater. Today: Proc., 2018, 5, 16065–16072 CAS.
- P. P. N. S. Garcia, M. F. B. Cardia, R. S. Francisconi, L. N. Dovigo, D. M. P. Spolidório, A. N. de Souza Rastelli and A. C. Botta, Microsc. Res. Tech., 2017, 80, 456–461 CrossRef CAS PubMed.
- N. Yahya, P. Puspitasari and N. R. A. Latiff, Characterization and Development of Biosystems and Biomaterials, Springer, 2013, pp. 9–32 Search PubMed.
- A. A. A. Aziz and A. M. S. Mostafa, Egypt. Dent. J., 2019, 65, 1741–1750 CrossRef.
- J. M. Guerreiro-Tanomaru, A. Trindade-Junior, B. C. Costa, G. F. da Silva, L. Drullis Cifali, M. I. Basso Bernardi and M. Tanomaru-Filho, Sci. World J., 2014, 2014, 975213 Search PubMed.
- M. S. Rad, TNT2012 is an EPS sponsored conference, 2012 Search PubMed.
- S. S. Shah, Pain, 2016, 105, 90–95 Search PubMed.
- M. R. Subhashini and J. D. Raj, Drug Invent. Today, 2018, 4, 3623–3629 Search PubMed.
- N. Mutoh and N. Tani-Ishii, Dent. Mater. J., 2011, 1103080120 Search PubMed.
- C. Poggio, M. Lombardini, M. Colombo, A. Dagna, E. Saino, C. R. Arciola and L. Visai, Int. J. Artif. Organs, 2011, 34, 908–913 CrossRef CAS PubMed.
- G. Singh, I. Gupta, F. M. Elshamy, N. Boreak and H. E. Homeida, Eur. J. Dent., 2016, 10, 366–369 CrossRef PubMed.
- S. N. Kozuszko, M. A. Sánchez, M. I. G. de Ferro, A. M. Sfer, A. P. M. Madrid, K. Takabatake, K. Nakano, H. Nagatsuka and A. P. Rodríguez, J. Hard Tissue Biol., 2017, 26, 311–318 CrossRef CAS.
- M. Kazemipoor, R. Hakimian and L. Akhoondzadeh, J. Biomed., 2019, 7, 1–13 Search PubMed.
- P. Nicolai, M. Mensi, F. Marsili, M. Piccioni, S. Salgarello, E. Gilberti and P. Apostoli, Acta Otorhinolaryngol. Ital., 2015, 35, 93 CAS.
- K. M. Trichês, J. S. Júnior, J. B. Calixto, R. Machado, T. Pereira Rosa, N. L. S. Emmanuel João and L. Pascoal Vansan, Microsc. Res. Tech., 2013, 76, 1292–1296 CrossRef PubMed.
- E. F. Rahman and S. Christiono, Dent. J., 2019, 6, 113–117 Search PubMed.
- S. Markan, G. Lehl and S. Kapoor, J. Dent. Oral. Biol., 2017, 2, 21067 Search PubMed.
- A. Shrestha, S. Zhilong, N. K. Gee and A. Kishen, J. Endod., 2010, 36, 1030–1035 CrossRef PubMed.
- J. R. Paul, Effectiveness of zinc-oxide nanoparticles compared to NaOCl/EDTA treatment in eliminating Enterococcus faecalis from infected root canals, Saint Louis University, 2012 Search PubMed.
- M. S. Rad, A. Kompany, A. K. Zak, M. Javidi and S. Mortazavi, J. Nanopart. Res., 2013, 15, 1925 CrossRef.
- M. J. Alves, L. Grenho, C. Lopes, J. Borges, F. Vaz, I. P. Vaz and M. H. Fernandes, Mater. Sci. Eng., C, 2018, 92, 840–848 CrossRef CAS PubMed.
- M. Zarei, M. Javidi, M. Gharechahi, M. Joybari, P. Tajzadeh and M. Arefnejad, J. Dent. Mater. Tech., 2018, 7, 167–173 Search PubMed.
- W. M. ElKateb, A. G. Massoud, N. A. Mokhless and T. I. Shalaby, J. Am. Sci., 2015, 11, 111–122 Search PubMed.
- M. Javidi, M. Zarei, N. Naghavi and M. Mortazavi, Contemp. Clin. Dent., 2014, 5, 20 CrossRef CAS PubMed.
- F. Parnia, J. Yazdani, V. Javaherzadeh and S. M. Dizaj, J. Pharm. Pharm. Sci., 2017, 20, 148–160 CAS.
- S. Kulshrestha, S. Khan, R. Meena, B. R. Singh and A. U. Khan, Biofouling, 2014, 30, 1281–1294 CrossRef CAS PubMed.
- Y. Xiang, X. Liu, C. Mao, X. Liu, Z. Cui, X. Yang, K. W. K. Yeung, Y. Zheng and S. Wu, Mater. Sci. Eng., C, 2018, 85, 214–224 CrossRef CAS PubMed.
- E. H. Abdulkareem, K. Memarzadeh, R. Allaker, J. Huang, J. Pratten and D. Spratt, J. Dent., 2015, 43, 1462–1469 CrossRef CAS PubMed.
- K. Memarzadeh, M. Vargas, J. Huang, J. Fan and R. Allaker, Key Eng. Mater., 2012, 493, 489–494 Search PubMed.
- L. D. Trino, L. F. Dias, L. G. Albano, E. S. Bronze-Uhle, E. C. Rangel, C. F. Graeff and P. N. Lisboa-Filho, Ceram. Int., 2018, 44, 4000–4008 CrossRef CAS.
- K. Memarzadeh, A. S. Sharili, J. Huang, S. C. Rawlinson and R. P. Allaker, J. Biomed. Mater. Res., Part A, 2015, 103, 981–989 CrossRef PubMed.
- S. Goel, P. Dubey, S. Ray, R. Jayaganthan, A. B. Pant and R. Chandra, Mater. Technol., 2019, 34, 32–42 CrossRef CAS.
- Y. Li, X. Liu, L. Tan, Z. Cui, X. Yang, K. W. Yeung, H. Pan and S. Wu, Mater. Sci. Eng., C, 2017, 76, 50–58 CrossRef CAS PubMed.
- Y. Xiang, J. Li, X. Liu, Z. Cui, X. Yang, K. W. K. Yeung, H. Pan and S. Wu, Mater. Sci. Eng., C, 2017, 79, 629–637 CrossRef CAS PubMed.
- L. Hao, Y. Hu, Y. Zhang, W. Wenzhen, H. Xiaochen, G. Yiqiao, H. Xiyu and J. Dong, RSC Adv., 2018, 8, 27304–27317 RSC.
- D. M. Al-Deen, B. G. Ali and A. S. Al-Mizraqchi, Indian J. Public Health Res. Dev., 2019, 10, 1556–1562 CrossRef.
- Y. Zhu, X. Liu, K. W. Yeung, P. K. Chu and S. Wu, Appl. Surf. Sci., 2017, 400, 14–23 CrossRef CAS.
- E. A. Münchow, M. T. Albuquerque, B. Zero, K. Kamocki, E. Piva, R. L. Gregory and M. C. Bottino, Dent. Mater., 2015, 31, 1038–1051 CrossRef PubMed.
- K. K. Ghudhaib, L. Ibrahim, E. A. Salman and Z. A. Salman, IOSR J. Appl. Chem., 2016, 9, 57–63 CAS.
- N. Aminu, S. Y. Chan and S. M. Toh, J. Appl. Pharm. Sci., 2017, 7, 234–242 CAS.
- A. M. Dias, F. G. da Silva, A. P. de Figueiredo Monteiro, A. D. Pinzón-García, R. D. Sinisterra and M. E. Cortés, Mater. Sci. Eng., C, 2019, 103, 109798 CrossRef CAS PubMed.
- S. Talalabd, W. L. Abdulla, R. A. Salman and Z. A. Salman, Int. J. Sci. Res., 2017, 6, 2197–2200 Search PubMed.
- K. K. Ghudhaib, L. Ibrahim, E. A. Al-Rubaee and Z. A. Salman, Int. J. Adv. Res. Biol. Sci., 2016, 3, 179–186 CAS.
- T. Cherian, K. Ali, S. Fatima, Q. Saquib, S. M. Ansari, H. A. Alwathnani, A. A. Al-Khedhairy, M. Al-Shaeri and J. Musarrat, J. Microbiol. Methods, 2019, 166, 105716 CrossRef CAS PubMed.
- Ş. Şeker, A. E. Elcin, T. Yumak, A. Sınağ and Y. M. Elcin, Toxicol. In Vitro, 2014, 28, 1349–1358 CrossRef PubMed.
- J. Mou, Z. Liu, J. Liu, J. Lu, W. Zhu and D. Pei, Drug Delivery, 2019, 26, 179–187 CrossRef CAS PubMed.
- M. C. Bottino, Microsc. Microanal., 2016, 22, 996–997 CrossRef.
- A. Nasajpour, S. Ansari, C. Rinoldi, A. Shahrokhi Rad, T. Aghaloo, S. Ryon Shin, Y. Kumar Mishra, R. Adelung, W. Swieszkowski, N. Annabi, A. Khademhosseini, A. Moshaverinia and A. Tamayo, Adv. Funct. Mater., 2018, 28, 1703437 CrossRef.
- F. Hala, M. Mohammed and I. Thanaa, Alex. Dent. J., 2016, 41, 169–175 CrossRef.
- K. K. Ghudhaib, L. M. Ibrahim, E. A. Al-Rubaee and Z. A. Salman, Int. J. Adv. Res. Biol. Sci, 2016, 3, 85–91 CAS.
- F. Saeed, N. Muhammad, A. S. Khan, F. Sharif, A. Rahim, P. Ahmad and M. Irfan, Mater. Sci. Eng., C, 2020, 106, 110167 CrossRef CAS PubMed.
- A. Zaharia, V. Muşat, V. P. Ghisman and N. Baroiu, Eur. Polym. J., 2016, 84, 550–564 CrossRef CAS.
- M. M. Gad, A. M. Al-Thobity, S. Y. Shahin and B. T. Alsaqer, Int. J. Nanomed., 2017, 12, 5409 CrossRef CAS PubMed.
- A. K. Mallik, M. L. Habib, F. N. Robel, Md. Shahruzzaman, P. Haque, M. Mizanur Rahman, V. Devanath, D. J. Martin, A. Kumar Nanjundan, Y. Yamauchi, M. Takafuji and H. Ihara, ChemistrySelect, 2019, 4, 7954–7958 CrossRef CAS.
- M. R. Anaraki, A. Jangjoo, F. Alimoradi, S. M. Dizaj and F. Lotfipour, Pharm. Sci., 2017, 23, 207–214 CrossRef.
- M. Cierech, A. Kolenda, A. M. Grudniak, J. Wojnarowicz, B. Woźniak, M. Gołaś, E. Swoboda-Kopeć, W. Łojkowski and E. Mierzwińska-Nastalska, Int. J. Pharm., 2016, 510, 323–335 CrossRef CAS PubMed.
- B. A. Sabri, M. Satgunam, N. M. Abreeza and N. A. Abdulrahman, Cogent. Eng., 2021, 8, 1875968 CrossRef.
- D. Popović, R. Bobovnik, S. Bolka, M. Vukadinović, V. Lazić and R. Rudolf, Mater. Technol., 2017, 51, 871–878 Search PubMed.
- M. Cierech, I. Osica, A. Kolenda, J. Wojnarowicz, D. Szmigiel, W. Łojkowski, K. Kurzydłowski, K. Ariga and E. Mierzwińska-Nastalska, Nanomaterials, 2018, 8, 305 CrossRef PubMed.
- R. Pokrowiecki, J. Wojnarowicz, T. Zareba, I. Koltsov, W. Lojkowski, S. Tyski, A. Mielczarek and P. Zawadzki, Int. J. Nanomed., 2019, 14, 9235 CrossRef CAS PubMed.
- D. Charoenkijkajorn and S. Sanohkan, Eur. J. Dent., 2020, 14, 525–532 CrossRef PubMed.
- S. Vikram and N. G. Chander, Eur. Oral. Res., 2020, 54, 31 CrossRef CAS PubMed.
- A. K. Al-Noori, N. A. Hatim and A. A. Taqa, Int. J. Enhanced Res. Sci. Tech. Eng., 2015, 4, 210–217 Search PubMed.
- S. Abd Alwahab, J. M. Moosa and S. Muafaq, Indian J. Public Health Res. Dev., 2020, 11, 2047–2051 Search PubMed.
- S. Amin Mosavi, R. Gottasloo, A. Akbarzadeh, S. Sadighi and A. Khoramdel, J. Dent. Res. Dent. Clin. Dent. Prospects, 2019, 13, 11–18 CrossRef PubMed.
- N. Ahmad, Z. Jafri and Z. H. Khan, J. Oral. Biol. Craniofac. Res., 2020, 10, 189–193 CrossRef PubMed.
- N. Salahuddin, M. El-Kemary and E. Ibrahim, Int. J. Appl. Ceram. Technol., 2018, 15, 448–459 CrossRef CAS.
- S. Tahmasbi, F. Mohamadian, S. Hosseini and L. Eftekhar, Nanomed. J., 2019, 6, 11–18 CAS.
- H. Khatria, J. Orthod. Sci., 2016, 5, 127 CrossRef PubMed.
- A. K. Reddy, P. B. Kambalyal, S. R. Patil, M. Vankhre, M. Y. A. Khan and T. R. Kumar, J. Orthod. Sci., 2016, 5, 127 CrossRef.
- M. Riad, A. Y. Harhash, O. A. Elhiny and G. A. Salem, Life Sci. J., 2015, 12, 27–34 CAS.
- A. Mirhashemi, A. Bahador, M. Kassaee, G. Daryakenari, M. Ahmad-Akhoundi and A. Sodagar, J. Med. Bacteriol., 2013, 2, 1–10 CAS.
- S. Y. Hailan and M. M. Al-Khatieeb, J. Baghdad Coll. Dent., 2019, 31, 10–16 Search PubMed.
- M. Pourhajibagher, A. S. Vaziri, N. Takzaree and R. Ghorbanzadeh, Photodiagn. Photodyn. Ther., 2019, 25, 239–246 CrossRef CAS PubMed.
- S. Shibli, R. Remya and K. Chinchu, Mater. Res. Innovations, 2012, 16, 186–197 CrossRef CAS.
- B. Ramazanzadeh, A. Jahanbin, M. Yaghoubi, N. Shahtahmassbi, K. Ghazvini, M. Shakeri and H. Shafaee, J. Dent., 2015, 16, 200 Search PubMed.
- K. Teymoornezhad, H. Alaghehmand, G. Daryakenari, S. Khafri and M. Tabari, Electron. Physician, 2016, 8, 3289 CrossRef PubMed.
- A. Behroozian, M. Kachoei, M. Khatamian and B. Divband, J. Dent. Res. Dent. Clin. Dent. Prospects, 2016, 10, 106 CrossRef PubMed.
- M. Kachoei, A. Nourian, B. Divband, Z. Kachoei and S. Shirazi, Nanomedicine, 2016, 11, 2511–2527 CrossRef CAS PubMed.
- S. Yamagata, Y. Hamba, K. Nakanishi, A. B. E. Shigeaki, T. Akasaka, N. Ushijima, U. O. Motohiro, I. I. D. A. Junichiro and F. Watar, Nanoarchitecton. Biomed., 2012, 4, 11–17 Search PubMed.
- A. Jatania and B. Shivalinga, Eur. J. Dent., 2014, 8, 112–117 CrossRef PubMed.
- M. Nuri Sari, N. Rahmani, M. Araghbidi Kashani, G. Eslami Amirabadi, A. Akbari Sari and E. Seyedtabaii, J. Islamic Dent. Assoc. Iran, 2015, 27, 70–76 Search PubMed.
- A. Jatania and B. Shivalinga, Eur. J. Dent., 2014, 8, 112 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2021 |