Open Access Article
Open Access ArticleLigand-free iridium-catalyzed regioselective C–H borylation of indoles†
Zilong Panabc,
Luhua Liubc,
Senmiao Xu *b and
Zhenlu Shen
*b and
Zhenlu Shen *a
*a
aCollege of Chemical Engineering, Zhejiang University of Technology, Hangzhou 310014, China. E-mail: zhenlushen@zjut.edu.cn
bState Key Laboratory for Oxo Synthesis and Selective Oxidation, Center for Excellence in Molecular Science, Suzhou Research Institute, Lanzhou Institute of Chemical Physics, Chinese Academy of Science, Lanzhou 730000, China. E-mail: senmiaoxu@licp.cas.cn
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 29th January 2021
Abstract
We herein report a ligand-free Ir-catalyzed C–H borylation of N-acyl protected indoles. This simple protocol could tolerate a variety of functional groups, affording C3 borylated indoles in good yields with excellent regioselectivities. We also demonstrated that the current method is amenable to gram-scale borylation and the C–B bonds could be easily converted to C–C and C-heteroatom bonds.
Indoles are not only widespread subunits but also useful building blocks in drug discovery and synthetic chemistry.1 Thus, their functionalization and transformation have gained attention. In particular, borylated indoles are of significant importance because they can serve as useful synthons by converting C–B bonds into many other functionalities.2 As a result, a number of regioselective C–H borylation methods have been developed. In this context, regioselective transition-metal-catalyzed C–H borylation has emerged as a powerful tool for preparing borylated indoles in an atom- and step-economic way under mild reaction condition.3,4 Because the C–H borylation at the C2 positions of indoles are electronically more favorable,4f–i the reactions at the other positions are more challenging. In general, directing groups are usually required to realize regioselective C–H borylation reactions. For example, bulky directing groups at nitrogen atoms such as Boc and Si(i-Pr)3 could result in C3-selective C–H borylation enabled by dtbpy/Ir catalysis (dtbpy = 4,4′-di-tert-butyl-2,2′-bipyridine).5 The N-Bpin moiety could serve as a traceless directing group for the dtbpy/Ir- and Ni(IMes)2-catalyzed C–H borylation at C3 positions.6 The use of C2 substituted indoles enables nitrogen-directed dtbpy/Ir-catalyzed C7-selective C–H borylation.7 Hartwig and co-workers used N–SiEt2H as the directing group to achieve relay directed dtbpy/Ir-catalyzed C7-selective C–H borylation.8 Another attractive and simple approach is the metal-free C–H borylation, which can regioselectively provide borylated indoles at C2, C3, C4, and C7 positions under mild reaction conditions.9 Despite the fact, the compatibility of functional groups is still narrow and usually limited to halogens, alkyl, and alkoxy substituted indoles. Thus, it is still appealing to develop complementary methods in this area.
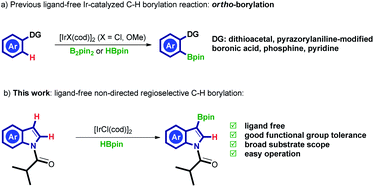
Ligand-free Ir-catalyzed regioselective C–H borylation of arenes have received growing interest. In this context, a judicious choice of directing group (DG) is crucial to promote the reaction. Usually, strong coordinating DGs are required. In this context, dithioacetal,10 pyrazorylaniline-modified boronic acid,11 phosphine,12 pyridine13 are commonly used for these transformations. It should be noted that all of the above-mentioned methods result in ortho-borylated products (Scheme 1A). In this work, we disclose the first example of ligand-free Ir-catalyzed C3-selective C–H borylation of N-acyl protected indoles. The current simple method could tolerate a variety of functionalities, including ester, ketone, nitro, and cyanide.
Our research commenced with the optimization of the reaction conditions. We chose N-isobutyryl indole 1a as our pilot substrate.14 The reaction 1a (0.20 mmol) with 1.5 equivalents of HBpin (pinacolborane) in the presence of a catalytic amount of [IrCl(cod)]2 (5.0 mol%) (cod: 1,5-cyclooctadiene) in n-hexane (1.0 mL) at 80 °C for 12 h affords C–H borylated product 2a in 79% yield with 97% C3 selectivity (Table 1, entry 1). GC/MS analysis of crude reaction mixture revealed that cod was reduced to cyclooctane, mono-borylated cyclooctane and mono-borylated cyclooctene.15 Replacement of [IrCl(cod)]2 to either [Ir(OMe)(cod)]2 or [IrCl(coe)2]2 (coe: cyclooctene) results in significantly decreased yields (Table 1, entries 2 and 3). We then survey the ligand effect on the reaction. We then used P(C6F5)3 (10 mol%) as the ligand which resulted in directed C2-borylated product 2a′ exclusively (Table 1, entry 4). The observed C2-selectivity is consistent with other C–H borylation using this ligand.16 The solvent effect showed that the reaction in THF yield and regioselectivity (Table 1, entry 5), which might be caused by coordinating nature of THF. Further examination of the reaction temperature indicates that the 80 °C is optimal in terms of both reactivity and regioselectivity (Table 1, entry 1 vs. entries 6 and 7). The significant loss of reactivity in the presence of a droplet of mercury indicates that the current catalytic process is more likely nanoparticle catalysis.17
| Entry | Variation from standard conditions | 2a/2a′b | Yield of 2ac (%) |
|---|---|---|---|
| a Unless otherwise noted, all the reactions were carried out with 1a (0.20 mmol), HBpin (0.30 mmol) in n-hexane (1.0 mL) at 80 °C for 12 h.b The ratio of 2a/2a′ was determined by GC analysis.c Isolated yield of 2a. | |||
| 1 | None | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
79 |
| 2 | 5 mol% [Ir(OMe)(cod)]2 in lieu of [IrCl(cod)]2 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
43 |
| 3 | 5 mol% [IrCl(coe)]2 in lieu of [IrCl(cod)]2 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
65 |
| 4 | 10 mol% P(C6F5)3 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
— |
| 5 | THF in lieu of n-hexane | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
47 |
| 6 | 70 °C instead of 80 °C | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
73 |
| 7 | 60 °C instead of 80 °C | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
31 |
With optimized reaction conditions in hand (Table 1, entry 1), we then determined the additional substrate scope of the current ligand-free regioselective Ir-catalyzed of C–H borylation of indoles as shown in Table 2. Generally, all of the substrates underwent reactions smoothly, giving most of borylated indoles with greater than 95% C3 selectivity. The reactions of substrates with substituents including F, Cl, Br, Me, MeO, and CF3, at C5, and C6 positions gave C3-selective borylated indoles 2b–f, 2n–o, 2p–s, and 2w in moderate to good isolated yields (65–91%) with constantly excellent regioselectivities (>95%). The C6-Br and C7-Me substituted indole 1p and 1w gave the C3-borylated product 2p and 2w in 80% and 88% yields with slightly diminished regioselectivities (92% and 93%, respectively) compared to their C5- and C6-counterparts. Substrates with Ar groups at C5 positions gave products 2g and 2h in 75% and 65% yields with 92% and 94% regioselectivities, respectively. Interestingly, the current method could also well tolerate a variety of sensitive substituents including ketone (1i and 1t) ester (1j, 1k, 1u, and 1x), and nitro (1l), cyanide (1m and 1v) at indoles' C5, C6, and C7 positions, furnishing corresponding C3-borylated products 2i–m, 2t–v, and 2x in moderate to good isolated yields (56–88%) with excellent regioselectivities (94–99%).
Interestingly, the reaction of C2-methyl substituted indole 3a under standard conditions could give C3-borylated product 4a exclusively, albeit with 40% isolated yield (eqn (1)). In contrast, no reaction was observed when C3-methyl or C2,C3-dimethyl substituted indoles 3b and 3c was employed (eqn (2)).
In order to demonstrate the synthetic utility of the current protocol, a gram-scale reaction of 1a and several transformations of 2a were conducted as shown in Fig. 1. The reaction of 1a (1.00 g) with 2.5 mol% [IrCl(cod)]2 for 24 h afforded 2a in 79% isolated yield (1.32 g) with 97% C3 selectivity, which is almost identical with that obtained from the small-scale reaction. The C–B bond could be transferred to other functional groups bearing C–Br (5), C–I (6), C–CN (8), and C–Ph (10) bonds under various reaction conditions in good to excellent yields (70–90%).18–21 Interestingly, prolonging the reaction time of iodination and cyanation could ultimately result in deacylation products 7 and 9 in both 80% yields.
 | ||
| Fig. 1 Gram-scale C–H borylation of 1a and synthetic application of borylated product 2a (DEMEDA = N,N′-dimethylenediamine). | ||
Conclusions
In conclusion, we have developed a ligand-free Ir-catalyzed C–H borylation under mild reactions for the first time. This easy-to-operate method could tolerate a variety of functional groups, affording C3 borylated products in good to excellent yields. We have also demonstrated that the obtained borylated product could be used in a series of C–C and C-heteroatom bond-forming reactions. Further exploration of ligand-free regioselective C–H borylation is currently underway in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank National Natural Science Foundation of China (21776260 and 21873261) for the generous support.Notes and references
- (a) M. A. Corsello, J. Kim and N. K. Garg, Chem. Sci., 2017, 8, 5836 RSC; (b) J. Vaca, F. Salazar, A. Ortiz and E. Sansinenea, J. Antibiot., 2020, 73, 798 CrossRef CAS PubMed; (c) A. Kumari and R. K. Singh, Bioorg. Chem., 2019, 89, 103021 CrossRef CAS PubMed; (d) A. K. Clarke, H. E. Ho, J. A. Rossi-Ashton, R. J. K. Taylor and W. P. Unsworth, Chem.–Asian J., 2019, 14, 1900 CrossRef CAS PubMed; (e) N. K. Kaushik, N. Kaushik, P. Attri, N. Kumar, C. H. Kim, A. K. Verma and E. H. Choi, Molecules, 2013, 18, 6620 CrossRef CAS PubMed.
- (a) D. Leonori and V. K. Aggarwal, in Synthesis and Application of Organoboron Compounds, ed. E. Fernández and A. Whiting, Springer International Publishing, Cham, 2015, pp. 271–295 Search PubMed; (b) H.-Y. Sun, and D. G. Hall, in Synthesis and Application of Organoboron Compounds, ed. E. Fernández and A. Whiting, Springer International, Cham, Switzerland, 2015, pp 221–242 Search PubMed; (c) S. Panda and J. M. Ready, J. Am. Chem. Soc., 2017, 139, 6038 CrossRef CAS PubMed.
- (a) L. Xu, G. Wang, S. Zhang, H. Wang, L. Wang, L. Liu, J. Jiao and P. Li, Tetrahedron, 2017, 73, 7123 CrossRef CAS; (b) I. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy and J. F. Hartwig, Chem. Rev., 2010, 110, 890 CrossRef CAS PubMed; (c) J. F. Hartwig, Chem. Soc. Rev., 2011, 40, 1992 RSC; (d) A. Ros, R. Fernández and J. M. Lassaletta, Chem. Soc. Rev., 2014, 43, 3229 RSC.
- (a) Y. Pang, T. Ishiyama, K. Kubota and H. Ito, Chem.–Eur. J., 2019, 25, 4654 CrossRef CAS PubMed; (b) S. Kawamorita, H. Ohmiya and M. Sawamura, J. Org. Chem., 2010, 75, 3855 CrossRef CAS PubMed; (c) K. Mertins, A. Zapf and M. Beller, J. Mol. Catal. A: Chem., 2004, 207, 21 CrossRef CAS; (d) P. Harrisson, J. Morris, T. B. Marder and P. G. Steel, Org. Lett., 2009, 11, 3586 CrossRef CAS PubMed; (e) I. A. I. Mkhalid, D. N. Coventry, D. Albesa-Jove, A. S. Batsanov, J. A. K. Howard, R. N. Perutz and T. B. Marder, Angew. Chem., Int. Ed., 2006, 45, 489 CrossRef CAS PubMed; (f) T. Ishiyama, J. Takagi, J. F. Hartwig and N. Miyaura, Angew. Chem., Int. Ed., 2002, 41, 3056 CrossRef CAS; (g) T. Furukawa, M. Tobisu and N. Chatani, Chem. Commun., 2015, 51, 6508 RSC; (h) T. Furukawa, M. Tobisu and N. Chatani, J. Am. Chem. Soc., 2015, 137, 12211 CrossRef CAS PubMed; (i) N. G. Léonard, M. J. Bezdek and P. J. Chirik, Organometallics, 2017, 36, 142 CrossRef; (j) M. R. Smith, R. Bisht, C. Haldar, G. Pandey, J. E. Dannatt, B. Ghaffari, R. E. Maleczka and B. Chattopadhyay, ACS Catal., 2018, 8, 6216 CrossRef CAS PubMed; (k) V. A. Kallepalli, K. A. Gore, F. Shi, L. Sanchez, G. A. Chotana, S. L. Miller, R. E. Maleczka and M. R. Smith, J. Org. Chem., 2015, 80, 8341 CrossRef CAS PubMed; (l) H. Zhang, S. Hagihara and K. Itami, Chem. Lett., 2015, 44, 779 CrossRef CAS; (m) A. Das, P. K. Hota and S. K. Mandal, Organometallics, 2019, 38, 3286 CrossRef CAS.
- (a) J. Takagi, K. Sato, J. F. Hartwig, T. Ishiyama and N. Miyaura, Tetrahedron Lett., 2002, 43, 5649 CrossRef CAS; (b) V. A. Kallepalli, F. Shi, S. Paul, E. N. Onyeozili, R. E. Maleczka and M. R. Smith, J. Org. Chem., 2009, 74, 9199 CrossRef CAS PubMed.
- (a) S. M. Preshlock, D. L. Plattner, P. E. Maligres, S. W. Krska, R. E. Maleczka and M. R. Smith, Angew. Chem., Int. Ed., 2013, 52, 12915 CrossRef CAS PubMed; (b) Y.-M. Tian, X.-N. Guo, Z. Wu, A. Friedrich, S. A. Westcott, H. Braunschweig, U. Radius and T. B. Marder, J. Am. Chem. Soc., 2020, 142, 13136 CrossRef CAS PubMed.
- (a) S. Paul, G. A. Chotana, D. Holmes, R. C. Reichle, R. E. Maleczka and M. R. Smith, J. Am. Chem. Soc., 2006, 128, 15552 CrossRef CAS PubMed; (b) F. Shen, S. Tyagarajan, D. Perera, S. W. Krska, P. E. Maligres, M. R. Smith and R. E. Maleczka, Org. Lett., 2016, 18, 1554 CrossRef CAS PubMed; (c) W. F. Lo, H. M. Kaiser, A. Spannenberg, M. Beller and M. K. Tse, Tetrahedron Lett., 2007, 48, 371 CrossRef CAS.
- D. W. Robbins, T. A. Boebel and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 4068 CrossRef CAS PubMed.
- (a) V. Bagutski, A. D. Grosso, J. A. Carrillo, I. A. Cade, M. D. Helm, J. R. Lawson, P. J. Singleton, S. A. Solomon, T. Marcelli and M. J. Ingleson, J. Am. Chem. Soc., 2013, 135, 474 CrossRef CAS PubMed; (b) T. Stahl, K. Müther, Y. Ohki, K. Tatsumi and M. Oestreich, J. Am. Chem. Soc., 2013, 135, 10978 CrossRef CAS PubMed; (c) J. L. Lavergne, A. Jayaraman, L. C. M. Castro, É. Rochette and F.-G. Fontaine, J. Am. Chem. Soc., 2017, 139, 14714 CrossRef PubMed; (d) M.-A. Légaré, M.-A. Courtemanche, É. Rochette and F.-G. Fontaine, Science, 2015, 349, 513 CrossRef PubMed; (e) S. Zhang, Y. Han, J. He and Y. Zhang, J. Org. Chem., 2018, 83, 1377 CrossRef CAS PubMed; (f) Q. Zhong, S. Qin, Y. Yin, J. Hu and H. Zhang, Angew. Chem., Int. Ed., 2018, 57, 14891 CrossRef CAS PubMed; (g) S. A. Iqbal, J. Cid, R. J. Procter, M. Uzelac, K. Yuan and M. J. Ingleson, Angew. Chem., Int. Ed., 2019, 58, 15381 CrossRef CAS PubMed; (h) J. Lv, X. Chen, X.-S. Xue, B. Zhao, Y. Liang, M. Wang, L. Jin, Y. Yuan, Y. Han, Y. Zhao, Y. Lu, J. Zhao, W.-Y. Sun, K. N. Houk and Z. Shi, Nature, 2019, 575, 336–340 CrossRef CAS PubMed; (i) É. Rochette, V. Desrosiers, T. Soltani and F.-G. Fontaine, J. Am. Chem. Soc., 2019, 141, 12305 CrossRef PubMed.
- L. Liu, G. Wang, J. Jiao and P. Li, Org. Lett., 2017, 19, 6132 CrossRef CAS PubMed.
- (a) T. Yamamoto, A. Ishibashi and M. Suginome, M. Org. Lett., 2019, 21, 6235 CrossRef CAS PubMed; (b) T. Yamamoto, A. Ishibashi and M. Suginome, Org. Lett., 2017, 19, 886 CrossRef CAS PubMed.
- K. M. Crawford, T. R. Ramseyer, C. J. A. Daley and T. B. Clark, Angew. Chem., Int. Ed., 2014, 53, 7589 CrossRef CAS PubMed.
- Y. Yang, Q. Gao and S. Xu, Adv. Synth. Catal., 2019, 361, 858 CrossRef CAS.
- See the ESI Table S1† for more details.
- (a) P. Nguyen, H. P. Blom, S. A. Westcott, N. J. Taylor and T. B. Marder, J. Am. Chem. Soc., 1993, 115, 9329 CrossRef CAS; (b) T. Ishiyama, J. Takagi, K. Ishida and N. Miyaura, J. Am. Chem. Soc., 2002, 124, 390 CrossRef CAS PubMed.
- I. Sasaki, J. Taguchi, S. Hiraki, H. Ito and T. Ishiyama, Chem.–Eur. J., 2015, 21, 9236 CrossRef CAS PubMed.
- V. M. Chernshev, A. V. Astahov, I. E. Chikunov, R. V. Tyurin, D. B. Eremin, G. S. Ranny, V. N. Khrustalev and V. P. Ananikov, ACS Catal., 2019, 9, 2984 CrossRef.
- H. L. Li, Y. Kuninobu and M. Kanai, Angew. Chem., Int. Ed., 2017, 56, 1495 CrossRef CAS PubMed.
- J. Szyling, A. Franczyk, P. Pawluć, B. Marciniec and J. Walkowiak, Org. Biomol. Chem., 2017, 15, 3207 RSC.
- Y. Ye, Y. Wang, P. Liu and F. Han, Chin. J. Chem., 2013, 31, 27 CrossRef CAS.
- Y.-C. Hu, D.-W. Ji, C.-Y. Zhao, H. Zheng and Q.-A. Chen, Angew. Chem., Int. Ed., 2019, 58, 5438 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra10211c |
| This journal is © The Royal Society of Chemistry 2021 |