Open Access Article
Open Access ArticleCreation of Mo/Tc@C60 and Au@C60 and molecular-dynamics simulations†
Tsutomu Ohtsuki *a,
Aaditya Manjanath*b,
Kaoru Ohno
*a,
Aaditya Manjanath*b,
Kaoru Ohno *c,
Makoto Inagaki
*c,
Makoto Inagaki a,
Shun Sekimotoa and
Yoshiyuki Kawazoed
a,
Shun Sekimotoa and
Yoshiyuki Kawazoed
aInstitute for Integrated Radiation and Nuclear Science, Kyoto University, Asashiro-Nishi, Kumatori-cho, Sennan-gun, Osaka 590-0494, Japan. E-mail: ohtsuki.tsutomu.5c@kyoto-u.ac.jp
bInstitute of Chemistry, Academia Sinica, 128 Academia Road, Section 2, Nankang, Taipei 11529, Taiwan. E-mail: aadityam@gate.sinica.edu.tw
cDepartment of Physics, Yokohama National University, 79-5 Tokiwadai, Hodogaya-ku, Yokohama 240-8501, Japan. E-mail: ohno@ynu.ac.jp
dNew Industry Creation Hatchery Center, Tohoku University, 6-6 Aramaki, Aoba, Sendai 980-8579, Japan
First published on 1st June 2021
Abstract
The formation of middle- and/or high-weight atom (Mo, Au)-incorporated fullerenes was investigated using radionuclides produced by nuclear reactions. From the trace radioactivities of 99Mo/99mTc or 194Au after high-performance liquid chromatography, it was found that the formation of endohedral and/or heterofullerene fullerenes in 99Mo/99mTc and 194Au atoms could occur by a recoil process following the nuclear reactions. Furthermore, the 99mTc (and 194Au) atoms recoiled against β-decay remained present inside these cages. To confirm the produced materials experimentally, ab initio molecular dynamics (MD) simulations based on an all-electron mixed-basis approach were performed. The possibility of the formation of endohedral fullerenes containing Mo/Tc and Au atoms is verified; here, the formation of heterofullerenes is excluded by MD simulations. These findings suggest that radionuclides stably encapsulated by fullerenes could potentially play a valuable role in diagnostic nuclear medicine.
Introduction
Foreign-atom-encapsulating fullerenes are interesting materials because of their variety of functional uses. However, their utility depends strongly on the specific encapsulated elemental species. Therefore, the chemical affinity between C60 and different elements is becoming an important field of research as new applications are identified.To date, numerous experimental and theoretical studies of endohedrally (and references therein)1 or exohedrally doped2–4 fullerenes and heterofullerenes5–8 with foreign atoms have been performed by utilizing arc-desorption or laser-vaporization techniques. However, it has also become possible to synthesize heterofullerenes in which foreign atoms are incorporated into the carbon cage.9,10 Heterofullerenes doped with foreign atoms such as B,11,12 N,13–15 and Si16,17 have been reported. In our previous studies, we investigated not only the endohedral doping of 7Be,18 79Kr, and 127Xe,19 but also the substitutional doping of 11C,20 13N,21 69Ge, and 72As22 using a recoil-implantation process following nuclear reactions.
Despite extensive research, the formation process and the materials produced with respect to the nature of the chemical interaction between a foreign atom and a fullerene cage have been only partially clarified. This is because the synthesis of such new foreign-atom-encapsulating fullerene complexes is a complicated, multi-step process. Therefore, it is important to investigate their properties to advance the production of these complexes in several research fields. For example, Li@C60 and N@C60 are expected to be useful in molecular switch23 and molecular qubit24 applications, respectively.
In this paper, we present evidence of heavy Mo/Tc and Au atom-encapsulating fullerenes after collision between a C60 cage and a Mo or Au atom. The collision was generated from recoil processes following nuclear reactions using the produced radionuclides, namely 99Mo/99mTc and 194Au. We performed ab initio molecular dynamics (AIMD) simulations to determine whether the Mo/Tc and Au atoms can be encapsulated in fullerenes with endohedral doping: Mo@C60/Tc@C60 and Au@C60, respectively. Studies on the chemical properties of these fullerenes encapsulating specific radioisotopes have been conducted,25–27 and will be helpful in tailoring such promising materials for nuclear medical applications. We consider the most frequently used radionuclides in diagnostic nuclear medicine,28–30 especially 99mTc, which is generally obtained by the decay of its parent radionuclide, 99Mo. Another heavy element, Au (e.g., 198Au or 194Au), can be regarded as a new candidate in related fields.31
Experimental procedure
High-energy bremsstrahlung or charged-particle irradiation was used according to the source nuclide used.(1) To produce 99Mo/99mTc-doped fullerenes, approximately 10 mg of C60 fullerene powder was mixed homogeneously with CS2 and 10 mg of a 97%-enriched 100Mo metal powder; the mixture was used as the target material. The samples were irradiated with a bremsstrahlung (high-energy γ-rays) of Emax = 30 MeV, which originated from the bombardment of a Pt plate of 2 mm in thickness with an electron beam at an electron linear accelerator (LINAC), Laboratory of Nuclear Science, Tohoku University. The radioisotope of 99Mo can be produced by a photonuclear reaction, namely (γ,n), by irradiating the enriched 100Mo. The irradiation time was set to approximately 8 h, and the average beam current was approximately 120 μA. The sample was then cooled in a water bath during the irradiation. To confirm 99Mo production, the characteristic γ-rays from nuclear decay at 740 and 181 keV were measured using a Ge detector. The produced 99Mo decayed to 99mTc with a 66 h half-life, and sequentially, 99mTc decayed to 99Tc, emitting γ-rays at 140.5 keV. The decay process was confirmed using a Ge detector.
(2) To produce 194Au-doped fullerenes, approximately 10 mg of C60 fullerene powder was mixed homogeneously with 10 mg of a natural Pt metal powder (0.8–1.5 μm) and used as the target material. Deuteron irradiation with a beam energy of 16 MeV was performed at the Cyclotron Radio-Isotope Center, Tohoku University. The radioisotope 194Au can be produced by (d,2n) reactions induced by irradiation. The deuteron beam current was typically 3 μA, and the irradiation time was approximately 1 h. The target was cooled with circulated He gas during the irradiation. After irradiation, the samples were left for one day to allow the decay of short-lived radioactive byproducts. After one day of cooling, the radioactivity of 194Au could be measured with characteristic γ-rays at 328 keV, 294 keV, and so on.
The fullerene samples were dissolved in o-dichlorobenzene after filtration to remove insoluble materials through a membrane filter (pore size = 0.45 μm and/or 0.2 μm). The soluble fraction was injected into a high-performance liquid chromatograph (HPLC) equipped with a 5PBB (silica-bonded with the pentabromobenzyl group) column of 10 mm (inner diameter) × 250 mm (length), at a flow rate of 2 mL min−1 for the 99Mo/99mTc and 3 mL min−1 for 194Au samples. The eluted solution was passed through an ultraviolet (UV) detector, the wavelength of which was adjusted to 290 nm to measure the quantity of fullerenes and their derivatives.
The fraction was collected at 30 s intervals, and the γ-ray activities of each fraction were measured with a Ge-detector coupled to a 4096-channel pulse-height analyzer with a conversion gain of 0.5 keV per channel. Therefore, the existence of 99Mo/99mTc and 194Au radioisotopes could be confirmed by their characteristic γ-rays.32
Results and discussion
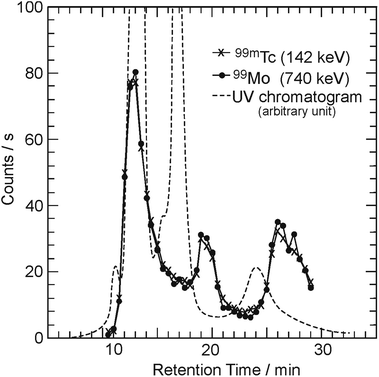
Fig. 1 shows three elution curves of the C60 sample irradiated by a bremsstrahlung of Emax = 30 MeV. The horizontal axis indicates the retention time after injection into the HPLC system. The vertical axis indicates the γ-counting rate of the 99Mo/99mTc radioactivity, as measured with a Ge detector (counts per s), as well as the absorbances monitored continuously by a UV detector (dashed line, arbitrary unit). The solid circles and cross symbols represent the radioactivities of 99Mo and 99mTc, respectively. A strong absorption peak was observed for a retention time of 12–14 min in the elution curve (dashed line: saturated), as measured using a UV detector. This peak position corresponds to the retention time of C60 (ultramarine blue in HPLC solution), which was confirmed in the calibration run using the C60 sample before irradiation. Following the first peak, two broad peaks at approximately 16–18 min (navy blue) and 22–26 min (brown) were observed in the UV chromatogram. The colors were reproduced well in each case.20,36 Although there is a delay in the elution peaks of the radioactivities with respect to those of the UV absorption peaks, the elution behaviors appear similar. As 99mTc is related to its radioactive equilibrium with 99Mo, the radioactive amount of 99mTc is almost the same as that of 99Mo.To characterize the components, the fraction corresponding to the second peak in Fig. 1 was collected and examined using matrix-assisted laser desorption ionization time-of-flight (MALDI TOF) mass spectrometry. The mass spectrum of the fraction exhibited a series of peaks at m/z = 1440 − 24n (n = 1–4) corresponding to the molecular ion peak of C120−nC2, in addition to the peak for C60 as a base peak.36 This indicates that the second and smaller third peaks can be assigned to C60 dimers and C60 trimers, respectively. These materials can be produced by the interaction between C60 molecules in coalescence reactions after ionization by incident γ-rays or charged particles.20
The elution curves shown by the curve without symbols and by the curve with circles in Fig. 2 indicate the absorbance monitored continuously by a UV detector and the γ-counting rate of 194Au measured by a Ge-detector, respectively. The horizontal and vertical axes are the same as those shown in Fig. 1. Four populations of 194Au appear at retention times of 9 min, 10–11 min, approximately 13 min, and 17–19 min in Fig. 2. The ultramarine blue of C60 in the second fraction, the third fraction (navy blue), and the fourth fraction (brown) in the UV chromatogram can be attributed to C60 monomers, dimers, and trimers, respectively, as indicated by the elution behavior of 99Mo/99mTc. The first peak (transparent) is due to the unknown byproducts of irradiation or UV spectrometry, despite the appearance of γ counts of 194Au. Therefore, this result indicates that radioactive fullerene monomers and their polymers (dimers and trimers) labeled with 194Au may exist in the final fractions. In our previous study, a similar trend was observed in the elution curves of the Kr, Xe, Ge, and As cases.19,22 The quantity of the Mo/Tc-incorporated radioactive fullerenes produced here is estimated to be approximately 1010–1012 molecules.
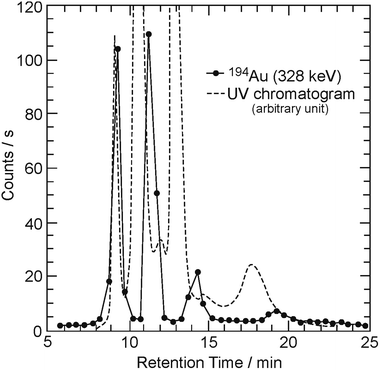 | ||
| Fig. 2 Similar to Fig. 1, except that the counting rate is for radioactivities of 194Au (solid circle). | ||
Here, it should be noted that no evidence of exohedral molecules was observed by extraction in the soluble portion. Exohedral molecules can be removed during solvation. Therefore, two possible molecular types should be considered in the present results: (1) endohedrally Mo/Tc atom-doped fullerenes, 99Mo@C60/99mTc@C60 and 194Au@C60,18,19 and (2) substitutional atom-doped heterofullerenes as a part of the cage, 99MoC59/99mTcC59 and 194AuC59, as a result of As*C-59, As*C-69 (As* = As-71, As-72, As-74), (GeC59)–Ge-69, and their polymers.22
To investigate the possibility of the formation of endohedral fullerenes and/or heterofullerenes in 99Mo/99mTc and 194Au atoms, we performed AIMD simulations to achieve greater clarity and distinction. The simulation configuration consisted of one C60 molecule and one Mo or Au atom. We assigned Mo (Au) with an initial Ekinetic of 40 eV (80 eV) at a distance of 5 Å from the C60 center along the z-direction impinging at the center of the topmost six-membered ring (x, y) = (0, 0), where x and y are the directions toward the center of a double bond and toward a carbon atom, respectively.
We employed the all-electron mixed-basis code TOMBO,19,33,34 in which both plane waves (PWs) and numerical atomic orbitals (AOs) are used as basis functions. Local density approximation was used for the Mo + C60 simulation, and the local spin-density approximation was used for the Au + C60 simulation. The Perdew–Zunger form of the exchange–correlation potential35 was used for both. A simple cubic unit cell with a 14 Å edge length was used. The cutoff energy for the PWs was 7 Ry, and the 1s, 2s, and 2p AOs were used for the C atoms, although the 2s and 2p AOs were confined inside the non-overlapping atomic sphere (NOAS) by subtracting a simple polynomial satisfying the matching condition at the surface of the NOAS. The subtracted smooth part that continues the original AO outside the NOAS can be simply expressed by a linear combination of PWs. The 1s, 2s, 2p, 3s, 3p, 3d, 5s, and 5p AOs were used for Mo, and the 1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p, 4d, 4f, 5s, and 5p AOs were used for Au. This treatment ensures that two- or three-center integrals are not required between adjacent AOs and completely avoids the so-called basis-set superposition error. Moreover, the problem of overcompleteness was significantly reduced using this procedure.35
Fig. 3 shows the time snapshots of the simulation, where the Mo atom attacks the C60 molecule with a kinetic energy (Ekinetic) of 40 eV. It hits the center of the six-membered ring at t = 21 fs (Fig. 3(b)) and then slightly penetrates the C60 cage at t = 60 fs (Fig. 3(c)), where the six-membered ring expands to accommodate the incoming Mo atom while maintaining three C–C bonds. After a while (t = 118–190 fs), the cage structure is gradually recovered (Fig. 3(d)–(f)), and the Mo atom is fully encapsulated.
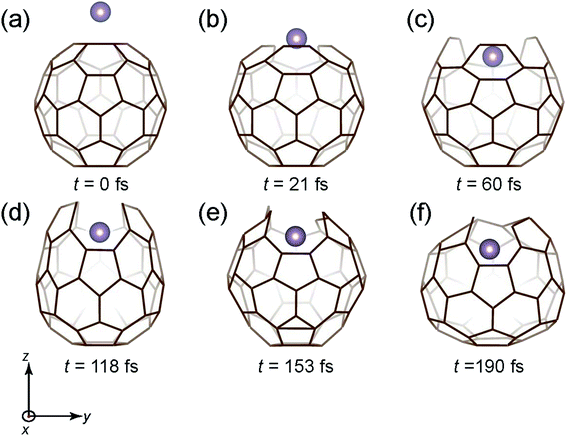 | ||
| Fig. 3 Time snapshots of the Mo + C60 → Mo@C60 simulation when the Mo atom vertically collides at the center of a six-member ring with an initial Ekinetic of 40 eV. | ||
However, if Ekinetic is increased to 80 eV, the Mo atom is not encapsulated, as shown in Fig. 4. The situation is similar to that of the 40 eV case (Fig. 3) up to 21 fs, where the cage structure opens up. Beyond 21 fs, owing to the high Ekinetic of the atom, the cage structure is gradually destroyed (Fig. 4(c)–(f)), eventually leading to no encapsulation of the atom.
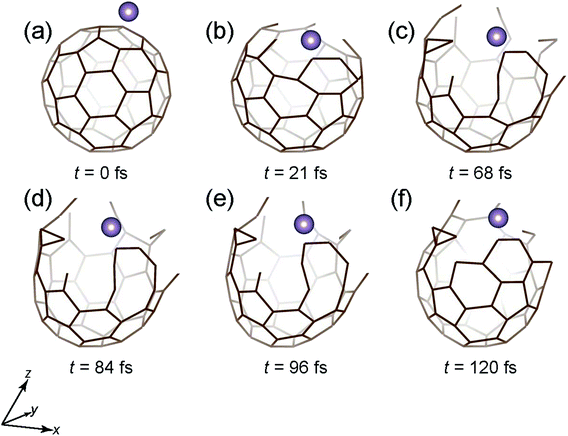 | ||
| Fig. 4 Time snapshots of the Mo + C60 → Mo + C60 (broken) simulation when the Mo atom vertically collides at the center of a six-member ring with an initial Ekinetic of 80 eV. | ||
In contrast, when we impose the same Ekinetic of 80 eV on the Au atom toward the center of the six-membered ring, the structure of the cage is preserved with Au encapsulation.
Fig. 5 shows the time snapshots of this simulation for Au + C60 → Au@C60. It hits the center of the six-membered ring at t = 27 fs (Fig. 5(b)), and then slightly goes inside at t = 60 fs (Fig. 6(c)), when the six-membered ring expands while keeping three C–C bonds, similar to the case of Mo encapsulation at 40 eV (Fig. 5(c)). The cage structure is recovered at t = 130–170 fs (Fig. 5(d) and (e)), and the Au atom penetrates deep inside the cage at t = 249 fs (Fig. 5(f)).
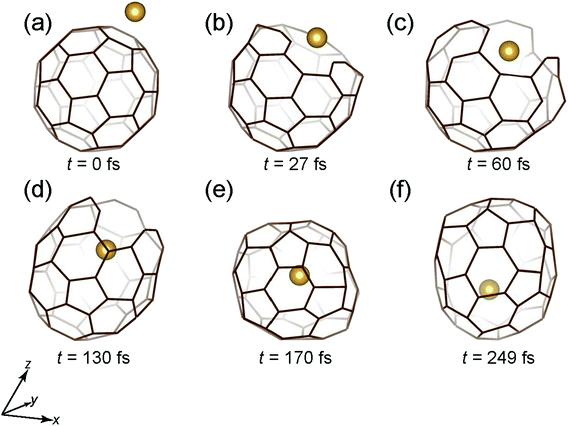 | ||
| Fig. 5 Time snapshots of the Au + C60 → Au@C60 simulation when an Au atom vertically collides at the center of a six-member ring with an initial Ekinetic of 80 eV. | ||
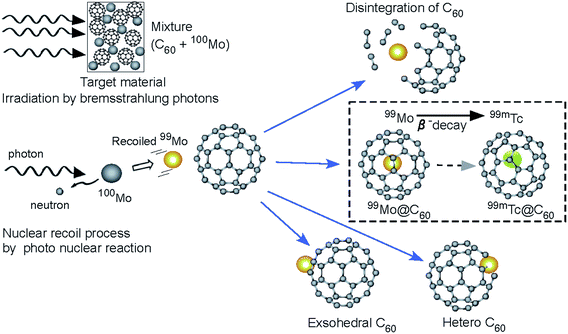 | ||
| Fig. 6 Schematic image in the Mo + C60 for the recoil process by photonuclear reactions (γ,n) is shown using the 100Mo irradiated by bremsstrahlung photons (high-energy photons: γ). | ||
To verify whether the Mo/Au atom continued to remain encapsulated or destroyed the cage, we performed MD simulations for much longer durations. We found that the atoms moved around in the cage owing to their Ekinetic values, but remained encapsulated. Therefore, it is reasonable to expect that at timescales longer than the picosecond regime, there would be no change in the encapsulation scenario. This fact supports the identification of the experimentally observed incorporated fullerenes as endohedral fullerenes of 99Mo/99mTc and 194Au atoms.
For a more comprehensive understanding, a schematic view of the reaction process in the target material is shown in Fig. 6. The experimental results presented here support the following scenario (here, for example, in the case of 100Mo (γ,n)99Mo → β− → 99mTc). Several radioactive nuclides are produced by (γ,n) (and (d,2n) for 194Au) reactions. The Ekinetic values of the radionuclides are of similar orders of magnitude, even in different nuclear reactions. The form of the emitted neutron spectrum is expected to be approximately Maxwellian in distribution and the average neutron Ekinetic seems to be approximately 2–3 MeV, while the initial Ekinetic of the recoiled nuclides is estimated to be approximately a few hundred kilo-electron volts, even if the reaction is accompanied by two neutron emissions. The energetic nuclides should destroy the fullerene cages because Ekinetic is estimated to be of a different order of magnitude than the energies (some electron volts) of molecular bonding. Therefore, the atoms being produced escape from their own material owing to the Ekinetic of approximately a few hundred kilo-electron volts. Then, Ekinetic is reduced in the sample to a magnitude appropriate for incorporation. Finally, the radionuclides hit the C60 cages, as shown in Fig. 3, and stop in the cage, thus forming endohedral fullerenes or heterofullerenes. Furthermore, the shock produces fullerene polymers by inducing interactions with neighboring fullerene cages.18–22
After encapsulation, recoil destruction may occur inside the fullerene; specifically, the radioactive species 99Mo/99mTc and 194Au can decay via β or γ emission to daughter nuclides via the reactions 99Mo → β− → 99mTc (radioactive equilibrium) and 99mTc → γ → 99Tc (140.5 keV; ground state), and 194Au → β+ → 194Pt (stable nuclide). If we consider the nuclear recoil by β-decay and/or γ-ray from an energetic perspective, we must consider the following important issues: (1) the contribution of the nuclear recoil process to reducing energy inside the C60 and (2) the reaction pathway for the radionuclide in exiting the C60 and destroying the host cages. The maximum energy of the β-ray in 99Mo was 1.214 MeV. Therefore, the maximum recoil energy of the radionuclides (i.e., 99mTc with β-decay and subsequent γ-ray emissions from 99Mo) is estimated to be much lower than a few tens of electron volts,37–40 even in 194Au → β+ → 194Pt and subsequent γ-ray emissions. Here, the recoil process occurs inversely from within the C60 cage to exit, and the motion is similar to that during the insertion process from the outside. The radioactive equilibrium between 99Mo and 99mTc was complete, and the radioactive amounts were approximately the same (see Fig. 1). This fact may indicate that the 99mTc atoms (and even those in 194Pt) recoiled against β-decay (β+-decay), and subsequently, γ-rays remained inside these cages to create 99mTc (194Pt) endohedral C60. Here, electronegativity may play an important role in the incorporation of middle- and/or heavy-weight atoms. Finally, it is interesting to note that if 99Mo/99mTc and 194Au can be successfully carried by a C60 cage to a certain organ, it may be effectively used for diagnosis and treatment. Furthermore, radioisotopes used in nuclear medicine (not only 99Mo/99mTc or 194Au, but also, 47Sc, 64,67Cu, 68Ga, 105Rh, 177Lu, and 188Re)29 are intriguing for encapsulation applications. These findings further indicate that radionuclide-encapsulating fullerenes have high potential for applicability in radioimmunotherapy; in particular, the physical and/or chemical properties of the radionuclide-encapsulating fullerenes could be superior to those of conventional chelating chemicals for medical use.
Conclusions
The formation of (Mo, Au)-encapsulating fullerenes was investigated using radionuclides produced by nuclear reactions. From the trace radioactivities of 99Mo/99mTc or 194Au after HPLC, it was observed that endohedral fullerenes and/or heterofullerene in 99Mo/99mTc and 194Au atoms can form through a recoil process following nuclear reactions. To verify the production of these materials, AIMD simulations based on an all-electron mixed-basis approach were performed. Our combined experimental and theoretical results strongly support the interpretation that endohedral fullerenes encapsulating Mo/Tc and Au atoms were formed. Furthermore, we found that the 99mTc (and even 194Au) atoms recoiled against β-decay can remain inside these cages. Fullerenes could potentially play a valuable role in diagnostic nuclear medicine by stably encapsulating radionuclides.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the NEDO project (No. 16101402-0), Kakenhi (No. 16K15579, No. 18H04150, No. 19K22596, No. 18H01939 in Grants-in-Aid for Scientific Research), and KAN-GENKON. We are also indebted to the HPCI promoted by MEXT for the use of the supercomputer SR16000 at Hokkaido University and at IMR, Tohoku University (Project IDs. hp170268 and hp170190). We are grateful for the assistance of the technical staff of the Laboratory of Nuclear Science and the Cyclotron, Tohoku University, for beam handling.Notes and references
- A. A. Popov, S. Yang and L. Dunsch, Chem. Rev., 2013, 113, 5989–6113 CrossRef CAS PubMed.
- L. M. Roth, Y. Huang, J. T. Schwedler, C. J. Cassady, D. Ben-Amotz, B. Kahr and B. S. Freiser, J. Am. Chem. Soc., 1991, 113, 6298–6299 CrossRef CAS.
- Y. Huang and B. S. Freiser, J. Am. Chem. Soc., 1991, 113, 9418 CrossRef CAS.
- S. W. McElvany, Journal of Physical Chemistry Chemical Physics, 1992, 96, 4935–4937 CAS.
- S. Ostrowski, M. H. Jamrz and J. Cz. Dobrowolski, Tetrahedron: Asymmetry, 2013, 24, 1097–1109 CrossRef CAS.
- R. Yu, M. Zhan, D. Cheng, S. Yang, Z. Liu and L. Zheng, Journal of Physical Chemistry Chemical Physics, 1995, 99, 1818–1819 CAS.
- Y. Hashikawa, M. Murata, A. Wakamiya and Y. Murata, J. Am. Chem. Soc., 2016, 138, 4096–4104 CrossRef CAS PubMed.
- O. Vostrowsky and A. Hirsch, Chem. Rev., 2006, 106, 51915207 CrossRef PubMed.
- S. Jalife, J. Arcudia, S. Pan and G. Merino, Chem. Sci., 2020, 11, 6642–6652 RSC.
- K. Ohno, A. Manjanath, Y. Kawazoe, R. Hatakeyama, F. Misaizu, E. Kwon, H. Fukumura, H. Ogasawara, Y. Yamada, C. Zhang, N. Sumi, T. Kamigaki, K. Kawachi, K. Yokoo, i S. Ono and Y. Kasama, Nanoscale, 2018, 10, 1825–1836 RSC.
- T. Guo, C. Jin and R. E. Smalley, Journal of Physical Chemistry Chemical Physics, 1991, 95, 4948–4950 CAS.
- H.-J. Muhr, R. Nesper, B. Schnyder and R. Kötz, Chem. Phys. Lett., 1996, 249, 399–405 CrossRef CAS.
- T. Pradeep, V. Vijayakrishnan, A. K. Santra and C. N. R. Rao, Journal of Physical Chemistry Chemical Physics, 1991, 95, 10564–10565 CAS.
- J. F. Christian, Z. Wan and S. L. Anderson, Journal of Physical Chemistry Chemical Physics, 1992, 96, 10597–10600 CAS.
- H. Huang, G. Zhang, D. Wang, N. Xin, S. Liang, N. Wang and L. Gan, Angew. Chem., 2013, 52, 5037–5040 CrossRef CAS PubMed.
- M. Pellarin, C. Ray, P. Mélinon, J. Lermé, J. L. Vialle, P. Kéghélian, A. Perez and M. Broyer, Chem. Phys. Lett., 1997, 277, 96–104 CrossRef CAS.
- C. Ray, M. Pellarin, J. L. Lermé, J. L. Vialle, M. Broyer, X. Blase, P. Mélinon, P. Kéghélian and A. Perez, Phys. Rev. Lett., 1998, 80, 5365–5368 CrossRef CAS.
- T. Ohtsuki, K. Masutomo, K. Ohno, Y. Maruyama, Y. Kawazoe, K. Sueki and K. Kikuchi, Phys. Rev. Lett., 1996, 77, 3522–3524 CrossRef CAS PubMed.
- T. Ohtsuki, K. Ohno, K. Shiga, Y. Kawazoe, Y. Maruyama and K. Masutomo, Phys. Rev. Lett., 1998, 81, 967–970 CrossRef CAS.
- T. Ohtsuki, K. Masutomo, K. Sueki, K. Kobayashi and K. Kikuchi, J. Am. Chem. Soc., 1995, 117, 12869–12870 CrossRef CAS.
- T. Ohtsuki, K. Masutomo, K. Sueki, K. Shikano and T. Shigematsu, J. Radioanal. Nucl. Chem., 1999, 239, 365–370 CrossRef CAS.
- T. Ohtsuki, K. Ohno, K. Shiga, Y. Kawazoe, Y. Maruyama and K. Masumoto, Phys. Rev. B, 1999, 60, 1531–1534 CrossRef CAS.
- H. J. Chandler, M. Stefanou, E. E. B. Campbell and R. Schaub, Nat. Commun., 2019, 10, 2283 CrossRef PubMed.
- S. R. Plant, M. Jevric, J. J. L. Morton, A. Ardavan, A. N. Khlobystov, G. A. D. Briggs and K. Porfyrakis, Chem. Sci., 2013, 5, 2971 RSC.
- M. D. Diener, J. M. Alford, S. J. Kennel and S. Mirzadeh, J. Am. Chem. Soc., 2007, 129, 5131–5138 CrossRef CAS PubMed.
- Z. Chen, L. Ma, Y. Liu and C. Chen, Theranostics, 2012, 2, 38–50 Search PubMed.
- R. D. Bolskar, Encyclopedia of Nanotechnology, Fullerenes for Drug Delivery, 2016, pp. 1267–1281 Search PubMed.
- M. Inagaki, S. Sekimoto, T. Tadokoro, Y. Ueno, Y. Kani and T. Ohtsuki, J. Radioanal. Nucl. Chem., 2020, 324, 681–686 CrossRef CAS.
- M. Inagaki, S. Sekimoto, W. Tanaka, T. Tadokoro, Y. Ueno, Y. Kani and T. Ohtsuki, J. Radioanal. Nucl. Chem., 2019, 322, 1703–1709 CrossRef CAS.
- J. Jang, H. Kikunaga, S. Sekimoto, M. Inagaki, T. Kawakami, T. Ohtsuki, S. Kashiwagi, K. Takahashi, K. Tsukada, K. Tatenuma and M. Uesaka, Nucl. Instrum. Methods Phys. Res., Sect. A, 2021, 987, 164815 CrossRef CAS.
- D. Maccora, V. Dini, C. Battocchio, I. Fratoddi, A. Cartoni, D. Rotili, M. Castagnola, R. Faccini, I. Bruno, T. Scotognella, A. Giordano and I. Venditti, Appl. Sci., 2019, 9, 1–23 Search PubMed.
- S. Y. F. Chu, L. P. Ekström and R. B. Firestone, Table of Isotopes, The Lund/LBNL Nuclear Data Search, Version 2.0, 1999 Search PubMed.
- K. Ohno, F. Mauri and S. G. Louie, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 56, 1009–1012 CrossRef CAS.
- S. Ono, Y. Noguchi, R. Sahara, Y. Kawazoe and K. Ohno, Comput. Phys. Commun., 2015, 189, 20–30 CrossRef CAS.
- J. P. Perdew and A. Zunger, Phys. Rev. B: Condens. Matter Mater. Phys., 1981, 23, 5048–5079 CrossRef CAS.
- T. Ohtsuki, K. Masutomo, T. Tanaka and K. Komatsu, Chem. Phys. Lett., 1999, 300, 661–666 CrossRef CAS.
- K. Sueki, K. Akiyama, K. Kikuchi, H. Nakahara and K. Tomura, Chem. Phys. Lett., 1998, 288, 179–182 CrossRef.
- G. E. Gadd, P. Schmidt, C. Bowles, G. McOrist, P. J. Evans, J. Wood, L. Smith, A. Dixon and J. Easey, J. Am. Chem. Soc., 1998, 120, 10322–10325 CrossRef CAS.
- T. Braun and H. Rausch, Chem. Phys. Lett., 1998, 288, 179–182 CrossRef CAS.
- T. A. Carlson, Phys. Rev., 1963, 132, 2239–2242 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra10196f |
| This journal is © The Royal Society of Chemistry 2021 |