Open Access Article
Open Access ArticleA comprehensive review on antimicrobial face masks: an emerging weapon in fighting pandemics
Gayathri Pullangott†
a,
Uthradevi Kannan†
a,
Gayathri S.
a,
Degala Venkata Kiran
b and
Shihabudheen M. Maliyekkal
 *a
*a
aDepartment of Civil and Environmental Engineering, Indian Institute of Technology Tirupati, Andhra Pradesh 517619, India. E-mail: shihab@iittp.ac.in; sm.maliyekkal@gmail.com; Fax: +91 877 2503004; Tel: +91 877 2503164
bDepartment of Mechanical Engineering, Indian Institute of Technology Tirupati, Andhra Pradesh 517619, India
First published on 8th February 2021
Abstract
The world has witnessed several incidents of epidemics and pandemics since the beginning of human existence. The gruesome effects of microbial threats create considerable repercussions on the healthcare systems. The continually evolving nature of causative viruses due to mutation or re-assortment sometimes makes existing medicines and vaccines inactive. As a rapid response to such outbreaks, much emphasis has been placed on personal protective equipment (PPE), especially face mask, to prevent infectious diseases from airborne pathogens. Wearing face masks in public reduce disease transmission and creates a sense of community solidarity in collectively fighting the pandemic. However, excessive use of single-use polymer-based face masks can pose a significant challenge to the environment and is increasingly evident in the ongoing COVID-19 pandemic. On the contrary, face masks with inherent antimicrobial properties can help in real-time deactivation of microorganisms enabling multiple-use and reduces secondary infections. Given the advantages, several efforts are made incorporating natural and synthetic antimicrobial agents (AMA) to produce face mask with enhanced safety, and the literature about such efforts are summarised. The review also discusses the literature concerning the current and future market potential and environmental impacts of face masks. Among the AMA tested, metal and metal-oxide based materials are more popular and relatively matured technology. However, the repeated use of such a face mask may pose a danger to the user and environment due to leaching/detachment of nanoparticles. So careful consideration is required to select AMA and their incorporation methods to reduce their leaching and environmental impacts. Also, systematic studies are required to establish short-term and long-term benefits.
1. Introduction
Outbreaks of communicable diseases are not uncommon and have existed since humankind's hunter-gatherer days. However, they became more prominent when the world shifted towards settled community life. Pathogenic organisms such as viruses, bacteria, protozoa, helminths, and fungi are the major causes of infectious diseases in humans and animals. It is estimated that one-fourth of annual global deaths are due to these pathogenic organisms.1 Mainly, flu viruses and non-flu viruses like Flavivirus, Filoviridae, and human coronavirus are responsible for major epidemics and pandemics starting from Russian flu-1889 to the present Coronavirus disease (COVID-19). The four communicable diseases that rattled the world are Influenza, Ebola, Acquired immunodeficiency syndrome (AIDS), and COVID-19. According to the World Health Organisation (WHO), the respiratory diseases from annual seasonal influenza alone results in 290![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–650
000–650![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths each year.2,3 Also, the rapidly evolving influenza viruses cause annual epidemics and occasional pandemics (3 to 4 times per century).4 The fatality rate in Ebola is estimated to be as high as 50%.5 AIDS, with no complete cure, continues to be a significant global public health issue,6 and the present COVID-19 pandemic has already resulted in more than 95 million cases worldwide (till 17th January 2021).7
000 deaths each year.2,3 Also, the rapidly evolving influenza viruses cause annual epidemics and occasional pandemics (3 to 4 times per century).4 The fatality rate in Ebola is estimated to be as high as 50%.5 AIDS, with no complete cure, continues to be a significant global public health issue,6 and the present COVID-19 pandemic has already resulted in more than 95 million cases worldwide (till 17th January 2021).7
The emergence of more resistant strains with high infection potential happens because of mutation or re-assortment of pre-existing microbial strains, which sometimes makes the vaccines and antibiotics incompetent. Therefore, to control or delay the spread of the infection, WHO and the Centers for Disease Control and Prevention (CDC) suggests various non-pharmaceutical interventions such as hand washing, personal protective equipment (PPE), isolation, quarantine, personal hygiene, use of disinfectants, and social distancing. For instance, the use of respiratory protective devices (RPDs) has gained massive impetus during COVID-19. This intervention is highly beneficial in pandemic situations to reduce exposure to infectious respiratory aerosols and droplets and slow down the spread of the disease.8–12 Realising the fact, most of the countries have enforced or advised its citizen to wear face masks in public places.13
However, there are several concerns associated with the use of conventional face masks such as the survivability of microorganisms on the face mask surface,14,15 re-aerosolisation of settled particles,16 safe management and disposal of used face masks,17 and fomite transmission.18 Some of these concerns have led to the development of face masks with inherent antimicrobial properties to impart surface contact killing/deactivation property in addition to particle filtration.
Antimicrobial face masks pave the way for reusability and considerably reduces the surging demand for single-use face masks, especially during outbreaks in highly populated, developing, and middle-income countries. Moreover, the ongoing pandemic has further stressed the significance of such development. Thorough knowledge of suitable antimicrobial agents (AMA) and incorporation methods are required to develop potential antimicrobial face masks. This is one of the prospective areas evolving through research and gaining much interest among scientists and researchers. The lucrative market steers the entrepreneurs and industrialists to focus on improvising face masks to meet consumers' safety and quality demands, especially in the COVID-19 and post COVID-19 scenarios. More clarity on various aspects of the antimicrobial mask is imminent at this juncture, realising the importance of a review on antimicrobial face masks.
The paper presents a comprehensive review of the face mask with a particular emphasis on antimicrobial face masks as an emerging weapon in containing the spread of infectious diseases. The topics such as AMA used in face masks and their methods of incorporation, filtration efficacy, and current and future market potential are discussed. A detailed description of the evolution of face masks and their role in preventing the spread of infectious disease, potential issues with the conventional face masks, and possible environmental challenges associated with the usage of face masks are also included. The review also gives a brief insight into the history of pandemics, epidemics, and their socio-economic impacts. The authors feel that this review is vital in the present scenario of COVID-19 and post COVID-19 due to the recurring nature of infectious diseases, to acquaint the readers with the relevant aspects of face mask in general and antimicrobial face masks in particular.
2. Major epidemics and pandemics in history
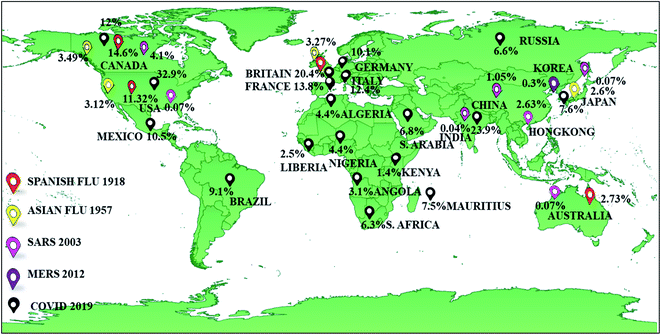
The occurrence of pandemics and epidemics have been reported throughout history. It is accepted that pandemics occur at unpredictable intervals with no specific occurrence pattern.14 The years (in A.D.) 1510, 1557, 1580, 1593, 1732–1733, 1767, 1781–1782, 1802–1803, 1830–1833, 1836–1837, 1847–1848, 1850–1851, 1855, 1857–1858 and 1874–1875 have been recorded as pandemic years. The reports on epidemics and pandemics outbreak before the 12th century are defective, unreliable, or does not warrant definite conclusions.19 Among the pandemics, the Bubonic plague or Black death (AD 1347 to 1351) was the deadliest in terms of total death (75 to 200 million) and fatality rate (30 to 100%).20 In the last 20 decades, the diseases due to flu viruses (Influenza), primarily transmitted through respiratory aerosols, are prevailing worldwide. Influenza takes a notable place among various infectious diseases because of its pronounced pandemic character than others.19Spanish flu-1918 pandemic was the most fatal among various flu diseases, with 10 and 26.8% fatality rate (total number of death × 100/total number of infected individuals) and infection rate (total number of infected individuals × 100/population), respectively. Asian flu-1957 and Hong Kong flu-1968 pandemics had a fatality rate of 0.2 to 0.8%. The infection rate of Asian flu-1957 and Hong Kong flu-1968 was 17.4 and 14.1%, respectively. The fatality rate (0.01 to 0.04%) of Swine flu-2009 pandemic was low, but the infection rate was as high as 21%.21,22 The COVID-19 has also resulted in a catastrophic fatality rate of approximately 2.2% and an infection rate of 1.2%.7,23 This data reveals that COVID-19 is the worst pandemic since the Spanish flu-1918 in terms of fatality rate.
Among the non-flu diseases, AIDS, Ebola, Severe acute respiratory syndrome (SARS, 2003) and the Middle East respiratory syndrome (MERS, 2012) were of much concern to the entire world. AIDS is given a special term ‘global pandemic’ with almost 76 million people infected, and 33 million deaths since its first reported occurrence in 1981.24 AIDS is one of the significant infectious viral diseases in terms of fatality rate (43.5%), followed by Ebola (39.6%).21,22,24 SARS-2003 and MERS-2012 with a fatality rate of 9.6% and 34.4%, respectively are epidemics that target the human respiratory system.25–27 Even though the fatality rate is higher for SARS-2003 and MERS-2012, the infected rate is 3 or 4 orders lesser than COVID-19. COVID-19 has resulted in 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 016
016![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 073 cases, 2
073 cases, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 032
032![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 deaths affecting 219 countries and territories worldwide, plus two international conveyances (till 17th January 2021).7 A summary of the well-documented deadliest pandemics in history is presented in Fig. 1.
342 deaths affecting 219 countries and territories worldwide, plus two international conveyances (till 17th January 2021).7 A summary of the well-documented deadliest pandemics in history is presented in Fig. 1.
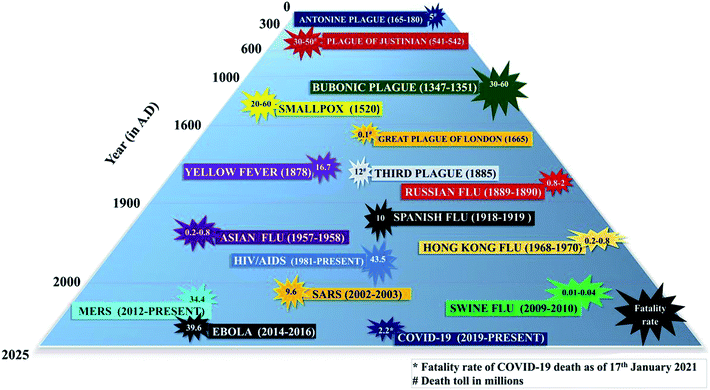 | ||
| Fig. 1 Timeline of various deadly pandemics in history. This figure is adapted from the ref. 29 with modifications and the data is from the ref. 7, 20, 21, 24–26, 28 and 29. | ||
3. Socio-economic impacts of epidemics and pandemics
The impacts of the pandemic are far-reaching, disrupting the social, economic, and political conditions of the affected nations. During the Spanish flu-1918 wave, cities worldwide ran out of space to bury the dead, and several villages perished.4 The vast difference in fatality rate between the high and low-income countries was also noticed during the Spanish flu times. Social inequality is an unnoticed factor associated with pandemics, as evident from the fact that fatality rates are highest for individuals with the lowest socio-economic status.30,31 The recent COVID-19 pandemic has resulted in massive and simultaneous shutdowns/lockdowns worldwide with the suspension of social gatherings and drastic changes in the work and education culture. More than half of the world population were under complete lockdown by the first week of April 2020. Drastic behavioural changes and long-term social stigma arising out of the isolation of the infected and suspected individuals were prevalent during Spanish flu-1918. Similar manifestations are observed in the present COVID-19 as well. Mood changes, insomnia, psychiatric issues, and fatigue were also reported for 40% of the survivors of SARS-2003 in Hong Kong, and maybe the case with all other pandemics.32Pandemics can also increment the anxieties of nations with a weak political framework.30 For example, during the Cholera pandemic-1832, deaths of people in a particular community were linked to some conspiracy theories, resulting in communal violence.33 In light of COVID-19, publishing in the newspaper was banned in some countries, and political instability surged in some places.34,35 These are examples of the influence of pandemic in arenas like sovereignty, civil rights, and democracy.
Apart from social and political repercussions, pandemic and its aftermath affect all major sectors of the economy – trade, agriculture, tourism, and industrial activities. These can be through multiple channels, including short-term fiscal shocks and long-term impact on economic growth. The major incursion of the direct and indirect costs include setting up proper diagnostic and treatment facilities, imparting the preventive steps, strengthening core public-health infrastructure, disinfecting the affected regions, promoting research on developing vaccines, and most essentially, providing essential amenities to the unemployed poor people.30 Economic impacts can scale up to an increased cost of living and a wider gap between the rich and the poor. Pandemics can reduce the capacity of the infected and recovering population to undertake income-generating activities, eroding them financially. It is estimated that a pandemic with 1.4 million deaths could result in 0.8% gross domestic product (GDP) loss.36 Fig. 2 shows the financial losses in terms of GDP loss (%) associated with some of the major pandemics and epidemics.36–42
Spanish flu-1918 has impacted the European region the most. The GDP losses in Great Britain, Canada and the United States (US) were 16.91, 14.68 and 11.32%, respectively. Asian flu-1957 has incurred a GDP loss of 2.6, 3.1, 3.3 and 3.5% in Japan, the US, Great Britain and Canada, respectively.36 Most cases of SARS-2003 were reported in regions of China, Hong Kong, Taiwan, Vietnam, Singapore, Canada, and the US.43 SARS-2003 displayed a negative impact on China's economy, with a total loss of 25.3 billion USD and 1 to 2 percentage point reduction in GDP rate than expected.44 After the Spanish flu-1918, the worst impact on the economy is due to the ongoing COVID-19. The GDP losses (%) are in two digits range for the major economies of the world with the US, India, United Kingdom (UK), France, Italy, Canada, and Germany incurring 32.9, 23.9, 20.4, 13.8, 12.4, 12, and 10.6%, respectively. In contrast, the Chinese economy grew by 3.2% in the April to June quarter of 2020.41 One can see that China's economy has shown a V-shaped recovery after the first quarter. The fast recovery is attributed to increased medical exports, manufacturing, and industrial production.45,46
Individual behavioural changes such as aversion for work environments hamper the financial progress of the country. The disrupted domestic and international distribution channels and consumer hoarding during pandemics can heighten the price of agricultural and other commodities, thereby reducing the household purchasing power. The International Labour Organisation has predicted that unemployment could rise by 5.3 to 24.7 million due to COVID-19.37 When young people in the age group of 15 to 54 are affected by pandemics, it can significantly decline the GDP growth rate. The world economic outlook report by the International Monetary Fund predicted that the global economy would contract by 3% in 2020, which is much worse than the 2008–09 financial crisis. Moreover, a resurgence of COVID-19 in 2021 could leave the economies to wrestle for posterity.47,48 The World Bank estimates that the occurrence of any pandemic in the future with high severity, similar to Spanish flu-1918, might decline the GDP by 5%.36 If the severe effects continue, even food security would be at threat, especially in low-income countries.37 Considering various socio-economic impacts of the pandemic, a transdisciplinary approach should be adopted to study them. Such a study in which several factors are considered to prepare the pandemic preparedness plan would reduce the burden of disease.31
4. Role of face mask in preventing the spread of infectious diseases
The spread of infectious diseases from person to person and the degree of transmission vary based on the aetiology and mode of transmission. Usually, the degree of transmission is estimated by a mathematical number know as reproduction number. It determines the number of infections caused by a single infected individual. It combines factors such as duration of infectiousness, the speed of contact to vulnerable individuals per unit time, and the probability of transmission per contact.49 The transmission ways are mainly classified into the following three: (i) direct transmission – infected droplet/aerosol transmission, (ii) airborne route – inhaling infected respiratory aerosols, and (iii) contact transmission through secretions on fomites or directly such as through physical touch resulting in hand-to-mouth, hand-to-eye or hand-to-nose transmission.50,51 The information regarding the causative agent, transmitting host, transmission route, and reproduction number help in implementing a robust and effective protocol to control infectious diseases. Table 1 summarises major viral diseases declared by WHO as a public health emergency in the affected countries with their causative agents, hosts, and mode of transmission.52| Causative virus | Transmitting hosts | Mode of transmission | Reproductive number |
|---|---|---|---|
| SARS coronavirus (SARS-CoV)9 | Bats, Civets | Respiratory droplets | 4 |
| MERS coronavirus (MERS-CoV)9 | Bats, Camels | Respiratory droplets, cough | 1 |
| Ebola9 | Nonhuman primates | Contact with body fluids | 1.5 to 2.5 |
| Seasonal Influenza (H3N2, H1N1)10 | Pig | Respiratory droplets | 2 |
| SARS-CoV-2 (ref. 11 and 12) | Bats | Respiratory droplets | 1.5 to 3.5 |
A face mask as an RPD helps prevent airborne, droplet and aerosol transmission where the primary control mechanism lies in reducing exposure. Face mask help in filtering out particles of different sizes through various mechanisms:53,54 (i) inertial impaction by which particles with more mass and inertia are diverted from the streamline around the filter fibre, (ii) interception by filter fibres of larger particles, (iii) diffusion which results from the collision with air molecules or fibres resulting in deviation from streamline on account of the Brownian movement of fine particles, and (iv) electrostatic attraction between oppositely charged particles and filter media, without any size distinction of particles. Diffusion mechanism mainly comes into play when the particle sizes are lesser than 0.1 μm, and a combination of diffusion and interception applies for particle size between 0.1 to 0.5 μm. Particles greater than 0.5 μm are removed by interception and inertial impaction.53 The diameter of bacteria is typically 0.4 μm.55 Diameter of viruses usually ranges between 0.01 to 0.20 μm.56 Diagrammatic representation of filtration mechanisms and filter efficiency versus particle diameter are shown in Fig. 3(A) and (B), respectively. In case of antimicrobial face masks, in addition to the physical entrapment, the AMA deactivates/kills the microorganisms that are deposited or captured. Herein, deactivation or killing is mainly based on the mode of action of AMA and is discussed in Section 6.
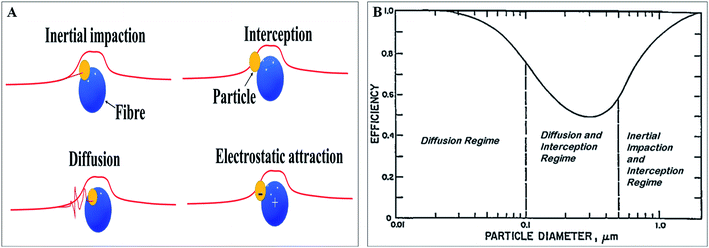 | ||
| Fig. 3 (A) Particles filtration mechanisms in face masks; (B) face mask filter efficiency versus particle diameter. (B) is reproduced from the ref. 53 (open source subjected to creative common attribution 3.0 license). | ||
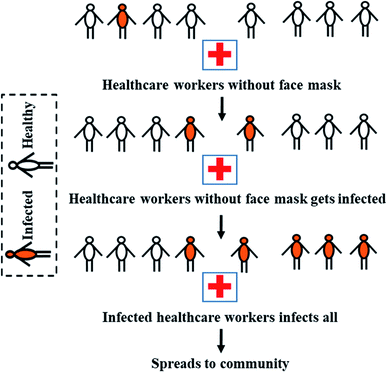
A recent study in Hong Kong has revealed 96.6% general public complying with face mask use during COVID-19. The incidence rates reported in Hong Kong were significantly lower than Spain, Italy, Germany, France, the US, the UK, Singapore, and South Korea,57 emphasising the importance of face mask in reducing community-wide transmission. The use of respiratory protection in healthcare first started gaining prominence during the 1990 tuberculosis outbreak.58,59 Extensive use of surgical face masks was recorded during the SARS-2003, Avian bird flu-2007 in Japan, and Swine flu-2009 in the US and Mexico.60 It was reported that none of the health care workers in hospitals in Hong Kong and China was infected during SARS-2003, who followed appropriate and consistent use of face masks, gloves, and gowns.30
Additionally, virus isolation rates were considerably reduced for 76% of the general public who wore face masks during SARS-2003.61 The air pollution incident in China in 2012 has led to the widespread manufacturing and use of N95 and KN90 respirators to filter out fine particulates.62 Eventually, face masks became an integral part of PPE for health workers to protect themselves from the particles.53 It is now globally accepted that usage of surgical face masks or respirators produces a lesser incidence of virus infections.63 The face mask is inevitably an emerging weapon for the frontline healthcare workers in the COVID-19 war. A diagrammatic representation of the importance of face mask in reducing transmission in healthcare settings is shown in Fig. 4.
5. Evolution of face mask
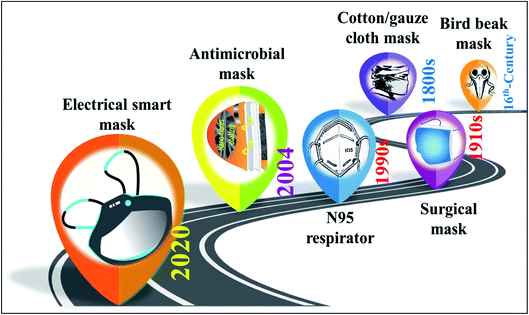
The earliest records of the use of face mask-like cloth coverings are visible on doors of Persian tombs. A 13th-century travelogue describes the use of silk scarves by the servants of the Emperor of the Yuan dynasty, possibly to prevent their breath from affecting the food being prepared.62 The development of functional face mask-like objects was noticed during the 16th century when the Great plague hit the city of London. The mask was popularly known as beak masks and was believed to be filled with herbs such as clove, cinnamon, or camphor to mask unpleasant odours and was used by doctors.64,65 During the same period, it is reported that Leonardo Da Vinci promoted the use of wet clothes as respiratory filters.62 Before the 18th century, loose animal bladder skins and wet clothes were also known to be used to filter dust and harmful agents.58,66 In 1877, the Nealy Smoke face mask was patented,67 which used a series of water-saturated sponges and a bag of water attached to the neck strap to filter out smoke. In 1899, a French doctor created a face mask out of six-layer gauze, which is considered the starting point of modern time tie or string face masks.62,68 These face masks were also combined with additional impervious layers and held on metal frames.69With the onset of chemical warfare associated with World War I, the need for better respiratory protection like an emergency escape breathing apparatus was observed.67,69 The spread of Manchurian plague-1910 and Spanish flu-1918 boosted the widespread use of face mask-like objects among healthcare workers and the general public.69 The respirator certification program was initiated in 1919 by the US Bureau of Mines. In 1920, the agency certified the first respirator known as Gibbs Respirator, mainly used by miners, soldiers, and firefighters.67 During the 1930s, single-use paper face masks were prevalent, and towards the 1960s, disposable face masks made of fleece70 or nonwoven synthetic fibre materials69 came into use. This new type of face mask material facilitated massive transformation by filtering incoming and outgoing air and preventing droplet spread along with better fit and comfort.69 Some other face masks present in the period included a layered gauze face mask with a six-inch rubber piece, a Jel face mask with gauze and filter, and a cellulose-based face mask incorporating plastacele and cotton pledgets.71
Eventually, several factors, like adequate protection, comfort, and physical convenience in wearing, service-life, and materials used, were evaluated for regulation or standardising higher quality respiratory protection.58 Occupational Safety and Health Administration (OSHA) and National Institute of Occupational Safety and Health (NIOSH) were promulgated in the 1970s to assist in safety recommendations and regulation in the workplace, respiratory protection, and other areas of PPE. The factors such as fit and tightness of the face masks also started gaining importance during these times.
Modern face masks are typically made of nonwoven polymeric fibres of different thickness and porosity. The major types of face masks used in the market are respirators and surgical face masks. Respirators are used primarily by healthcare workers to reduce exposure to aerosols and provide leak-proof protection. Respirators should comply with the standards of NIOSH or any other internationally accepted organisations. Respiratory filters are typically made of polypropylene (PP) wool felt fabric or fibreglass paper.66 Surgical face masks are not necessarily designed to provide proper fit or seal to the face; however, its primary purpose is to intercept larger droplets from being expelled by the wearer into the environment. They are designed to be fluid resistant and usually discarded after single-use.53 Surgical face masks are typically made from PP filter layers placed between nonwoven fabrics.72 Polyester, fibreglass, polycarbonate, polyethylene (PE) are also employed in the manufacture.72–74
The ever increasing safety concerns and need for adequate microbial protection have led to the development of antimicrobial compound(s) infused face masks and smart electrical masks. After SARS-2003, much research and development activities have been reported in the area of antimicrobial face mask. According to the report, the Li and team have published the first article on the antimicrobial effect of nanoparticle (NP) coated face masks.75 However, a patent filed in 2004 claims to have developed a face mask based on carbon material having anti-virus and anti-germ effect.76 Since the incidence of COVID-19, the need for the antimicrobial face mask has increased and reached new heights. For instance, recently introduced Guardian G-Volt face mask exploits antimicrobial properties of graphene and electric charge to repel and deactivate microorganisms coming in contact and provide sterilisation and reuse potential.77 The face mask technology has now advanced to include artificial intelligence and facial recognition facilities to create next-generation smart face masks. Fig. 5 summarises the significant milestones in the evolution of face masks.
6. Antimicrobial face masks
The use of a face mask as an RPD is instrumental in preventing airborne, droplet, and aerosol transmission, where the primary control mechanism lies in reducing exposure. However, there are serious concerns about the usage of conventional disposable face masks. When infected individual coughs or sneezes, the pathogen laden droplets can splash out and adheres to the immediate contact surfaces, and remain viable for several days.78 Many studies have proven the presence of SARS-CoV-2 on various material surfaces, including inner and outer layers of face masks for a period of 4 and 7 days, respectively.79,80 These contaminated face mask surfaces, in turn, becomes a source of fomite transmission.81 Besides, the warm and humid conditions inside the face mask due to breathing and saliva creates a favourable environment for the intercepted microorganisms to grow and flourish. The moist conditions will induce a capillary action due to which the intercepted microorganisms transfer further into the inner layers via suction, thus endangering the health of the wearer.75 Eventually, these microorganisms will aggregate as an extracellular polymeric matrix containing polysaccharides, proteins, and deoxyribonucleic acid (DNA) resulting in the formation of biofilms. In these situations, an unexpected danger arises due to the re-aerosolisation of the settled particles during intense sneezing or coughing by the wearer.16Microbial survival and re-growth on conventional face masks after usage and improper storage can also lead to secondary infections in humans.14,15 So, face masks are typically discarded after a single-use to avoid the inoculation and spread of highly infectious pathogens. This type of single-use and discard culture can lead to its massive shortage and the generation of a large quantum of hazardous waste, especially during pandemic times.82,83 The gap in supply and demand coupled with unaffordability, disposal challenges, and possible adverse impact on the environment calls for reusable face masks.
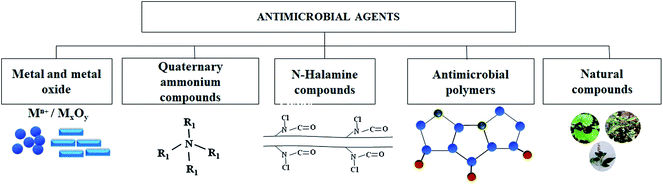
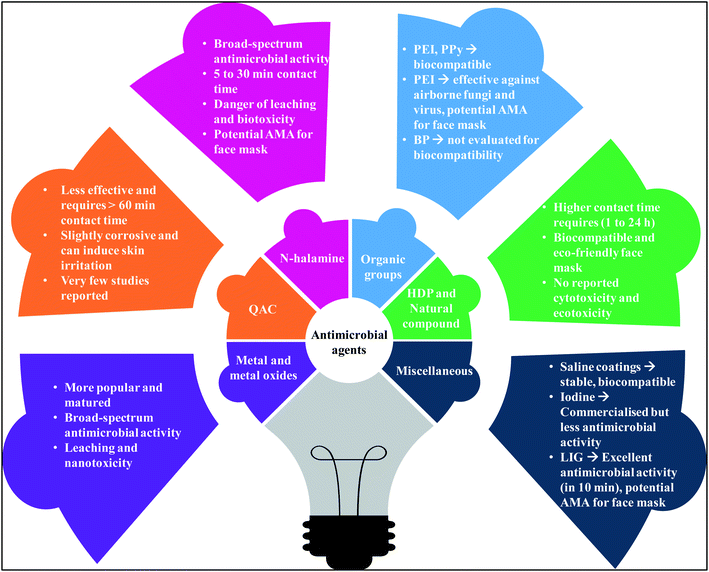
Antimicrobial face masks look attractive over conventional face mask and can address some of the concerns associated with single-use face masks by providing in situ real-time antimicrobial protection. Several AMA are developed over the years and are available in different forms, including films, coatings, beads, and NPs. The surfaces coated AMA are reported to be effective in deactivating/killing microorganisms and preventing the formation of biofilms. The active moieties which impart the biocidal qualities include metal and metal oxides,75,84–95 quaternary ammonium or phosphonium groups,96–100 antimicrobial polymers,101–107 N-halamine compounds,108–112 antimicrobial peptides,105 and natural compounds,82,113–121 as depicted in Fig. 6.
Given the wide choice of the AMA, a comprehensive understanding of AMA would help in the selection of right material-technology combination for the development of efficient, sustainable, and reusable face masks. The materials presented below include the AMA that are tested on the face masks and other promising materials studied for similar applications.
6.1. Metal and metal oxides
Metals such as silver (Ag),122–126 copper (Cu),84,85,87,127,128 zinc oxide (ZnO),95,128 tungsten oxide/carbide,94,129,130 magnesium peroxides,91 and their combinations are employed as AMA in fabrics or face masks.Since ancient times, Ag is a widely used metal for antimicrobial applications. Ag, especially in nano-forms, and its derivatives are known to exhibit antibacterial, antiviral, and antifungal activities.122–126 The interaction of Ag and its compounds with microorganisms is a complex process, and they are known to exhibit a multifaceted mechanism of deactivation. The prominent deactivation pathways include penetration into the cell, interaction with the thiol groups, the formation of free radicals, anchoring with soft bases such as sulphur and phosphorus compounds, and modulation of microbial signal transduction pathways.131,132 Various Ag and Ag-based compounds used in face masks and their potential ability in deactivating microorganisms are reviewed below.
Aloufy and El-Messiry (2013) demonstrated a simple method of soaking nylon-6 nanofibres in silver nitrate (AgNO3) solution to yield Ag incorporated nylon-6 nanofibres. The nanofibres used in the study were fabricated by electrospinning, followed by loading with silver nanoparticles (AgNPs). A 24% (w/w) polymer yielded smooth and uniform nanofibres of diameter 150 to 250 nm. It was reported that after 1 to 2 h of incubation, Escherichia coli (E. coli) colonies were vanished from the material surface due to the antibacterial effect of Ag, as determined from the spread plate method.89 However, Ag-loaded fabrics generally wear off with time due to the detachment of active Ag from the material or often result in agglomeration of the NPs.133–135 The processes such as electrospinning133,136 and melt-blowing133,137 can be employed to strengthen the attachment of biocidal components in the nanofibres. In a study, Ag has been used as an antimicrobial agent to yield an eco-friendly electrospun composite of Acacia lignin with polyvinyl alcohol (PVA).138 The electrospun PVA–lignin–AgNP hybrids nanofibres were tested for antimicrobial activity using agar well diffusion assay. The nanofibre mat of 1 × 1 cm in size showed inhibition zones of 1.1 ± 0.05 and 1.3 ± 0.08 cm against E. coli and Bacillus circulans after 24 h of incubation. The study reveals that the antimicrobial activity is mainly due to AgNPs, which inhibit cell permeability and respiration. Also, the lignin, which is a phenolic compound, results in cell damage and leak of cellular components.
In another study, in situ AgNP incorporated polyacrylonitrile (PAN) was electrospun onto a melt-blown PP layer to develop an antimicrobial bi-layer fabric.90 Dimethylformamide (DMF) was used as a solvent for the polymer and reducing agent for the formation of AgNPs. The developed antimicrobial bi-layer composite filter demonstrated biocidal activity against E. coli and Staphylococcus aureus (S. aureus). The PAN–Ag-15% fabric showed 66 and 78% reduction against S. aureus and E. coli after incubation for 6 h, as determined from the spread plate technique. Bacterial filtration efficiency (BFE) was conducted according to American Society for Testing and Materials (ASTM) F2101.01 (ref. 139) using S. aureus as the biological aerosol. It was observed that the spun-bonded PP nonwoven fabric of 50 to 55 GSM (g m−2), electrospinning time of 2 to 2.5 h, and 10 to 12.5% (w/w) of PAN–Ag was sufficient to achieve a BFE of 99%. A similar course of steps was followed to create a bi-layer micro-nano fibrous membrane.133 In situ AgNP incorporated polyurethane was electrospun onto a melt-blown PP layer. DMF was used as a solvent for electrospinning polyurethane and as a reducing agent for the formation of AgNPs. Disk diffusion assays performed on the material showed large zones of inhibition against E. coli and S. aureus. The study reveals that AgNPs release Ag+ ions, which damages the cell membrane resulting in leakage of cellular components and disruption of DNA. According to a report, electrospun or melt-blown nanofibres alone form a very soft and fragile substrate, but combining it with a nonwoven polymer substrate layer enhances the mechanical property and applicability in developing face masks.90 Also, such a bi-layer formation helps to achieve adequate filtration performance without significant pressure drop.133
In a study, the antimicrobial activity of melt-blown nonwoven PP was evaluated by incorporating Microban® (Ag-based) and Viroksan (magnesium monoperoxyphthalate), separately.137 Microban® and Viroksan were added to the fibre forming head part of the melt-blowing machine to obtain good mixing and even distribution of the compound in the fabric during the fibre formation process. It was observed that 1% (w/w) of Microban® produced complete E. coli reduction in 4 h. In contrast, Viroksan 0.86% (w/w) produced complete killing immediately after the deposition on test fabric. The magnesium monoperoxyphthalate has profound storage stability, low toxicity, and is approved by the Environmental Protection Agency (EPA) for use in consumer products.140 However, assessment should be carried out for determining BFE, antiviral activity, and skin compatibility to confirm the practical utility of such surface-modified face mask.
In addition to Ag immobilised on fabrics, a few studies are reported on Ag immobilised face masks. A commercially available face mask was treated with a starch capped colloidal Ag solution prepared by the chemical reduction method in a study.92 The face mask was soaked for 5 to 7 h in 50 and 100 mg L−1 colloidal Ag solution followed by drying. From the well-diffusion assay against the E. coli and S. aureus, the zone of inhibition was found to be >650 and 1000 mm2 for 50 and 100 mg L−1 of Ag colloidal solution, respectively. The study demonstrated that the mask treated with colloidal AgNPs solution inhibits the growth and multiplication of Gram-positive and Gram-negative bacteria. However, the presence of broad peaks in ultraviolet-visible (UV-Vis) absorption spectra for colloidal Ag and lumps in scanning electron microscope (SEM) images revealed an uneven distribution and aggregation of Ag.
Compared to physical attachment through a dip (soak) coating and spray coating, the surface modification mediated coating renders long-term attachment of AgNPs.16,134,135,137 In a study, the surface of an N95 respirator was treated with a surfactant, sodium oleate, which imparted carboxylic functionalities to the surface.16 The carboxylic sites are reported to be responsible for improved hydrophilicity and served as reaction sites for attachment for AgNPs. Microscopic observations of the fabric using Field Emission SEM after the spread plate tests revealed that the material effectively inhibited the growth of two model airborne bacterial strains, S. aureus and Pseudomonas aeruginosa (P. aeruginosa). However, this study mandates further works to assess the antimicrobial performance of the material and stability of the coating to evaluate the effectiveness of such surface-mediated coatings.
Metallic Cu, copper nanoparticles (CuNPs), copper(II) oxide (CuO), copper(I) oxide (Cu2O), copper iodide (CuI) are found to be effective in inactivating human immunodeficiency virus, influenza virus, bacteriophages, H9N2 virus, poliovirus, bronchitis virus, and herpes simplex virus.60,87,127 Cu ions interact with the negatively charged bacterial cell membrane through electrostatic interaction. The inactivation is through redox cycling between Cu2+ and Cu+ at the cell surface, which catalyses the hydroxyl radicals and superoxide anions. The generation of free radicals damages the lipids, protein, and DNA of bacteria and viruses.83 The antiviral activity of Cu is because of the interaction of Cu2+ with DNA, where the nucleotide and the base pair of the DNA offer the binding sites.141 The ROS formed during redox recycling also results in the degradation fragment of viral proteins, hemagglutinin and neuraminidase.87 It is said that the virucidal activity of Cu is enhanced in the presence of peroxide. Peroxide opens the protein coat of the virus and facilitates the entry of Cu ions to denature nucleic acids.85,87 Cu ions also interfere with the cell permeability resulting in the loss of potassium ions and cell death.83
Cu oxo-hydroxide NPs is reported to be better virucidal agents than other Cu combinations.93 The stability of CuNPs could be improved by functionalisation using carboxylate ligands. CuO and Cu oxo-hydroxide can be coated on face masks, surgical gowns and gloves, respirator filters, polymeric materials, and other medical equipment fabrics without losing its antiviral property.84,87,93,142 A four-layered NIOSH approved N95 respirator was modified with CuO particles to impart antimicrobial property.60 The first and fourth-layers were prepared using spun-bonded PP impregnated in situ with 2% (w/w) CuO particles. The second-layer was made up of a melt-blown PP with 2.2% (w/w) of CuO particles, and the third-layer was fabricated with polyester. The bioaerosol filtration tests revealed that 99.85% of H1N1 and H9N2 viruses were filtered under simulated breathing conditions with no infectious viral titers recovery within 30 min, by employing a method customised from ASTM F2101.01 for the virus.139 Moreover, the CuO coated face mask successfully passed BFE as per EN 14683:2005 and ASTM F2101 standards.139,143 The modified face mask also passed differential pressure (EN 14683:2005),143 latex particle challenge (ASTM F2299),139 and resistant to penetration by synthetic blood tests (ASTM F1862)144 and NIOSH N95 standards. Furthermore, it was claimed that no skin sensitisation or irritation was observed for the outer layers of the face mask as determined from animal studies.
In yet another study, Hashmi and coworkers (2019) reported a polymer solution made from PAN (8% w/w) in DMF and CuO. The composite solution was electrospun to develop an antimicrobial face mask. Disk diffusion assay showed that CuO (1% w/w) incorporated face mask was effective against both Bacillus subtilis (B. subtilis) and E. coli. Based on the breathability test (ASTM E96),146 air permeability test, and antimicrobial activity test, the authors claimed that the face mask is useful for breath mask applications. The nanofibre structure is intact even after contact with water for 72 h, and a concentration beyond 1% (w/w) of CuO is toxic.145
Compared to individual metal and metal oxides, combinations with other metal and metal oxides have shown complete inactivation in lesser contact time.75,88 A combination of different AMA also reduces the chances of resistant strains of microorganisms.75 In 2012, Davison developed BioFriend™ and BioMask™ masks, which comprised four layers of standard filtration materials approved for facemasks.88 The first and fourth-layer were made up of spun-bonded PP. The first-layer was treated with citric acid to create a low-pH hydrophilic coating, which can induce structural rearrangements in the lipid layer of viruses. The second-layer was fabricated with cellulose/polyester infused with Cu and zinc ions, which can bind the virus through sulfhydryl and carboxyl groups, destroying it. The third-layer was made up of melt-blown PP. The outer surface of both the mask was challenged with bacterial (initial 105 to 107 CFU mL−1) and viral (initial 5.35 to 7.79 log10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TCID50) suspensions for 5 to 180 min. The samples were then tested for the remaining virus titers using the Spearman–Karber method. Bacterial colonies were tested according to the American Association of Textile Chemists and Colorists (AATCC)-100 method.147 These masks showed reductions of 99.93% @ 30 min, 89.22% @ 60 min, 90.15% @ 60 min, 88.97% @ 60 min, 99.99% @ 8 h against methicillin-resistant S. aureus, Streptococcus pneumoniae, Haemophilus influenzae, Mycobacterium terrae, and Staphylococcus epidermis (S. epidermis), respectively. These masks showed ≥4 log10 reductions within 5 min of contact for 19 different subtypes of viruses, including influenza A and B viruses, paramyxovirus, SARS-CoV, and herpes simplex virus. Both the face masks conformed to ASTM2100:11/EN14683:2005 (ref. 143 and 148) and EN 149:2000+A1:2009 (ref. 149) standards. Additionally, both the face masks demonstrated no dermal irritation or sensitisation and allergenic potential under simulated breathing conditions.
TCID50) suspensions for 5 to 180 min. The samples were then tested for the remaining virus titers using the Spearman–Karber method. Bacterial colonies were tested according to the American Association of Textile Chemists and Colorists (AATCC)-100 method.147 These masks showed reductions of 99.93% @ 30 min, 89.22% @ 60 min, 90.15% @ 60 min, 88.97% @ 60 min, 99.99% @ 8 h against methicillin-resistant S. aureus, Streptococcus pneumoniae, Haemophilus influenzae, Mycobacterium terrae, and Staphylococcus epidermis (S. epidermis), respectively. These masks showed ≥4 log10 reductions within 5 min of contact for 19 different subtypes of viruses, including influenza A and B viruses, paramyxovirus, SARS-CoV, and herpes simplex virus. Both the face masks conformed to ASTM2100:11/EN14683:2005 (ref. 143 and 148) and EN 149:2000+A1:2009 (ref. 149) standards. Additionally, both the face masks demonstrated no dermal irritation or sensitisation and allergenic potential under simulated breathing conditions.
In another study, the first-layer of a surgical face mask fabric was coated with 0.4 mg cm−2 NP emulsion consisting of a mixture of 2 g L−1 aluminium oxide, 2 g L−1 AgNO3, 1.5 g L−1 titanium dioxide, 2 g L−1 Ca2+, 2 g L−1 Mg2+, and 60 g L−1 oleophobol C employing a textile finishing machine.75 Oleophobol C was added to ensure homogenous dispersion of the NP emulsion on the face mask surface. Minimum inhibitory concentration (MIC) and minimum bactericidal concentrations (MBC) for E. coli were found to be 1/128. The MIC and MBC for S. aureus were found to be 1/512 and 1/64 dilutions of the NP emulsion, respectively. The antibacterial tests revealed a complete reduction of E. coli and S. aureus, and no skin irritation was observed for volunteers who wore the face mask. The higher MBC for S. aureus can be attributed to the thick peptidoglycan layer for the Gram-positive bacteria. It was concluded that the biocidal effect arises upon damage to the plasma membrane and leakage of cytoplasmic contents. This study claims to be the first report on the antimicrobial effect of NP coated face mask.
Apart from the above-discussed metal and metal oxides or their combinations, tungsten oxide,94 magnesium peroxide,91 and ZnO95 are also antimicrobial. According to a report, magnesium peroxide coated cotton exhibited antimicrobial activity up to 70 washing cycles.91 It is reported that tungsten oxide and tungsten oxide composite microparticles are also used on face masks, filters, gloves, and other medical appliances.94 Studies have proved excellent antiviral property of zinc oxide nanoparticles (ZnONPs) and polyethylene glycol (PEG)-coated ZnONPs against influenza virus strains.95 However, the stability and effectiveness of these compounds need to be evaluated to establish its practical utility.
6.2. Antimicrobial polymer
Antimicrobial polymers are a versatile class of material with tunable surface chemistries, size, and multiple scale lengths; that can be combined with different matrices or surfaces and tailored to yield contact-killing abilities.150 There are two broad classes of antimicrobial polymers viz., bio-passive and bio-active. Bio-passive polymers are used for repelling surfaces to prevent bacterial adhesion. The polymers functionalised with bio-active agents are called bio-active polymers, and these are capable of killing the microorganisms adhered to the polymer surface.150,151 The active moieties include metals, metal oxides, N-halamines, quaternary ammonium or phosphonium compounds, antimicrobial peptides, and antibiotics.151In a study, a simple soaking method was employed to modify the PP surface with N-halamine.110 The melt-blown PP nonwoven-fabrics (22 and 50 GSM) were soaked in N-halamine 1-chloro-2,2,5,5-tetramethyl-4-imidazolidinone (MC), for 10 min and then padded and dried. The antimicrobial PP fabric with 0.47% (w/w) oxidative chlorine loading showed complete inactivation (6 log10) of S. aureus and E. coli in 5 and 10 min of contact time for 50 and 22 GSM fabric, respectively as determined from the spread plate technique. The higher GSM fabric provided greater surface area, higher chlorine availability, and rapid antimicrobial activity. Further, the bioaerosol filtration studies conducted as per ASTM F2101.01 (ref. 139) revealed that no viable cells were recovered up to 3 h exposure against S. aureus and E. coli aerosol. However, the N–Cl bond is prone to photo-dissociation. Hence, all the N-halamine coated fabrics are recommended for dark storage. A similar soaking, padding, and drying procedure were followed to coat PET fabric with 5% (w/w) terpolymer, poly(hydantoinyl acrylamide-co-glycidyl methacrylate-co-2-(methacryloyloxy)ethyl trimethylammonium chloride). After soaking, the fabric was halogenated using 10% (w/w) Clorox solution (bleach), followed by washing and drying. The material demonstrated a complete reduction of S. aureus and E. coli cells (6 log10 reduction) within 2 min of contact time for swatches having 0.19% (w/w) oxidative chlorine concentration, as determined from the spread plate technique.111 The rapid inactivation was partly due to the hydrophilicity imparted by the cetyltrimethylammonium chloride groups.
The incorporation of N-halamine precursors through graft copolymerisation resulted in better and permanent coating compared to physical coating through blending, soaking or spraying.109,152 In a study, N-halamine precursors such as acrylamide, and methacrylamide were attached separately to the PP through graft copolymerisation.109 The modified PP was processed into fibres through an extrusion process. The fibres were then halogenated by soaking in chlorine bleach (∼1500 mg L−1 of available chlorine) followed by washing and drying. The material demonstrated a complete reduction of E. coli within 30 min, tested as per AATCC-100 method.147 Also, both antimicrobial fabrics demonstrated >1.48 log10 reduction against S. aureus.
In a study by Tan and Obendorf (2007), N-halamine additives [chlorinated 5,5-dimethyl hydantoin (CDMH), chlorinated 2,2,5,5-tetramethyl-imidozalidin-4-one (CTMIO) and chlorinated 3-dodecyl-5,5-dimethyl hydantoin (CDDMH)] were separately incorporated into nylon-6 dope and electrospun to develop nanofibrous antimicrobial fabrics.108 Nylon 6-CDMH, nylon 6-CTMIO, and nylon 6-CDDMH fabrics with an active chlorine content of 1125 mg L−1 achieved a total reduction of E. coli and S. aureus within 10, 30, and 30 min, respectively (tested as per AATCC-100 method).147 The difference in the inactivation is due to the N–Cl bond of the three compounds. The dissociation rate is in the order of imide > amide > amine type halamine. In another study, N-halamine compound MC was introduced into PAN polymer dissolved in DMF solvent and electrospun to yield nanofibres (PAN/MC-5%).112 From the antibacterial assays, it was found that PAN/MC-5% with 0.29% oxidative chlorine loading inactivated all S. aureus and E. coli cells within 1 and 10 min of contact, respectively, tested as per AATCC-100 method.147 Also, PAN/MC-5% achieved >6 log10 reduction against S. aureus aerosols after 3 h of exposure, tested as per ASTM F2101.01.139 Moreover, the breathability of these membranes was reported to be 27.3 mm s−1, which is higher than the commercial nano-N95 and nano-surgical face mask.
In contrast to physical coating methods, covalent attachment and in situ incorporation via electrospinning eliminate the chances of leaching of N-halamines from the fabric surface.108,109,152,157 Even though few studies are reported on the leaching of N-halamine modified fabrics in water,108 the direct release of N-halamine from fabric or face mask surface is yet to be evaluated to assess its effect on the skin. Since the AMA generally coated on middle filtration layers of face mask, direct contact with skin is unlikely. Though studies reveal the excellent antibacterial activity of N-halamine incorporated fabrics, there is no literature on application of this compound in developing antimicrobial face mask. Performing antibacterial and antiviral assays, BFE and VFE would establish the practical utility of N-halamine incorporated face mask.
Studies have reported the immobilisation of QACs on glass surfaces,160 polymer surfaces,161 surgical face masks,100 and nonwoven fabrics96,98 through surface-mediated coatings, grafting, melt-blowing and spray coating to kill/deactivate airborne and aerosol borne microorganisms. QACs such as cetyltrimethylammonium bromide (CTAB) can form reactive oxygen species (ROS) and increases the superoxide mediated stress resulting in biocidal activity.162 Superoxide anions, hydrogen peroxide, and hydroxyl radicals are produced by penetrating oxygen into the cells during the intracellular enzymatic reactions. Montazer et al. (2010) developed antimicrobial polyester, PP, and viscose nonwoven fabrics coated with CTAB and fluorochemical using a padding procedure (bath coating).99 PP and polyester exhibited 6 log10 reductions of E. coli and S. aureus and >2 log10 reductions of P. aeruginosa with 1% CTAB solution, as determined from the spread plate technique. In contrast, nonwoven viscose fabric was unable to achieve the expected results due to the negative influence of adhesive used in fabric production. It is reported that fluorochemical was added to reduce the hydrophilicity of the surface to achieve good water or blood repellent property.
In another work, Gutarowska and coworkers (2014) studied the antimicrobial effect of bath (soak) and spray coating of Sanitised® T 99-19 (commercially available QAC) on needled nonwoven polyester, and melt-blown nonwoven PP.96 Sanitised® T 99-19 was sprayed and soaked to obtain 0.7 to 2% deposition on polymer fibres. Better antimicrobial activity is obtained for needled nonwoven polyester due to higher migration of active compound to fibre surface when compared to melt-blown nonwoven PP. It is attributed that the compact surface of a melt-blown nonwoven PP facilitates the only surface distribution of the active compound. The bathing method offered better antimicrobial activity than spray coating since it wets the entire volume of the material. It is reported that needled nonwoven polyester subjected to bath coating with 2% of Sanitized® T 99-19 was biocidal against E. coli, S. aureus, Candida albicans (C. albicans), and Aspergillus niger (A. niger), tested as per AATCC-100 method.147 Whereas, for melt-blown nonwoven PP, the biocidal values were not much significant. Further, paraffin oil mist and airflow resistance of the material was tested according to the EN 149:2001+A1:2009 standards.149 However, bioaerosol filtration studies have to be performed to understand the efficacy of the material for its applicability in preparing an RPD.
Studies have shown that perlite (volcanic glass) carriers could enhance the hydrophilicity, antimicrobial activity, and flexibility of PP to bond with various chemical agents.98,163 Bio-perlite and bio-bentonite refer to alkylammonium microbicides (QAC compound) on perlite and bentonite (aluminosilicate base) carriers, respectively. These compounds were used in a study to impart biocidal quality to PP fibres.163 The compounds were added in powder form to the fibre forming head to obtain thorough mixing with PP during the fibre formation process. Subsequent melt-blowing resulted in uniform distribution of the biocidal agent on the nonwoven PP. It is reported that melt-blown PP with 15% bio-perlite (0.69% of microbicide) is biocidal and achieved 96.8% and 74.1% BFE against E. coli and S. aureus, respectively. The study utilised the electrostatic interaction between the positively charged microbicide and the negatively charged mineral carriers to create bio-perlite and bio-bentonite. The mineral carriers remained attached to the fibres, while the microbicide remained free and capable of antimicrobial activity. The study was further extended to explore the antimicrobial respiratory protection using a three-layer half-mask model by Gutarowska and coworkers in 2010.98 The first-layer was made from needling nonwoven fabric consisting of Bico biocomponent (Inatex, Poland) and an acrylic fibre (Unia, Poland). The second-layer was an antimicrobial filter layer made of nonwoven PP with bio-perlite. The third-layer was made from needled nonwoven polyester and Bico biocomponent. The filtering half-mask with a bio-active nonwoven PP with 0.37% of the active compound (8% bio-perlite) achieved a complete reduction of E. coli and S. aureus, tested as per AATCC-100 method.147 The bio-active nonwoven PP fabrics were also effective against P. aeruginosa and K. pneumoniae, Mariniluteicoccus flavus, and B. subtilis. Further, it was established that the filtration efficiency of the material was excellent against liquid particles (>95% against paraffin oil mist aerosol), solid particles (>98% against 0.6 μm NaCl aerosol), and microorganisms (99.9% for E. coli and S. aureus suspended in 0.85% NaCl solution).
Majchrzycka et al. (2017) developed a biocidal agent, namely Gemini surfactant (QAC-based salt) on a halloysite carrier that exhibited temporal biocidal activity triggered by humidity.164 Varying amounts of 1,2-propanediol was added to regulate the humidity, resulting in temporal activation of the biocidal agent. The biocidal agent was added to the semi-liquid polymer during the melt-blowing process of PP, resulting in a permanent bonding. The material with 0.336% of the Gemini surfactant exhibited 97.46, 87.85, 94.53, and 46.78% reduction against bacteria E. coli, Pseudomonas fluorescens, moulds A. niger and Penicillium chrysogenum, respectively, tested as per AATCC-100 method.147 This filtering nonwoven PP is a potential material in producing reusable RPDs. Further, the temporal release of the particular biocide is impressive from safety aspects as it would reduce the skin contact upon continuous use of RPDs.
Tseng et al. (2016) modified a US Food and Drug Administration (US FDA) approved three layers surgical mask by spraying QAC based Goldshield-5 solution (GS5, 1%), which gave 0.1 mL cm−2 coating.100 The GS5 coated face mask was challenged with settled aerosol through an aerosol test system (tested with an aerosol test system modified from ASTM 2721-10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 165), which generated 108 CFU mL−1 of bacterial strains. The test results showed a 2.32 to 5.02 log10 reduction of colonies in 60 min contact with GS5 coated mask for Acinetobacter baumannii (A. baumannii), Enterococcus faecalis (E. faecalis), and S. aureus. Further bioaerosol filtration study revealed >98% efficiency against all the three bacterial aerosols for 60 min contact time. However, in the study, simplified spraying method is used for coating GS5. Spray coating would not ensure complete penetration of antimicrobial compound into the crevices or shadowed regions, thus warranting proper investigation into coating efficiency and stability of the coating. Also, various QAC compounds are known to be corrosive and pose health issues such as work-related asthma and skin dermatitis.166,167 Therefore, a detailed evaluation of the biocompatibility is recommended.
165), which generated 108 CFU mL−1 of bacterial strains. The test results showed a 2.32 to 5.02 log10 reduction of colonies in 60 min contact with GS5 coated mask for Acinetobacter baumannii (A. baumannii), Enterococcus faecalis (E. faecalis), and S. aureus. Further bioaerosol filtration study revealed >98% efficiency against all the three bacterial aerosols for 60 min contact time. However, in the study, simplified spraying method is used for coating GS5. Spray coating would not ensure complete penetration of antimicrobial compound into the crevices or shadowed regions, thus warranting proper investigation into coating efficiency and stability of the coating. Also, various QAC compounds are known to be corrosive and pose health issues such as work-related asthma and skin dermatitis.166,167 Therefore, a detailed evaluation of the biocompatibility is recommended.
In another study, polyester fabrics were incorporated with polypyrrole nanoparticles and investigated for antimicrobial activity.106 The antimicrobial activity of polypyrrole is due to the positive charges introduced along its backbone during the polymerisation process.168 Dispersions of stable polypyrrole nanoparticles, produced by chemical oxidative polymerisation, were coated on polyester using an ultrasound-assisted coating process. A polypyrrole loading of 4.33 ± 0.13 g m−2 on polyester showed a complete reduction of S. aureus and E. coli cells, as determined from the spread plate technique.
Hong and Sun (2009) probed the incorporation of photosensitisers such as benzophenone (BP) into polymers and textiles to render them antimicrobial. In the study, polymer solutions of PP, PE, PVA, and PS were thoroughly mixed with BP solution and melted on glass slides and cast into films separately.169 Initially, the microbial suspension was placed on the film and subjected to irradiation with UV light for 1 h. Bacterial concentration remaining was determined by the conventional spread plate technique. Complete reduction of E. coli and S. aureus cells was achieved for PVA/BP (0.25% w/w), PE/BP (1% w/w), and PS/BP (1% w/w). Among various polymers tested, PVA showed the highest antimicrobial activity. Polymers such as PVA with BP groups are potential self-decontaminating material with proven application in hygiene gloves, protective face masks, and surgical gowns. When BP is photo-irradiated, it forms BP ketyl radicals by accepting hydrogen atoms due to the interaction of amines and alcohols groups present in the supporting polymer (i.e., PVA). Upon exposure to oxygen, the BP ketyl radicals immediately reoxidised to form BP and H2O2. The formed reactive radicals and H2O2 play a significant role in the antimicrobial action.169 However, the production of reactive radicals and H2O2 is of concern. Hence, biocompatibility and skin sensitisation tests have to be conducted to ensure the safety of the wearer.
Natural host defence peptides (HDPs) are part of our innate immune system, which deactivates a broad spectrum of pathogens, playing a vital role in the host response system. Biocidal cationic polymers inspired by HDPs are biocompatible,105 do not possess cytotoxicity and ecotoxicity,170 and has excellent applicability in antimicrobial surfaces.171 An antimicrobial nanofibrous substrate was prepared by grafting L-cysteine onto PP, followed by electrospinning to form fibres and cross-linked by Cys-LC-LL-37.105 Antibacterial assays on the fabric were performed according to ASTM E 2180-01 standard test method172 and showed 1.6 and 6 log10 reduction against P. aeruginosa and S. aureus after 24 h contact time. The material was reported to be effective against nosocomial pathogens and their spread to the community. Due to their biocompatibility, antimicrobial peptides could be potential candidates for developing novel antimicrobial face masks with least environmental concern.
Apart from the aforementioned groups, natural polymers, polymers with aromatic or heterocyclic groups (imidazole derivatives), polyacrylamides, polyacrylates, poly-siloxanes, poly-ionenes, poly-oxazolines, hyperbranched and dendritic polymers (poly(amidoamine) and poly(propylene imine) dendrimers) and polymers with guanidine groups, possess intrinsic antimicrobial qualities.153 The antimicrobial abilities of these groups after incorporating on face mask are yet to be evaluated.
6.3. Natural compounds
Several antimicrobial compounds extracted from medicinal plants are found to be effective against bacteria and viruses. Unlike chemical polymeric or nano-based materials, the natural compounds do not produce toxic effects. Extracts of Vitex trifolia,173,174 Punica granatum,175–177 Allium sativum,178–180 Acacia nilotica,181 Andrographis paniculata,182,183 Sphaeranthus indicus,184 Strobilanthes cusia,185,186 Chromolaena odorata,187 Aloe barbadensis,188–190 and Azadirachta indica113,191 have antimicrobial properties. Also, the combination of eugenol, eugenol acetate, carvacrol, thymol, and vanillin,115 Melaleuca alternifolia,116 ginkgo leaf extract,117 and ginkgo extract in combination with sumac (Anacardiaceae family)118 have been studied for their antiviral properties. These compounds are found to be potential agents that could be applied to face mask, air filters with plastic and nonwoven polymer fabrics.Mangosteen extracts (α-mangostin (46.36%) and γ-mangostin (5.45%)) were spray-coated on commercially available melt-blown PP layers to develop a three-layer antimicrobial mask with improved hydrophilic character.119 BFE was conducted as per ASTM F2101.01 (ref. 139) and was reported to be 97.9 ± 0.2% for the face mask containing 5% (w/v) mangosteen extract. Antimicrobial studies as per AATCC-100 method147 revealed a 5 and 2 log10 reductions in S. aureus and Mycobacterium tuberculosis, respectively, after 24 h exposure. The 97.2% antimicrobial activity of the extract was retained even after 21 days of storage and did not compromise the mechanical attributes of the filter. The coated face mask did not exhibit any toxicity as revealed from indirect cytotoxicity studies based on the viability of L929 mouse fibroblasts. In mangosteen extract, α-mangostin and γ-mangostin are the bioactive components, which inhibits the bacterial activity by inducing rupture of the cytoplasmic cell membrane resulting in leakage of intracellular components.119
A recent study reports the development of a three-layer antibacterial face mask consisting of a nonwoven PP outer layer, antibacterial filter layer and an inner medical cotton yarn layer.120 Microcapsule made from the extract of Scutellaria baicalensis was used as an antimicrobial agent in face mask. The microcapsules were incorporated to filter clothes such as cotton, polyester and nonwoven PP by soaking followed by plasma mediated surface modification. Antibacterial studies as per AATCC-100 method147 revealed that all filter clothes exhibited >96% and >95% reduction against E. coli and S. aureus, respectively. Further, nonwoven PP, cotton, and polyester exhibited BFE of 97.31, 95.44, and 91.7%, respectively. It was observed that oxygen plasma surface treatment helped in the long-term attachment of microcapsules (up to 100 cycles) on filter clothes when compared to the untreated surface. It is reported that the antibacterial activity of Scutellaria baicalensis is due to the bioactive components present like wogonin, skullcapflavone, baicalein, and baicalin.192 However, its mechanism of action is not studied in detail.193
In another study, oil of a Vietnamese medicinal plant, Folium Plectranthii amboinicii, and sustainable filter paper was used to develop a seven-layer antimicrobial face mask.82 The first, second, sixth, and seventh-layer were made from filter paper; third and fifth-layer with blotter paper; and fourth-layer with waterproof paper. The third and fifth-layers contained activated carbon, 5% standardised BSI (Benzoic acid, Salicylic, and Iode) solution and 0.5% Plectranthii amboinicii plant oil extract. This face mask is reported to be qualified by the Directorate for Standards, Metrology, and Quality of Vietnam criteria. The fractional concentration of nitric oxide in exhaled breath (FENO), a relevant inflammatory biomarker of the respiratory system, was found to be significantly lower (p = 0.01) after the usage of the face mask. Also, the wearing of the mask resulted in clearer nasal passageways (p < 0.0001), fewer throat symptoms (p < 0.020), and fewer respiratory symptoms (p < 0.020). The antibacterial effect of Plectranthii amboinicii is accounted due to terpinen-4-ol and carvacrol, which inhibits bacterial proliferation.194
Chen et al. (2020) explored the suitability of biocompatible amino acid-grafted enzymatic hydrolysis lignin derivatives with both cationic/anionic groups as AMA for face masks. Antimicrobial tests on the middle layer of commercial N95 face mask soaked in arginine modified enzymatic hydrolysis lignin (33% w/w) revealed that the number of viable E. coli cells decreases to a non-measurable level from an initial load of 107 CFU mL−1, as determined by a method of the National Committee for Clinical Laboratory Standards Institute.121 The enzymatic hydrolysis lignin has plenty of phenolic and carboxylic groups which disturbs the bacterial cell membrane. Besides, the grafting of amino acid groups enhances the positive charges and causes cell membrane rupture.121
Catel-Ferreira et al. (2015) developed a novel bio-based antiviral face mask through the laccase enzyme-mediated modification of the nonwoven cellulosic fibre filter with catechin polyphenols. The modified filter targets the viruses and produced 5 log10 reductions against T4D bacteriophage in 1 h, determined using a laboratory aerosol filtration setup with an effective filtration area of 15.9 cm2. According to the report, the best virus capture factor was obtained when two numbers of this modified cellulosic filter are placed inside the medical face mask. This bio-based antiviral face mask also showed excellent antiviral activity against the T4D bacteriophage virus of E. coli B.195
6.4. Miscellaneous agents
Quan et al. (2017) found that saline coatings can enhance the hydrophilic character of face mask surfaces, which promotes microbial adhesion.196 NaCl was incorporated as an active moiety in the middle PP layer of a three-ply surgical face mask in the study. Saline loading of 18.6 mg cm−2 showed 85% filtration efficiency against H1N1 influenza virus aerosol (2.5 to 4 μm), using a laboratory aerosol filtration setup. Saline coated filters demonstrated complete hemagglutinin activity (HA) loss of adsorbed virus (physical destruction of the virus) within 5 min contact time. In vivo experiments on mice exposed to viral aerosol derived from saline coated filters showed higher survival against the H1N1 influenza virus and other strains, including A/Puerto Rico/08/1934 (PR/34 H1N1) and A/Vietnam/1203/2004 (VN/04 H5N1). Further, it was observed that the saline remains coated on the filters retaining its performance even after storing for 15 days at 37 °C and 70% relative humidity. Even though a change in grain orientation was observed, the stability of coating was ensured even under harsh environmental conditions. When bioaerosols come in contact with salt, it will cause the salts to dissolve locally and the osmotic pressure imbalance happens to the bioaerosol. Due to evaporative drying, the salt concentration increases and attains super-saturation, resulting in recrystallisation of salt. The physical damage due to membrane perturbation and protein denaturation during recrystallisation and osmotic imbalance results in inactivation of the bioaerosol. The physical damage is linked to the HA activity loss of the virus in the above study.196,197Iodine, along with an element in period 4 to period 6 and group 8 to group 15 (Cu, Ag, antimony, iridium, germanium, tin, titanium, palladium, bismuth, gold, iron, zinc cobalt, nickel, indium and mercury) are active antiviral agents. It is reported that these antiviral agents can be immobilised on fibres of face masks, filters, clothes, and polymers such as polyester, PE, PET, PAN, PP, PVA, polyvinyl chloride (PVC), polytetramethylene terephthalate, polybutylene terephthalate polyamide, polyacrylic acid, polytetrafluoroethylene, kevlar, poly(methyl methacrylate), cupra, polynosic, tinsel, rayon, wool, cotton, silk, hemp, and bamboo to yield potential antimicrobial fabrics.198 Heimbuch et al. (2006) incorporated a thin coating of iodine releasing polymer on P95 face masks and found 4.3 log10 reduction in MS2 coliphage.199 Iodine based antimicrobial masks such as iodine-based flat-panel N95, iodine-based cup-shaped P95, and NIOSH approved SafeLife N95 filtering facepiece respirator (FFR) with Triosyn (iodine) agent have been studied for their antimicrobial efficacy.9,10 In a study, it was observed that both the antimicrobial FFR, i.e. iodine-based flat-panel N95 (AM-N95) and iodine-based cup-shaped P95 with exhalation valve (AM-P95) demonstrates no significant difference in physical and viable penetration tests. This implies that the performance is based on physical effects rather than antimicrobial qualities.9 Both the iodine-based antimicrobial FFR did not show enhanced performance over conventional N95 and P100 FFRs.
Since the discovery of graphene in 2004, it has been a widely used nanomaterial for several applications, including antimicrobial application. The first report on the antimicrobial activity of graphene was published in 2010. According to data, 85 μg mL−1 of graphene oxide has shown a 98.5% reduction in E. coli load from an initial load of 107 CFU mL−1.200 The antimicrobial activity of graphene is attributed to the physical destruction of the cell membrane upon contact with the sharp edges of graphene. According to Kumar et al. (2019), the generation of ROS and the consequent exertion of oxidative stress that damages the proteins and lipids of the cell membrane is the major pathway of antimicrobial action.201 Among various nanoscale carbon derivatives, graphene,202 graphene oxide,203,204 and reduced graphene oxide205 are employed to make antimicrobial fabrics effective against bacteria and viruses.201,206,207
Laser-Induced Graphene (LIG) was discovered in 2014. The method uses an infrared laser scriber to convert carbonaceous materials into porous 3D graphene under an ambient environment. It is a surface graphitising technique used for patterning graphene in various shapes on desirable substrates.208,209 Recently, LIG technology is being explored to develop self-decontaminating face masks. Zhong et al. (2020) employed LIG technology to deposit graphene over a commercial surgical face mask.210 Contact angle measurements revealed graphene-coated mask provides a superhydrophobic surface (contact angle > 140°), which prevents the attachment of virus-laden droplets onto the face mask surface. From photothermal studies, it was revealed that on exposure to sunlight, the temperature of the graphene-coated face mask spikes to 70 °C in 40 s, with potential to eliminate any virus deposited on the face mask surface (any virus is expected to be inactivated at 70 °C). However, a thorough evaluation of the antimicrobial property and bioaerosol filtration efficiency need to be carried out to implement it in the real field applications. In a similar study, Huang and co-workers (2020) observed that LIG deposited commercial surgical face mask exhibited 81.57% reduction against E. coli load from an initial from an initial load of 107 to 108 CFU mL−1. The face mask demonstrated 100% inhibition against E. coli when irradiated at 0.75 kW m−2 sunlight for 10 min.211 The present environmental problems faced due to single-use face masks can be overcome by developing similar self-cleaning or self-sterilising mask, which assures the reusability of face masks. An overview of Section 6.1 to 6.4 regarding various AMA is given in Fig. 7.
6.5. Methods of incorporation of antimicrobial agents
AMA can be incorporated into a face mask through various methods such as dip coating, spray coating, melt-blowing, and electrospinning. The diagrammatic representations of these techniques are illustrated in Fig. 8.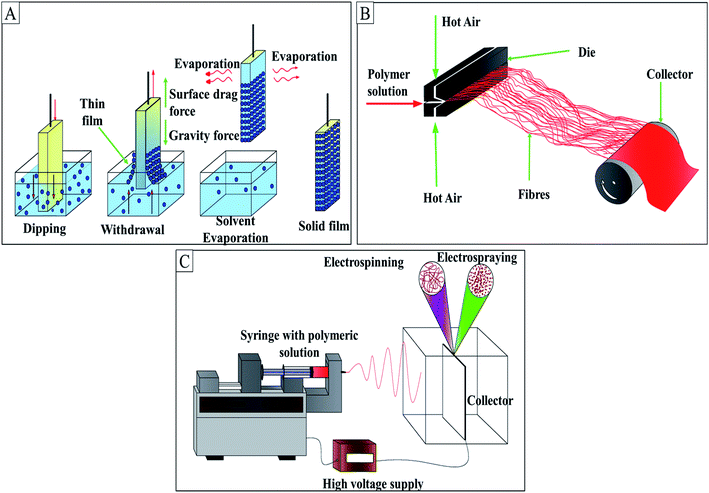 | ||
| Fig. 8 (A) Dip coating; (B) melt-blowing; (C) electrospinning; this figure is adapted from the ref. 212 and 213 with modifications. | ||
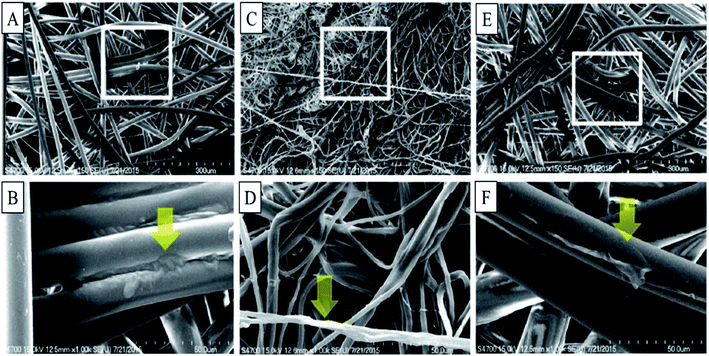
Dip coating is a method of depositing a thin homogenous layer of a solution containing particles of ceramic, metallic, fibrous materials, biomolecules, and polymer films to form a coating on the substrate. The process is done in 5 stages: immersion, start-up, and deposition, followed by drainage and evaporation. The thickness of the coating depends on the solution properties such as surface tension, viscosity, density, and processing parameters such as withdrawal speed, evaporation rate, and duration of immersion, start-up, and deposition.214 This technique is restricted to flat or smooth surfaces, and it is not suitable for selective coating a single side of the object. The instability of the precursor in case of fast condensation, large requirement of the coating solution, and difficulty in handling are the critical issues. Fig. 9(A)–(C) depicts SEM image of cotton, polyester and nonwoven fabric soaked with a microcapsule based on extractant of Scutellaria baicalensis. However, it is observed that the dip (soak) method has resulted in aggregation of microcapsules resulting in non-uniform distribution. Fig. 9C also shows that nonwoven fabric has randomly fashioned fibres with disordered and unsystematic features. Spin coating can address these issue and is a preferred method over dip coating for the said purpose.215 Spin coating is practised to incorporate compounds such as QACs, antimicrobial peptides, N-halamine, metal NPs, and phosphonium salts onto fabrics.216
 | ||
| Fig. 9 SEM images of fabrics incorporated with Chinese herb microcapsule: (A) cotton; (B) polyester; (C) nonwoven; this figure is reproduced from the ref. 120 (Gigvvy science open access publishing platform, subject to CC BY-SA 4.0 creative common attribution license). | ||
Spray coating employs liquid spray to coat the material surfaces. Cold spray and thermal spray are the two types of spray coating. Cold spray is a suitable method for ceramics, metals, nanostructured materials, polymers, and non-metallic substrates. The solution is accelerated over a velocity range of 300–1200 m s−1 via De-Laval nozzle.217 Since the particles are passed at high velocity, the plastic deformation on the surface disrupts the thin film. Metals like Cu cannot be used in this technique since the high impact energy disrupts the surface. Spray technique is used to incorporate antimicrobial agents like QACs, fluorochemicals, natural compounds onto the surface of face masks.100,116,119,218 Fig. 10 shows the SEM image of a commercial three-layer mask spray-coated with QAC-based compound GS5. The white and opaque coating over the fibres represents the GS5 coating. However, it is reported that spray coating remains mostly on outer surfaces without much penetration into crevices or shadowed region where microorganisms can get stuck.100
 | ||
| Fig. 10 SEM images: (A and B) first-layer; (C and D) second-layer; (E and F) third-layer of a commercial three-layered surgical mask coated with 1% GS5. (A), (C) and (E) have a magnification of 150×. White boxes in (A), (C), and (E) is magnified by 1000×, as shown in (B), (D), and (F). This figure is reproduced from ref. 100 with permission from Taylor & Francis, copyright 2016. | ||
In the melt-blowing technique, nanofibres (unbonded and self-bonded webs) are formed by blowing molten thermoplastic resin at high velocity from an extruder die tip to the collector.219 The diameter of the nanofibre (<0.1 μm, 0.5 to 10 μm, ≥10 μm) can be optimised based on the airflow velocity.220 The factors such as polymeric types, airflow velocity, die to collector distance, angle of approach, polymer throughput rate, and temperature play a role in forming fibres. Usually, PP, polybutylene terephthalate, PET, and polylactic acid-based nonwoven materials can be formed using this process.221 Melt-blowing helps in developing breathable fibres, which can act as efficient filters. Double quaternary ammonium salts,164 alkylammonium microbicides on perlite,98 Ag,222 natural compounds,119 and CuO60 have been incorporated into fabrics using this technique.
Electrospinning is a facile methodology that aids in the preparation of nonwoven nanofibres. The method is applicable for both natural and synthetic polymers, metals, ceramics, and polymer alloys. The polymers like PS, PVA, PAN, and polyvinyl pyrrolidone can be electrospun to yield nanofibres to prepare disposable face masks.223 In the electrospinning process, the polymeric solution in the syringe is passed through single or multiple needles at a constant rate with the help of a syringe pump.224 The droplets get charged under a high electric field, and the electrostatic repulsion overcomes the surface tension.225 The charged jet gets ejected and is directed towards the collector as thin nanofibres. The collector can be a flat plate, parallel plates/strips, or a rotating disc or drum. The diameter of the nanofibres is controlled by solution and process parameters.226 Most organic polymers, including natural polymers like chitosan, chitin, silk fibroin, dextran, collagen, alginate, gelatin, and synthetic polymers such as PS, PVC, PAN, PET, polyurethane, polyamide, are suitable for electrospinning.227 Biocompatible and biodegradable polymers such as polylactic acid, poly lactic-co-glycolic acid, conductive polymers such as polyaniline, polypyrrole, and functional polymers as polyvinylidene fluoride can also be electrospun.223 Electrospinning technique can be employed to develop air filtration membranes and protective clothing materials with antimicrobial substances like metal NP, antibiotics, biopolymers, carbon nanomaterials, and natural compounds.227,228
It has been observed that when compared to dip (soak) and spray coating, incorporation of AMA during electrospinning yield even distribution without aggregation. Fig. 11(A)–(C) shows randomly oriented, straight and continuous features of electrospun poly(methyl methacrylate) (PMMA) with 1,3-dichloro-5,5-dimethylhydantoin (DCDMH), 1,3-dibromo-5,5-dimethylhydantoin (DBDMH) and DCDMH/DBDMH. Here, DCDMH and DBDMH are N-halamine based antimicrobial compounds. From Fig. 11(D)–(F), it is clear that electrospinning PMMA along with DCDMH and DBDMH shows coarse features of the nanofibres. The transmission electron microscope (TEM) images of PMMA with DCDMH, DBDMH and DCDMH/DBDMH as shown in Fig. 11(G)–(I) reveals the uniform distribution of DCDMH and DBDMH without any noticeable aggregation, obtained through electrospinning.
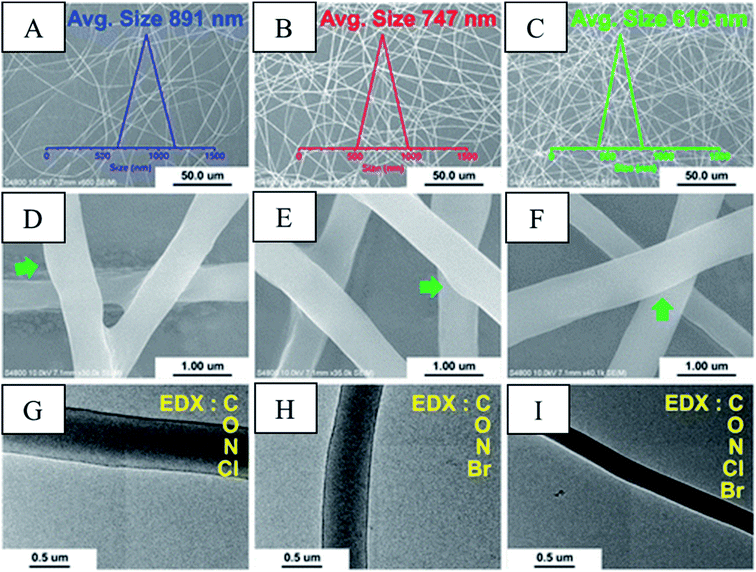 | ||
| Fig. 11 SEM image: (A and D) PMMA–DCDMH; (B and E) PMMA–DBDMH; (C and F) PMMA–DCDMH/DBDMH; TEM image: (G) PMMA–DCDMH; (H) PMMA–DBDMH; (I) PMMA–DCDMH/DBDMH nanofibres developed through electrospinning. This figure is reproduced from the ref. 229 with permission from American Chemical Society, copyright 2016. | ||
6.6. Antimicrobial face masks in the commercial market
Recent face masks launched in the market reveals that research and development are progressing towards creating novel antimicrobial face masks. Worldwide many start-ups are actively involved in the production of antimicrobial face masks/respirators. Table 2 provides the details of various commercially available antimicrobial face masks.| Sl no. | Name of the product | Developed by | Antimicrobial agent/technology | Performance efficacy | Remarks |
|---|---|---|---|---|---|
| 1 | SonoFace230,231 | Sonovia (Israel) | Nano metal-oxide such as ZnO and CuO | • 1 log10 reduction of SARS-CoV-2 | • >98% particle filtration (5 μm) |
| • 6 log10 reduction of viruses similar to SARS-CoV-2 | • Reusable and potent up to 100 wash cycles at 70 °C | ||||
| • Less toxic to human and the environment | |||||
| • Pack of three costs 159 USD | |||||
| 2 | MedCu's CuO face mask230 | MedCu Technologies (Israel) | CuO | 4 log10 reduction of viruses within 30 min | • Pose no hazard upon disposal |
| • Conformitè Europëenne (CE) and FDA certified | |||||
| • Costs less than a conventional N95 face mask | |||||
| 3 | Maya sticker230 | National Emergency Team of Israel and Israel Institute of Technology | Nanofibres coated with antiseptics | Blocks and neutralise viral particles | • This sticker can be attached to regular surgical face masks for the usage |
| 4 | ViriFace230 | Israel | Data not available | Viruses including SARS-CoV-2 | • Filters must be replaced every 12 h |
| • Wearable part can be washed and reused | |||||
| • Two filters and disposable envelopes cost 69 USD | |||||
| 5 | GS-3D N95 (ref. 232) | Gold Shield (China) | Organosilane based antimicrobial compound | 3 log10 reduction against bacteria and viruses like H1N1, methicillin-resistant S. aureus (MRSA), and SARS-CoV | • Five-ply 3D N95 face mask |
| • Effective against particulates, pollutants, and pollens | |||||
| • US FDA approved | |||||
| • Reusable up to 14 days | |||||
| 7 | Silver Shield Ag+™ (ref. 233) | Good Research Solutions LLC (US) | Silver ions | 2 log10 reduction against pathogens | • Reusable cotton face mask |
| • 5-Layer activated carbon filter present | |||||
| • Potent up to 50 wash cycles | |||||
| 8 | SpectraShield 9500 (ref. 234) | Nexera medical Inc. (Canada) | Sciessent's Ag–Cu based technology | • 4 log10 reduction of bacteria in 1 h | • Ag–Cu impounded to polyester fibres |
| • 5 log10 reduction of influenza viruses in 5 min | • Used up to 8 h continuously | ||||
| • NIOSH approved | |||||
| 9 | NanoHack235 | Copper Company (Chile) | Nano Cu | Antiviral activity against influenza viruses | • Three-layer nonwoven PP filter to embed nano-Cu |
| • Recyclable face mask made up of biocompatible polymer | |||||
| • Blocks 96.4% (1 μm) and 89.5% (0.02 μm) microorganisms | |||||
| 10 | Copper KN99 (ref. 236) | Copper Compression (US) | Cu | Filters 99% of airborne particles equivalent to KN99 | • Four-layer washable and reusable face mask made up of cotton fabrics |
| • Durable up to 50 wash cycles | |||||
| • Costs 29.56 USD per piece | |||||
| 11 | Noveko™ RD2 respirator237 | Noveko International Inc (Canada) | Data not available | H1N1, H5N1, S. aureus, K. pneumonia, MRSA, and endospores of Bacillus, Enterococcus, Acinetobacter | • Complies with EN149:2001+A1:2009 standard |
| • BFE 99.8% | |||||
| • VFE 99.7% | |||||
| 12 | Safe Life™ (ref. 238) | Fisher scientific (US) | Triosyn iodine-polymeric resin compound | 99.99% effective against airborne and droplet borne viruses | • Fluid resistance provides protection from blood, liquid splashes, and wet environmental contaminants |
| • Activated carbon layer combats organic vapours and odours | |||||
| 13 | A400 series N95 (ref. 239) | Safe Life Corp (US) | Iodine | Data not available | • Compact, flat-fold design |
| • Was widely distributed by healthcare during Swine flu-2009 | |||||
| 14 | Nsafe+240 | Nanosafe solutions and Indian Institute of Technology Delhi (India) | Data not available | Data not available | • Triple-layer filtration technology with antimicrobial layer |
| • BFE 99.2% | |||||
| • Complies with ASTM standard | |||||
| • Reuse potential up to 50 times | |||||
| 15 | MyClyns® (ref. 241) | ViralClyns (US) | Data not available | 95% effective against bacteria, viruses, spores, and protozoa | • Patented and EPA authorised antimicrobial agent |
| 16 | Guardian G-Volt77 | LIGC Applications (US) | Graphene and electrical charge | Data not available | • Electric charge repels particles deposited |
| • 99% effective against 0.3 μm particles | |||||
| 17 | Graphene-enhanced face mask242 | PlanarTECH & IDEATI's 2AM (UK) and Thailand | Graphene and other carbon nanomaterials | 99.95% effective against S. aureus within 24 h | • Reusable cotton face mask potent up to 10 cycles |
| • Repels the PM 2.5 dust | |||||
| • Costs 24.95 USD for a pack of three | |||||
| 18 | Leaf243,244 | Redcliffe medical Devices Inc. (US) | Ultraviolet C (UVC) | 99.99% reduction of influenza A virus | • FDA cleared UVC N99 face mask |
| • HEPA and active carbon filters | |||||
| • 99.9997% filtration of 0.3 μm size |
Worldwide companies are competing to develop novel antimicrobial face masks claiming to be safer, effective, and environmentally friendly than existing ones. The same can be comprehended from the current market potential of face masks discussed in Section 6 of the review.
7. Market potential of face masks
The face mask market depends on various factors such as decreasing air quality linked to air pollution, customer base, the number of manufacturing units for the production of face masks,245 the prevalence of respiratory diseases along with consumer health awareness, investment in the healthcare sector, and new launches in the market and its availability. The market is categorised based on usage (reusable, disposable), end-use (public, healthcare workers), and region (North America, Latin America, Europe, Asia Pacific, Middle East & Africa). A market research-based company, Zion, has analysed the global scenario on the PPE market, especially on face masks.246 It reports that the worldwide market for the N95 face mask reached around 822.6 million USD in 2019, and it can generate an income of about 1890.3 million USD by the end of the year 2026, representing an impressive compound annual growth rate (CAGR) of over 12.8% during the forecast period. COVID-19 and 2009 H1N1 outbreaks have surged the demand for face masks and respirators among the frontline workers and the general public. The Asia-Pacific region has the highest disposable market share of 33.9% in the year 2019, with a rising trend due to increasing demand for N95 face masks in highly populated countries like China and India.247China is the producer of half the world's face mask, even before COVID-19. Other countries are highly dependent on them to procure nonwoven PP fabrics.248 PP is of high demand as the nonwoven PP face masks are free from latex, PVC, and di (2-ethylhexyl) phthalate material. PP resists allergic reactions for consumers with sensitive skin.247 It is reported that during January to May 2020 in China, more than 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 companies have registered for face mask production or trade, bringing a 13.56 fold increase compared to 2019.249 More than 7000 new companies have registered to make or trade the melt-blown fabric incurring 23.77 times surge compared to 2019. The data support that COVID-19 has resulted in a rise in the investment, research, and development in the N95 face mask market. However, the high cost and disparity in the performance can restrict the global growth of the N95 market in the future.246
000 companies have registered for face mask production or trade, bringing a 13.56 fold increase compared to 2019.249 More than 7000 new companies have registered to make or trade the melt-blown fabric incurring 23.77 times surge compared to 2019. The data support that COVID-19 has resulted in a rise in the investment, research, and development in the N95 face mask market. However, the high cost and disparity in the performance can restrict the global growth of the N95 market in the future.246
The demand for disposable face masks is rising due to its widespread availability even in online shopping platforms like Amazon.com, Inc., and eBay Inc., and this trend is expected to continue in the near future.247 Besides, retail stores like supermarkets, hypermarkets, drug stores, and department stores also contribute to the global face mask market shares.250 However, fake brands are playing in the market where the customers are highly price-sensitive, which affects the trade of genuine products.251 Currently, the global key players dominating in respirators and face masks market are 3M, Hakugen, Vogface mask, Prestige Ameritech, Kimberly-Clark, Ansell, Sinotextiles, Dasheng, DACH Schutzbekleidung, Uvex group, Honeywell International Inc, Moldex-Metric, Inc., Kowa Company, Ltd., the Gerson Company, and SAS Safety Corp.252
The unprecedented situations brought by COVID-19 have increased the markets for antimicrobial face masks as well. It is reported that consumer awareness and health concerns due to escalating fungi and bacteria prone diseases drives the demand for breathable antimicrobial coatings. Incorporation of antimicrobials on face masks can enhance the reusability of face masks. It is reported that the reusable N95 respirators recorded the highest market share in 2019 due to its superior advantages over the disposable N95 respirators.246 In contrast, the UK and US promote single-use or disposable face masks, as they abolish the need for sterilisation after use and avoids cross-contamination with other reusable products.252 North America and Europe take up a significant share in the breathable antimicrobial coating, including antimicrobial face masks. The market potential in the Asia-Pacific region is expected to improve as they will exhibit a considerable consumption rise in the forecast period from 2019 to 2026.253
Major retailers like Amazon and Etsy have taken up the large customer base of antimicrobial face masks. The escalating trend also has boosted the activity of biotechnology companies. Studies demonstrating the quick deactivation of COVID-19 on Cu surfaces are definite to enhance the market potential of antimicrobial face masks.254 The superiority of antimicrobial face masks, with self-sanitising properties that can prevent unpleasant odours and cross-contamination, increases the likelihood of usage of antimicrobial face masks, as reported by the Chief Executive Officer of Noble Biomaterials, a company specialising in antimicrobials for soft surfaces.
8. Possible environmental impacts associated with the usage of face masks
In response to the control of infectious diseases, single-use PPEs, including face masks and respirators with secondary infection potential, opens up a serious question of their safe and effective disposal. Improper handling and disposal of face masks and respirators can lead to various environmental challenges soon. Medical face masks, which are rated for single-use, pose a challenging problem of managing a large volume of hazardous waste. Prata and team estimated that approximately 129 billion masks and 65 billion plastic gloves are used every month worldwide in the COVID-19 scenario.255 The increasing demand for face masks made of synthetic polymers such as PE, PP, polyurethane, PAN, polycarbonate, PS, and polyester adds to the plastic waste, which is harder to recycle256,257 and causing a significant burden on the environment.82 It is reported that improper disposal of even 1% of face masks can add 10 million face masks to the solid-waste stream and pose a significant threat to the environment.257New York Times reports that 80% of Americans wear face mask regularly when going out or in close contact with other people.258 Data reveal that 60 to 90% of the population wears face masks in public spaces in Spain, Italy, Germany, and France since July 2020.259 A report estimates that if every individual in the UK wears a single-use mask per day, it will mound to 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of contaminated waste plus 57
000 tonnes of contaminated waste plus 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of plastic packaging waste annually.260 Even in a normal situation, an adult in Japan uses 43 numbers of face masks per year on average.261 In highly populated countries like China and India, the volume of use is expected to be significantly high. According to Environment Pollution (Prevention and Control) Authority, New Delhi is presently producing more than 300 tonnes per day of biomedical waste, which is substantially higher than the installed combined capacity of 74 tonnes per day of the two Biomedical Waste Treatment Centres (BMWTCs) in the city.262,263 A recent report shows that COVID-19 pandemic has doubled the generation of biomedical waste from approximately 12 to 24 tonnes per day in Mumbai.262,264 On average, India produced 600 tonnes per day of biomedical waste, and the installed capacity of 198 BMWTCs is even inadequate to handle the waste generated during pre-COVID-19 times.262 Hospitals in Wuhan were producing 240 tonnes of biomedical waste per day at the peak of pandemic in February 2020, i.e. six times the no-COVID situation,265–267 necessitating the establishment of mobile waste treatment facilities for the mass influx of waste.268 Another study conducted in a few Asian cities during COVID-19 reported a 5-time daily increase in biomedical waste compared to pre-COVID-19 time.269
000 tonnes of plastic packaging waste annually.260 Even in a normal situation, an adult in Japan uses 43 numbers of face masks per year on average.261 In highly populated countries like China and India, the volume of use is expected to be significantly high. According to Environment Pollution (Prevention and Control) Authority, New Delhi is presently producing more than 300 tonnes per day of biomedical waste, which is substantially higher than the installed combined capacity of 74 tonnes per day of the two Biomedical Waste Treatment Centres (BMWTCs) in the city.262,263 A recent report shows that COVID-19 pandemic has doubled the generation of biomedical waste from approximately 12 to 24 tonnes per day in Mumbai.262,264 On average, India produced 600 tonnes per day of biomedical waste, and the installed capacity of 198 BMWTCs is even inadequate to handle the waste generated during pre-COVID-19 times.262 Hospitals in Wuhan were producing 240 tonnes of biomedical waste per day at the peak of pandemic in February 2020, i.e. six times the no-COVID situation,265–267 necessitating the establishment of mobile waste treatment facilities for the mass influx of waste.268 Another study conducted in a few Asian cities during COVID-19 reported a 5-time daily increase in biomedical waste compared to pre-COVID-19 time.269
Even though there are stringent regulations and disposal mechanisms for used PPEs in clinical settings,82 most countries worldwide are not equipped to handle the unprecedented volume of such wastes generated in a short period. Face masks littering creates a new form of environmental challenge for both terrestrial and aquatic ecosystems.17 As per a United Nations report, approximately 75% of the discarded face masks find their way to dump yards or water bodies.270 In the COVID-19 scenario, there have been alarming reports of washing up of face masks on beaches resulting in maritime littering,257 which reduces the aesthetic and recreational values of coastal areas impacting the tourism industry.271,272 The dispersed face masks in the aquatic system are a source of riverine and marine pollution with microplastics and microfibres.273 The microplastics in face masks often contain phthalate, polybrominated biphenyl ether, nonylphenol, organotin, and triclosan, which are toxic and can cause high risks when released during their disintegration.271 The microplastics leached from the face masks get ingested in marine animals like fishes and sharks.271,274 Through the fishes and other aquatic animals, the microplastics enter the food chain and pose health risks to living beings at higher trophic levels.17
The ongoing COVID-19 pandemics has also generated an unusually large quantum of household medical wastes, and their disposal is a mammoth task, especially for developing and populated countries like India. Data reveals that 3 billion people lack access to safe disposal mechanisms worldwide and relies on open, uncontrolled dumping and burning of used face masks and gloves. When mixed with municipal solid wastes, these wastes lead to a large mass of hazardous waste if not correctly segregated.273 Improper waste collection and management systems, together with ignorance and irresponsible behaviour of ordinary people, can further burden the environment. The only means of disposal of face mask for them remains dumping or burning. Piling up of used face masks on roadside and dump yards can inflict health threat to individuals, sanitation workers, animals, birds, and rodents coming in contact with contaminated face masks. In contrast, open burning or uncontrolled incineration practices of the petrochemical-derived face masks can release harmful gases, including dioxins and furans, into the environment.257,270
The conventional waste management systems like landfilling and incineration have limitations. The poorly maintained incinerators and overloaded landfills are not equipped to handle hazardous nature of face masks, causing emissions and leaching of toxins.257 On the other hand, the land disposal of face masks can result in the release of biohazards, microplastics, and AMA and contaminate the soil and water bodies.275 The carbon footprint exerted from the polymer resin production, conversion, and disposal stage is critically high.276 It is reported that 2.7 tonnes of greenhouse gas could be potentially released into the environment per tonne of face mask from the polymer resin production stage alone.276 Studies reveal that one tonne of PP, PE, PS, and PVC produces 1.5, 2.4, 3.1, and 2.2 tonnes of CO2 during the production stage and 1.7, 1.6, 2.1, and 0.9 tonnes of CO2 during incineration with energy recovery, respectively.277,278
Recently, efforts are made to improve the life and reusability of the face mask by incorporating AMA. Reusable antimicrobial face masks have been found useful for multiple cycles and can reduce the waste generation significantly. Though such face masks are promising in reducing the volume of waste generated and associated environmental impacts, experts are somehow worried about the fate of AMA at the service life of face masks. Since most of the AMA incorporated onto face masks are in nano-forms, the unpredictable interaction of these materials with the surrounding environment raises the issue of nanotoxicity.279 Waste treatment options such as landfilling, incineration, or even recycling do not nullify the harmful effects posed by the NPs.280 A portion of the NPs gets released through emissions and leaching and impacts the microenvironment. Moreover, there are chances that metal-based antimicrobial materials detach from the polymer matrix leaching into water bodies and soil matrix.134 For instance, during washing and reuse of face mask, the NPs leaching from the surface reduces the antimicrobial efficacy of face masks and finds their way into aqueous media.16,281 The fate of the AMA in water, soil, and air media has to be looked into to get a full picture of the environmental compatibility of antimicrobial face masks.
Apart from the environmental concerns, health impacts from the use of such novel AMA have to be studied. The health impacts arising out of face mask are of a high priority since the antimicrobial compounds will be in very close contact with the skin. Few studies express concern over the use of NPs in consumer products due to exposure to occupational and environmental hazards.282,283 The principal mechanism by which metal NPs induce toxicity is through the formation of ROS, followed by oxidative stress in cells and tissue.279,284 ROS in contact with skin is known to develop dermatological disorders.285 So it is highly recommended to conduct tests to evaluate skin irritation and user comfort for any combination of different classes of antimicrobials. While implementing novel technology, the evaluation of biocompatibility and cytotoxicity should be of prime concern. The data warrant an integrated approach for effective PPE waste management to reduce the microbial transmission with the least health and ecological implications.
9. Conclusion
The large-scale outbreaks of infectious diseases and associated public health crisis are not new to human history. Flu viruses, non-flu viruses, and human coronavirus are the significant causes of pandemics starting from Russian flu-1889 to COVID-19. These great pandemics of the past and the present have posed significant public health issues and negatively impacted society, economy, global security, and the environment to a great extent. With the onset of the COVID-19 pandemic, economies worldwide have weakened significantly due to prolonged lockdown. The pandemic has killed several thousands of people worldwide directly and indirectly. The pandemic has also taken away the opportunities and livelihoods and pushed several families to hunger and poverty, and caused irreversible damage to society.Countries across the world have taken several control measures to prevent the spread of COVID-19 and save the lives of their citizens. However, the advisories given to use face masks in public seems to be the most effective and practical short-term measures taken to prevent the spread of the disease. Face masks are proven effective in preventing the airborne, droplet, and aerosol transmission of pathogenic microorganisms. Its role in preventing the spread of infectious diseases are well recognised and time tested. The same is apparent in the ongoing COVID-19 pandemic as well. Given the role of face masks in pandemic preparedness, face masks have evolved in material and design to meet the increasing safety and comfort requirements from the cotton coverings of the middle ages to the recent hi-tech smart face masks. However, most of the face mask available in the commercial market are designed and developed for single-use. The widespread use of disposable face mask can pose a significant challenge to the environment due to the high generation of hazardous waste.
The risk of secondary transmission, cross-contamination, and the difficulty in managing a large quantum of plastic waste laden with biohazards has heightened the development of reusable face mask with inherent antimicrobial properties. The reusable antimicrobial face masks can also reduce the surge in demand and the stress in the supply chain. It is established that antimicrobial face masks exhibit enhanced performance over conventional face masks by providing in situ real-time antimicrobial control. However, choosing the right AMA is crucial for optimal performance. Now there are different classes of AMA that can be appropriately used in various combinations to yield a novel and effective antimicrobial face mask. Among various AMA studied, Ag and Cu infused antimicrobial face masks are more popular owing to their high reactivity and excellent antimicrobial efficacy in nano-forms. However, the detachment of the nanoparticles from the face mask and associated nanotoxicity are the concerns. Metal and metal oxide nanoparticles incorporated through electrospinning and melt-blowing techniques have shown higher stability and less leaching.
New classes of AMA such as antimicrobial polymers (with active moieties like antimicrobial peptides, QACs, N-halamine compounds), iodine, NaCl, and natural compounds such as mangosteen extracts, extracts of Scutellaria baicalensis extracts, Folium Plectranthii amboinicii oil, Vitex trifolia, Punica granatum, Allium sativum are identified as effective against various microorganisms including viruses. Polymers, which acts as the primary substrate for face masks, can be fine-tuned to impart bio-active and bio-passive properties. The active moieties like N-halamines, QACs, PEI, BP, polypyrrole, and inorganic groups (mostly metals) have been incorporated to yield various antimicrobial polymers suitable for making a reusable face mask. Among these, N-halamine and QACs are proven powerful against a broad spectrum of microorganisms. Bath coating, spray coating, and immobilisation via carriers have been employed to yield QACs modified antimicrobial fabrics. Direct polymerisation of monomers and covalent attachment of QACs may enhance the stability of the coating and the performance. All the studies pertaining to N-halamines reports high effectiveness in short contact times in antimicrobial fabrics. However, the real field applicability of N-halamine on face masks has not been explored yet. Natural compounds and antimicrobial peptides are promising AMA due to less ecotoxicity and proven antimicrobial properties. Besides these compounds, saline coatings and iodine (employed in NIOSH approved commercial face masks) have been investigated for their antimicrobial properties after incorporation on face masks. Lately, graphene-coated face masks are gaining much prominence owing to their self-decontaminating property.
Research on antimicrobial face masks started gaining momentum after the SARS-2003 epidemic. Further with the onset of COVID-19, the spike in demand for antimicrobial face masks has escalated its market share. The CAGR growth of 12.8% of the face mask market between 2020 and 2026 project the potential opportunities in investment, innovation, research, and development in the area. However, the mushrooming sales of antimicrobial masks, without adequate quality control, in online and offline shopping platforms is a cause of concern.
Though antimicrobial face masks seem superior over conventional face masks on multiple facets, a detailed study on various aspects associated with their usage and disposal should be performed to understand their short and long-term benefits and drawbacks. Besides, the methods reported for testing antimicrobial activities are different for different micro-organisms studied, assays used, and experimental setup. The lack of a standardised methodology makes it difficult to compare their performance. In this context, extensive research is also needed to develop a robust protocol and validating standards about the use of AMA, antimicrobial loading, and testing the antibacterial and antiviral activity of antimicrobial face mask. The performance of antimicrobial face masks should be evaluated for both antibacterial and antiviral activities, to establish the claim of “antimicrobial face mask” on more substantial grounds for developing a protective face mask. There should be a thorough evaluation of the biotoxicity and ecotoxicity associated with the AMA and the antimicrobial face masks. The risk of unknown toxicity calls for proper assessment of, skin compatibility, and stability of the antimicrobial coatings. Therefore, proper usage, reuse, and disposal protocol should be formulated to avail the full benefits. In short, there are ample opportunities for various stakeholders dealing with the antimicrobial mask to develop affordable, safe, and efficient antimicrobial face mask that can overcome the challenges present with the single-use face masks.
Abbreviations
| A. baumannii | Acinetobacter baumannii |
| A. niger | Aspergillus niger |
| AgNP | Silver nanoparticle |
| AIDS | Acquired immunodeficiency syndrome |
| AMA | Antimicrobial agents |
| ASTM | American Society for Testing and Materials |
| B. subtilis | Bacillus subtilis |
| BFE | Bacterial filtration efficiency |
| BMWTC | Biomedical waste treatment centre |
| BP | Benzophenone |
| BSI | Benzoic acid, Salicylic, and Iode |
| C. albicans | Candida albicans |
| CAGR | Compound annual growth rate |
| CDC | Centers for Disease Control and Prevention |
| CDDMH | Chlorinated 3-dodecyl-5,5-dimethyl hydantoin |
| CDMH | Chlorinated 5,5-dimethyl hydantoin |
| CE | Conformitè Europëenne |
| CFU | Colony-forming unit |
| COVID | Coronavirus disease |
| CTAB | Cetyltrimethylammonium bromide |
| CTMIO | Chlorinated 2,2,5,5-tetramethyl-imidozalidin-4-one |
| CuNP | Copper nanoparticle |
| DBDMH | 1,3-Dibromo-5,5-dimethylhydantoin |
| DCDMH | 1,3-Dichloro-5,5-dimethylhydantoin |
| DMF | Dimethylformamide |
| DNA | Deoxyribonucleic acid |
| E. coli | Escherichia coli |
| E. faecalis | Enterococcus faecalis |
| EN | European Nation |
| EPA | Environmental Protection Agency |
| FDA | Food and Drug Administration |
| FENO | Fractional concentration of nitric oxide in exhaled breath |
| FFR | Filtering facepiece respirator |
| GDP | Gross domestic product |
| GS5 | Goldshield 5 |
| GSM | Gram per metre square |
| HA | Hemagglutinin activity |
| HDP | Host defence peptide |
| K. pneumoniae | Klebsiella pneumoniae |
| LIG | Laser-induced graphene |
| MBC | Minimum bactericidal concentration |
| MC | 1-Chloro-2,2,5,5-tetramethyl-4-imidazolidinone |
| MERS | Middle East respiratory syndrome |
| MERS-CoV | Middle East respiratory syndrome coronavirus |
| MIC | Minimum inhibitory concentration |
| NIOSH | National Institute for Occupational Safety and Health |
| NP | Nanoparticle |
| OSHA | Occupational Safety and Health Administration |
| P. aeruginosa | Pseudomonas aeruginosa |
| PAN | Polyacrylonitrile |
| PE | Polyethylene |
| PEG | Polyethylene glycol |
| PEI | Polyethyleneimine |
| PET | Polyethylene terephthalate |
| PMMA | Poly(methyl methacrylate) |
| PPE | Personal protective equipment |
| PP | Polypropylene |
| PS | Polystyrene |
| PVA | Polyvinyl alcohol |
| PVC | Polyvinyl chloride |
| QAC | Quaternary ammonium compound |
| ROS | Reactive oxygen species |
| RPD | Respiratory protective device |
| S. aureus | Staphylococcus aureus |
| S. epidermis | Staphylococcus epidermis |
| SARS | Severe acute respiratory syndrome |
| SARS-CoV | Severe acute respiratory syndrome coronavirus |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscope |
| UK | United Kingdom |
| US | United States |
| USD | United States Dollar |
| UVC | Ultraviolet C |
| UV-Vis | Ultraviolet-visible |
| VFE | Viral filtration efficiency |
| w/v | Weight/volume |
| w/w | Weight/weight |
| WHO | World Health Organisation |
| ZnONP | Zinc oxide nanoparticle |
Credit authorship contribution statement
G. P., U. K., G. S., and S. M. M. designed and wrote the original draft, S. M. M. and D. V. K. reviewed and edited the paper, S. M. M. and D. V. K. secured the fund.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was financially supported by the Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Government of India (CVD/2020/000244). The authors thank Amara Raja Industries, Tirupati and IIT Tirupati for supporting the work. Author Uthradevi Kannan expresses gratitude to DST- Innovative in Science Pursuit for Inspired Research (INSPIRE), Government of India for the fellowship support (IF 160288) to pursue PhD at IIT Tirupati, India. All authors express their gratitude to M. S. V. Naga Jyothi, MS Scholar, Department of Civil and Environmental Engineering, IIT Tirupati for her help in creating world map using Geographic Information System and C. P. Ramesh, Graphic Designer, Dubai Civil Defense for digitizing the graphical abstract.References
- Y. Xue, H. Xiao and Y. Zhang, Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts, Int. J. Mol. Sci., 2015, 16, 3626–3655 CrossRef CAS.
- WHO, Up to 650 000 people die of respiratory diseases linked to seasonal flu each year, 2017, https://www.who.int/news-room/detail/14-12-2017-up-to-650-000-people-die-of-respiratory-diseases-linked-to-seasonal-flu-each-year Search PubMed.
- A. D. Iuliano, K. M. Roguski, H. H. Chang, D. J. Muscatello, R. Palekar and S. Tempia, et al., Estimates of global seasonal influenza-associated respiratory mortality: a modelling study, Lancet, 2018, 391, 1285–1300 CrossRef.
- M. S. Smolinski, M. A. Hamburg and J. Lederberg. Microbial Threats to Health: Emergence, Detection, and Response, National Academies Press, Washington, D.C., 2003, http://www.nap.edu/catalog/10636 Search PubMed.
- WHO, Ebola virus disease, 2020, https://www.who.int/news-room/fact-sheets/detail/ebola-virus-disease Search PubMed.
- WHO, HIV/AIDS, 2020. https://www.who.int/news-room/fact-sheets/detail/hiv-aids Search PubMed.
- Worldometer, Coronavirus Update (Live): 95,016,073 Cases and 2,032,342 Deaths from COVID-19 Virus Pandemic - Worldometer, 2021, https://www.worldometers.info/coronavirus/ Search PubMed.
- A. C. K. Lai, C. K. M. Poon and A. C. T. Cheung, Effectiveness of facemasks to reduce exposure hazards for airborne infections among general populations, J. R. Soc. Interface, 2012, 9, 938–948 CrossRef CAS.
- M. B. Lore, J. M. Sebastian, T. L. Brown, A. S. Viner, N. V. McCullough and S. H. Hinrichs, Performance of conventional and antimicrobial-treated filtering facepiece respirators challenged with biological aerosols, J. Occup. Environ. Hyg., 2012, 9, 69–80 CrossRef CAS.
- D. A. Harnish, B. K. Heimbuch, M. Husband, A. E. Lumley, K. Kinney and R. E. Shaffer, et al., Challenge of N95 filtering facepiece respirators with viable H1N1 influenza aerosols, Infect. Control Hosp. Epidemiol., 2013, 34, 494–499 CrossRef.
- N. H. L. Leung, D. K. W. Chu, E. Y. C. Shiu, K.-H. Chan, J. J. McDevitt and B. J. P. Hau, et al., Respiratory virus shedding in exhaled breath and efficacy of face masks, Nat. Med., 2020, 26, 676–680 CrossRef CAS.
- C. R. MacIntyre and A. A. Chughtai, A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients, Int. J. Nurs. Stud., 2020, 108, 103629 CrossRef.
- S. Feng, C. Shen, N. Xia, W. Song, M. Fan and B. J. Cowling, Rational use of face masks in the COVID-19 pandemic, Lancet Respir. Med., 2020, 8, 434–436 CrossRef CAS.
- A. L. Pasanen, J. Keinanen, P. Kalliokoski, P. I. Martikainen and J. Ruuskanen, Microbial growth on respirator filters from improper storage, Scand. J. Work, Environ. Health, 1993, 19, 421–425 CrossRef CAS.
- L. M. Brosseau, N. V. McCullough and D. Vesley, Bacterial survival on respirator filters and surgical masks, J. Am. Biol. Saf. Assoc., 1997, 2, 32–43 Search PubMed.
- C. R. Zheng, S. Li, C. Ye, X. Li, C. Zhang and X. Yu, Particulate respirators functionalized with silver nanoparticles showed excellent real-time antimicrobial effects against pathogens, Environ. Sci. Technol., 2016, 50, 7144–7151 CrossRef CAS.
- O. O. Fadare and E. D. Okoffo, Covid-19 face masks: A potential source of microplastic fibers in the environment, Sci. Total Environ., 2020, 737, 140279 CrossRef CAS.
- S. Rengasamy, E. Fisher and R. E. Shaffer, Evaluation of the survivability of MS2 viral aerosols deposited on filtering face piece respirator samples incorporating antimicrobial technologies, Am. J. Infect. Control, 2010, 38, 9–17 CrossRef.
- A. Hirsch, Handbook of Geographical and Historical Pathology, New Sydenham Society, 1883, p. 740 Search PubMed.
- WHO, Plague, 2020, https://www.who.int/westernpacific/health-topics/plague Search PubMed.
- M. Rosenwald, History's deadliest pandemics, from ancient Rome to modern America, Washington Post, 2020, https://www.washingtonpost.com/graphics/2020/local/retropolis/coronavirus-deadliest-pandemics/ Search PubMed.
- Worldometer, World Population by Year - Worldometer, 2020, https://www.worldometers.info/world-population/world-population-by-year/ Search PubMed.
- Worldometer, World Population Clock: 7.8 Billion People (2021) - Worldometer, 2021, https://www.worldometers.info/world-population/ Search PubMed.
- WHO. WHO|HIV/AIDS, WHO, World Health Organization, 2020, http://www.who.int/gho/hiv/en/ Search PubMed.
- CDC, SARS|Basics Factsheet|CDC, 2019, https://www.cdc.gov/sars/about/fs-sars.html Search PubMed.
- WHO, WHO|Middle East respiratory syndrome coronavirus (MERS-CoV), WHO, World Health Organization, 2020, http://www.who.int/emergencies/mers-cov/en/ Search PubMed.
- S. Hamid, M. Y. Mir and G. K. Rohela, Novel coronavirus disease (COVID-19): a pandemic (epidemiology, pathogenesis and potential therapeutics), New Microbes New Infect., 2020, 35, 100679 CrossRef.
- E. T. Ewing, La Grippe or Russian influenza: Mortality statistics during the 1890 Epidemic in Indiana, Influenza Other Respir. Viruses, 2019, 13, 279–287 CrossRef.
- N. LePan, Visualizing the History of Pandemics, Visual Capitalist, 2020, https://www.visualcapitalist.com/history-of-pandemics-deadliest/ Search PubMed.
- N. Madhav, B. Oppenheim, M. Gallivan, P. Mulembakani, E. Rubin and N. Wolfe, Pandemics: Risks, Impacts, and Mitigation, in Disease Control Priorities: Improving Health and Reducing Poverty, ed. D. T. Jamison, H. Gelband, S. Horton, P. Jha, R. Laxminarayan, C. N. Mock, et al., The International Bank for Reconstruction and Development/The World Bank, Washington, DC, 3rd edn, 2017, http://www.ncbi.nlm.nih.gov/books/NBK525302/ Search PubMed.
- S.-E. Mamelund, Social inequality – a forgotten factor in pandemic influenza preparedness, Tidsskr Den Nor Legeforening, 2017, https://tidsskriftet.no/2017/05/global-helse/social-inequality-forgotten-factor-pandemic-influenza-preparedness Search PubMed.
- Q. Fottrell, Another warning from the 1918 flu for COVID-19: ‘Survival does not mean that individuals fully recovered’, MarketWatch, 2020, https://www.marketwatch.com/story/another-warning-from-1918-spanish-flu-for-covid-19-survival-does-not-mean-that-individuals-fully-recovered-2020-08-18 Search PubMed.
- L. Spinney, How pandemics shape social evolution, Nature, 2019, 574, 324–326 CrossRef CAS.
- CPJ, Iran bans printing of all newspapers, citing spread of coronavirus, Committee to Protect Journalists, 2020, https://cpj.org/2020/03/iran-bans-printing-of-all-newspapers-citing-spread/ Search PubMed.
- I. Berman, Coronavirus & Iran: Regime's End?|National Review, 2020, https://www.nationalreview.com/2020/03/will-irans-regime-survive-coronavirus/ Search PubMed.
- W. McKibbin and A. Sidorenko, Global Macroeconomic Consequences of Pandemic Influenza, Centre for Applied Macroeconomic Analysis, Crawford School of Public Policy, The Australian National University, 2006, https://econpapers.repec.org/paper/eencamaaa/2006-26.htm Search PubMed.
- African Development Bank Group, African Economic Outlook 2020 - Supplement, African Development Bank - Building today, a better Africa tomorrow. African Development Bank Group, 2020, https://www.afdb.org/en/documents/african-economic-outlook-2020-supplement Search PubMed.
- EFE, MERS outbreak to reduce South Korean GDP by 0.3 percent in 2015, www.efe.com, 2015, https://www.efe.com/efe/english/business/mers-outbreak-to-reduce-south-korean-gdp-by-0-3-percent-in-2015/50000265-2640814 Search PubMed.
- IMF, World Economic Outlook, April 2020: The Great Lockdown, IMF, 2020, https://www.imf.org/en/Publications/WEO/Issues/2020/04/14/weo-april-2020 Search PubMed.
- UNDRR, The known and unknown economic and social consequences of pandemics, 2020, https://www.preventionweb.net/go/72070 Search PubMed.
- Business Today, Which top economies have suffered worst GDP fall due to COVID-19?, 2020, https://www.businesstoday.in/current/economy-politics/which-top-economies-have-suffered-worst-gdp-fall-due-to-covid-19/story/414683.html Search PubMed.
- WHO, WHO|Worldwide reduction in MERS cases and deaths since 2016, WHO, World Health Organization, 2020, http://www.who.int/emergencies/mers-cov/worldwide-reduction-in-mers-cases-and-deaths-since-2016/en/ Search PubMed.
- WHO, WHO|SARS (Severe Acute Respiratory Syndrome), WHO, World Health Organization, 2020, https://www.who.int/ith/diseases/sars/en/ Search PubMed.
- Z. Zhao, W. Hai, J. Wang and Z.-G. Hou, The Short-term Impact of SARS on the Chinese Economy, ResearchGate, 2004, https://www.researchgate.net/publication/24089622_The_Short-term_Impact_of_SARS_on_the_Chinese_Economy Search PubMed.
- BBC, Chinese economy bounces back into growth, BBC News, 2020, https://www.bbc.com/news/business-53399999 Search PubMed.
- K. Yao and G. Crossley, China's economy has rebounded after a steep slump - but challenges lie ahead, World Economic Forum, 2020, https://www.weforum.org/agenda/2020/07/chinas-economy-rebounds-after-steep-slump-u-s-tensions-weak-consumption-raise-challenges/ Search PubMed.
- IMF, World Economic Outlook Update, June 2020: A Crisis Like No Other, An Uncertain Recovery, IMF, 2020, https://www.imf.org/en/Publications/WEO/Issues/2020/06/24/WEOUpdateJune2020 Search PubMed.
- B. Winck, The IMF says its forecast for the COVID-19 recession might now be too optimistic, World Economic Forum, 2020, https://www.weforum.org/agenda/2020/04/imf-economy-coronavirus-covid-19-recession/ Search PubMed.
- M. M. Farley, 2009 H1N1 Influenza: A Twenty-first century pandemic with roots in the early twentieth century, Am. J. Med. Sci., 2010, 340, 202–208 CrossRef.
- N. J. Cox and K. Subbarao, Global epidemiology of influenza: Past and present, Annu. Rev. Med., 2000, 51, 407–421 CrossRef CAS.
- T. P. Weber and N. I. Stilianakis, Inactivation of influenza A viruses in the environment and modes of transmission: A critical review, J. Infect., 2008, 57, 361–373 CrossRef.
- C.-C. Lai, T.-P. Shih, W.-C. Ko, H.-J. Tang and P.-R. Hsueh, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges, Int. J. Antimicrob. Agents, 2020, 55, 105924 CrossRef CAS.
- L. Brosseau, ScD, Ann RB. N95 Respirators and Surgical Masks|Blogs|CDC, 2009, https://blogs.cdc.gov/niosh-science-blog/2009/10/14/n95/ Search PubMed.
- A. Tcharkhtchi, N. Abbasnezhad, M. Zarbini Seydani, N. Zirak, S. Farzaneh and M. Shirinbayan, An overview of filtration efficiency through the masks: Mechanisms of the aerosols penetration, Bioact. Mater., 2021, 6, 106–122 CrossRef CAS.
- P. A. Levin and E. R. Angert, Small but mighty: cell size and bacteria, Cold Spring Harbor Perspect. Biol., 2015, 7, 1–11 Search PubMed.
- A. Lošdorfer Božič, A. Šiber and R. Podgornik, Statistical analysis of sizes and shapes of virus capsids and their resulting elastic properties, J. Biol. Phys., 2013, 39, 215–228 CrossRef.
- V. C.-C. Cheng, S.-C. Wong, V. W.-M. Chuang, S. Y.-C. So, J. H.-K. Chen and S. Sridhar, et al., The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2, J. Infect., 2020, 81, 107–114 CrossRef CAS.
- CDC, 100 Years of Respiratory Protection History|NPPTL|NIOSH|CDC, 2019, https://www.cdc.gov/niosh/npptl/Respiratory-Protection-history.html Search PubMed.
- Tuberculosis I of M (US) C on ROE to, M. J. Field, Respiratory Protection and Control of Tuberculosis in Health Care and Other Facilities, Tuberculosis in the Workplace. National Academies Press (US), 2001, https://www.ncbi.nlm.nih.gov/books/NBK222460/ Search PubMed.
- G. Borkow, S. S. Zhou, T. Page and J. Gabbay, A Novel anti-influenza copper oxide containing respiratory face mask, PLoS One, 2010, 6, 1–8 Search PubMed.
- WHO, Nonpharmaceutical interventions for pandemic influenza, national and community measures, Emerging Infect. Dis., 2006, 12, 88–94 Search PubMed.
- Global Times. The evolution of face masks - Global Times, 2020, https://www.globaltimes.cn/content/1179358.shtml Search PubMed.
- R. MacIntyre and A. A. Chughtai, Facemasks for the prevention of infection in healthcare and community settings, BMJ, 2015, 350(h694), 1–12 Search PubMed.
- E. Blakemore, Why plague doctors wore those strange beaked masks, 2020, https://www.nationalgeographic.com/history/reference/european-history/plague-doctors-beaked-masks-coronavirus/ Search PubMed.
- V. Postrel, Pandemics come and go but medical masks are eternal, Bloomberg.com, 2020, https://www.bloomberg.com/opinion/articles/2020-04-10/medical-face-masks-an-illustrated-history Search PubMed.
- M. M. Weiss, P. D. Weiss, D. E. Weiss and J. B. Weiss, Disrupting the transmission of influenza A: Face masks and ultraviolet light as control measures, Am. J. Public Health, 2007, 97, S32–S37 CrossRef.
- D. Spelce, T. R. Rehak, R. W. Metzler and J. S. Johnson, Pre-World War I firefighter respirators and the U.S. Bureau of Mines involvement in WWI, J. Int. Soc. Respir. Prot., 2017, 34, 128–135 Search PubMed.
- S. Durn, A brief history of medical face masks, Gizmodo, 2020, https://gizmodo.com/a-brief-history-of-medical-face-masks-1843698852 Search PubMed.
- B. J. Strasser and T. Schlich, A history of the medical mask and the rise of throwaway culture, Lancet, 2020, 396, 19–20 CrossRef CAS.
- C. Matuschek, F. Moll, H. Fangerau, J. C. Fischer, K. Zänker and M. van Griensven, et al., The history and value of face masks, Eur. J. Med.Res., 2020, 25, 1–6 CrossRef.
- J. L. Spooner, History of Surgical Face Masks: The myths, the masks, and the men and women behind them, AORN J., 1967, 5, 76–80 CrossRef CAS.
- B. Henneberry, How surgical masks are made, tested and used, 2020, https://www.thomasnet.com/articles/other/how-surgical-masks-are-made Search PubMed.
- N. L. Belkin, The evolution of the surgical mask: Filtering efficiency versus effectiveness, Infect. Control Hosp. Epidemiol., 1997, 18, 49–57 CrossRef CAS.
- B. Hogan and L. P. Samaranayake, The surgical mask unmasked: A review, Oral Surg. Oral Med. Oral Pathol., 1990, 70, 34–36 CrossRef CAS.
- Y. Li, P. Leung, L. Yao, Q. W. Song and E. Newton, Antimicrobial effect of surgical masks coated with nanoparticles, J. Hosp. Infect., 2006, 62, 58–63 CrossRef CAS.
- S. Chou, Mask having anti-virus and anti-germ effect, US20050183727A1, 2005, https://patents.google.com/patent/US20050183727/en.
- I. Block, Guardian G-Volt masks use graphene and electrical charge to repel viruses, Dezeen, 2020, https://www.dezeen.com/2020/03/06/guardian-g-volt-face-mask-graphene-coronavirus-bacteria/ Search PubMed.
- N. van Doremalen, T. Bushmaker, D. H. Morris, M. G. Holbrook, A. Gamble and B. N. Williamson, Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1, N. Engl. J. Med., 2020, 1–3 Search PubMed.
- H. A. Aboubakr, T. A. Sharafeldin and S. M. Goyal, Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review, Transboundary Emerging Dis., 2020, 1–17 Search PubMed.
- A. W. H. Chin, J. T. S. Chu, M. R. A. Perera, K. P. Y. Hui, H.-L. Yen and M. C. W. Chan, et al., Stability of SARS-CoV-2 in different environmental conditions, Lancet Microbe, 2020, 1, e10 CrossRef CAS.
- WHO, Transmission of SARS-CoV-2: implications for infection prevention precautions, 2020, https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions Search PubMed.
- S. Duong-Quy, X. Ngo-Minh, T. Tang-Le-Quynh, T. Tang-Thi-Thao, B. Nguyen-Quoc and K. Le-Quang, et al., The use of exhaled nitric oxide and peak expiratory flow to demonstrate improved breathability and antimicrobial properties of novel face mask made with sustainable filter paper and Folium Plectranthii amboinicii oil: additional option for mask shortage during COVID-19 pandemic, Multidiscip. Respir. Med., 2020, 15, 664 Search PubMed.
- H. Wu, J. Huang, C. J. P. Zhang, Z. He and W.-K. Ming, Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures, EClinicalMedicine, 2020, 21, 100329 CrossRef.
- G. Borkow and J. Gabbay, Putting copper into action: copper-impregnated products with potent biocidal activities, FASEB J., 2004, 18, 1728–1730 CrossRef CAS.
- G. Borkow and J. Gabbay, Copper as a biocidal tool, Curr. Med. Chem., 2005, 12, 2163–2175 CrossRef CAS.
- A. Smith and J. Taylor, Antiviral metal impregnated activated carbon cloth components, US20110114095A1, 2011, https://patents.google.com/patent/US20110114095A1/en.
- Y. Fujimori, T. Sato, T. Hayata, T. Nagao, M. Nakayama and T. Nakayama, et al., Novel antiviral characteristics of nanosized copper(I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus, Appl. Environ. Microbiol., 2012, 78, 951–955 CrossRef CAS.
- A. M. Davison, Pathogen inactivation and filtration efficacy of a new anti-microbial and anti-viral surgical facemask and N95 against dentistry-associated microorganisms, International dentistry Australasian edition, 2012, 7, 6 Search PubMed.
- A. K. Aloufy and M. A.-M. El-Messiry, in Proceedings of World congress on Advances in Nano, Biomechanics, Robotics, and Energy Research, 2013, vol. 18, pp. 811–824 Search PubMed.
- A. K. Selvam and G. Nallathambi, Polyacrylonitrile/silver nanoparticle electrospun nanocomposite matrix for bacterial filtration, Fibers Polym., 2015, 16, 1327–1335 CrossRef CAS.
- R. Navik, L. Thirugnanasampanthan, H. Venkatesan, M. Kamruzzaman, F. Shafiq and Y. Cai, Synthesis and application of magnesium peroxide on cotton fabric for antibacterial properties, Cellulose, 2017, 24, 3573–3587 CrossRef CAS.
- C. B. Hiragond, A. S. Kshirsagar, V. V. Dhapte, T. Khanna, P. Joshi and P. V. More, Enhanced anti-microbial response of commercial face mask using colloidal silver nanoparticles, Vacuum, 2018, 156, 475–482 CrossRef CAS.
- C. A. P. Bastos, S. F. A. Bruggraber, N. J. R. Faria and J. J. Powell, Antibacterial compositions comprising copper oxo-hydroxide nanoparticles and their uses as biocidal agents, US Pat., US20180147177A1, 2018, https://patents.google.com/patent/US20180147177A1/en Search PubMed.
- K. Nakano, A. Sato, T. Kusaka, S. Kasamatsu, A. Sasaki and D. Fukushi, Antiviral material, antiviral film, antiviral fiber, and antiviral product, US10327445B2, 2019, https://patents.google.com/patent/US10327445B2/en.
- H. Ghaffari, A. Tavakoli, A. Moradi, A. Tabarraei, F. Bokharaei-Salim and M. Zahmatkeshan, et al., Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine, J. Biomed. Sci., 2019, 26, 70 CrossRef.
- B. Gutarowska, J. Skora, E. Nowak and I. Lysiak, Antimicrobial activity and filtration effectiveness of nonwovens with sanitized for respiratory protective equipment, Fibres & Textiles in Eastern Europe, 2014, 22(3(105)), 120–125 Search PubMed , https://www.researchgate.net/publication/262143415_Antimicrobial_Activity_and_Filtration_Effectiveness_of_Nonwovens_with_Sanitized_for_Respiratory_Protective_Equipment.
- L. Chang, X. Zhang, X. Shi, L. Zhao and X. Liu, Preparation and characterization of a novel antibacterial fiber modified by quaternary phosphonium salt on the surface of polyacrylonitrile fiber, Fibers Polym., 2014, 15, 2026–2031 CrossRef CAS.
- B. Gutarowska, B. Brycki, K. Majchrzycka and A. Brochocka, New bioactive polymer filtering material composed of nonwoven polypropylene containing alkylammonium microbiocides on a perlite carrier, Polimery, 2010, 55, 568–574 CrossRef CAS.
- M. Montazer, F. Rangchi and F. Siavoshi, Preparation of protective disposable hygiene fabrics for medical applications, in Medical and Healthcare Textiles, Elsevier, 2010, pp. 164–170, https://linkinghub.elsevier.com/retrieve/pii/B9781845692247500212 Search PubMed.
- C.-C. Tseng, Z.-M. Pan and C.-H. Chang, Application of a quaternary ammonium agent on surgical face masks before use for pre-decontamination of nosocomial infection-related bioaerosols, Aerosol Sci. Technol., 2016, 50, 199–210 CrossRef CAS.
- L. Cen, K. G. Neoh and E. T. Kang, Antibacterial activity of cloth functionalized with N-alkylated poly(4-vinylpyridine), J. Biomed. Mater. Res., Part A, 2004, 71, 70–80 CrossRef CAS.
- M. Ignatova, N. Manolova and I. Rashkov, Novel antibacterial fibers of quaternized chitosan and poly(vinyl pyrrolidone) prepared by electrospinning, Eur. Polym. J., 2007, 43, 1112–1122 CrossRef CAS.
- J. Lin, S. Qiu, K. Lewis and A. M. Klibanov, Mechanism of bactericidal and fungicidal activities of textiles covalently modified with alkylated polyethylenimine, Biotechnol. Bioeng., 2003, 83, 168–172 CrossRef CAS.
- K. Majchrzycka, M. Okrasa, A. Jachowicz, J. Szulc, B. Brycki and B. Gutarowska, Application of biocides and super-absorbing polymers to enhance the efficiency of filtering materials, Molecules, 2019, 24, 3339 CrossRef CAS.
- F. Nogueira, P. Teixeira and I. C. Gouveia, Electrospinning polypropylene with an amino acid as a strategy to bind the antimicrobial peptide Cys-LC-LL-37, J. Mater. Sci., 2018, 53, 4655–4664 CrossRef CAS.
- D. O. Sanchez Ramirez, A. Varesano, R. A. Carletto, C. Vineis, I. Perelshtein and M. Natan, et al., Antibacterial properties of polypyrrole-treated fabrics by ultrasound deposition, Mater. Sci. Eng., C, 2019, 102, 164–170 CrossRef CAS.
- G. Tiliket, D. L. Sage, V. Moules, M. Rosa-Calatrava, B. Lina and J. M. Valleton, et al., A new material for airborne virus filtration, Chem. Eng. J., 2011, 173, 341–351 CrossRef CAS.
- K. Tan and S. K. Obendorf, Fabrication and evaluation of electrospun nanofibrous antimicrobial nylon 6 membranes, J. Membr. Sci., 2007, 305, 287–298 CrossRef CAS.
- M. R. Badrossamay and G. Sun, Acyclic halamine polypropylene polymer: Effect of monomer structure on grafting efficiency, stability and biocidal activities, React. Funct. Polym., 2008, 68, 1636–1645 CrossRef CAS.
- B. Demir, I. Cerkez, S. D. Worley, R. M. Broughton and T.-S. Huang, N-Halamine-modified antimicrobial polypropylene nonwoven fabrics for use against airborne bacteria, ACS Appl. Mater. Interfaces, 2015, 7, 1752–1757 CrossRef CAS.
- I. Cerkez, H. B. Kocer, S. D. Worley, R. M. Broughton and T. S. Huang, Antimicrobial functionalization of poly(ethylene terephthalate) fabrics with waterborne N -halamine epoxides, J. Appl. Polym. Sci., 2016, 133(43088), 1–6 Search PubMed.
- C. Huang, Y. Liu, Z. Li, R. Li, X. Ren and T.-S. Huang, N-halamine antibacterial nanofibrous mats based on polyacrylonitrile and N-halamine for protective face masks, J. Eng. Fibers Fabr., 2019, 14, 155892501984322 Search PubMed.
- G. Thilagavathi and T. Kannaian, Dual antimicrobial and blood repellent finishes for cotton hospital fabrics, Indian J. Fibre Text. Res., 2008, 23–29 CAS http://nopr.niscair.res.in/handle/123456789/364.
- V. Balachandar, I. Mahalaxmi, J. Kaavya, G. Vivekanandhan, S. Ajithkumar and N. Arul, et al., COVID-19: emerging protective measures, Eur. Rev. Med. Pharmacol. Sci., 2020, 24, 3422–3425 CAS.
- H. Belbachir and J. Angelidis, Decontaminating composition having simultaneously bactericidal, fungicidal and virocidal properties, methods for obtaining and using said composition, US Pat., US8529968B2, 2013 Search PubMed.
- M. Reynolds, Antimicrobial compositions and applications therefore, US20100092398A1, 2010, https://patents.google.com/patent/US20100092398A1/en.
- H. J. Kim and C. J. Park, Composition for preventing infection of new influenza a (H1N1) virus comprising ginkgo extract, air filter comprising the same, and air cleaning device comprising the filter, US20120148507A1, 2012, https://patents.google.com/patent/US20120148507A1/en.
- H. J. Kim and C. J. Park, Composition for prevention of influenza viral infection comprising sumac extract, air filter comprising the same and air cleaning device comprising the filter, US9119814B2, 2015, https://patents.google.com/patent/US9119814B2/en.
- P. Ekabutr, P. Chuysinuan, S. Suksamrarn, W. Sukhumsirichart, P. Hongmanee and P. Supaphol, Development of antituberculosis melt-blown polypropylene filters coated with mangosteen extracts for medical face mask applications, Polym. Bull., 2019, 76, 1985–2004 CrossRef CAS.
- Y.-F. Wang, F. Kang, S.-J. You, C.-H. Tsai and G.-L. Lin, Preparation and characteristic of antibacterial facemasks with chinese herbal microcapsules, Aerosol Air Qual. Res., 2017, 17, 2120–2129 CrossRef.
- K. Chen, X. Qiu, D. Yang and Y. Qian, Amino acid-functionalized polyampholytes as natural broad-spectrum antimicrobial agents for high-efficient personal protection, Green Chem., 2020, 19, 6357–6371 RSC.
- J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri and J. T. Ramírez, et al., The bactericidal effect of silver nanoparticles, Nanotechnology, 2005, 16, 2346–2353 CrossRef CAS.
- K.-J. Kim, W. S. Sung, B. K. Suh, S.-K. Moon, J.-S. Choi and J. G. Kim, et al., Antifungal activity and mode of action of silver nano-particles on Candida albicans, BioMetals, 2009, 22, 235–242 CrossRef CAS.
- L. Lu, R. W.-Y. Sun, R. Chen, C.-K. Hui, C.-M. Ho and J. M. Luk, et al., Silver nanoparticles inhibit hepatitis B virus replication, Antiviral Ther., 2008, 13(2), 253 CAS.
- S. Park, H. H. Park, S. Y. Kim, S. J. Kim, K. Woo and G. Ko, Antiviral properties of silver nanoparticles on a magnetic hybrid colloid, Appl. Environ. Microbiol., 2014, 80, 2343–2350 CrossRef.
- G. Franci, A. Falanga, S. Galdiero, L. Palomba, M. Rai and G. Morelli, et al., Silver nanoparticles as potential antibacterial agents, Molecules, 2015, 20, 8856–8874 CrossRef CAS.
- M. Horie, H. Ogawa, Y. Yoshida, K. Yamada, A. Hara and K. Ozawa, et al., Inactivation and morphological changes of avian influenza virus by copper ions, Arch. Virol., 2008, 153, 1467–1472 CrossRef CAS.
- A. Brandelli, A. C. Ritter, F. F. Veras, Antimicrobial Activities of Metal Nanoparticles, in Metal Nanoparticles in Pharma, ed. M. Rai and R. Shegokar, Springer, Cham, 2017, pp. 337–363 Search PubMed.
- R. K. Matharu, L. Ciric, G. Ren and M. Edirisinghe, Comparative study of the antimicrobial effects of tungsten nanoparticles and tungsten nanocomposite fibres on hospital acquired bacterial and viral pathogens, Nanomaterials, 2020, 10, 1–16 CrossRef.
- U. Baig, M. A. Gondal, S. Rehman and S. Akhtar, Facile synthesis, characterization of nano-tungsten trioxide decorated with silver nanoparticles and their antibacterial activity against water-borne gram-negative pathogens, Appl. Nanosci., 2020, 10, 851–860 CrossRef CAS.
- S. Galdiero, A. Falanga, M. Vitiello, M. Cantisani, V. Marra and M. Galdiero, Silver nanoparticles as potential antiviral agents, Molecules, 2011, 16, 8894–8918 CrossRef CAS.
- T. Tashi, N. V. Gupta and V. B. Mbuya, Silver nanoparticles: Synthesis, mechanism of antimicrobial action, characterization, medical applications, and toxicity effects, J. Chem. Pharmaceut. Res., 2016, 8(2), 526–537 CAS.
- H. J. Kim, S. W. Han, M. K. Joshi and C. S. Kim, Fabrication and characterization of silver nanoparticle-incorporated bilayer electrospun–melt-blown micro/nanofibrous membrane, Int. J. Polym. Mater. Polym. Biomater., 2017, 66, 514–520 CrossRef CAS.
- A. Pasricha, S. L. Jangra, N. Singh, N. Dilbaghi, K. N. Sood and K. Arora, et al., Comparative study of leaching of silver nanoparticles from fabric and effective effluent treatment, J. Environ. Sci., 2012, 24, 852–859 CrossRef CAS.
- X. Zhu, L. Tang, K.-H. Wee, Y.-H. Zhao and R. Bai, Immobilization of silver in polypropylene membrane for anti-biofouling performance, Biofouling, 2011, 27, 773–786 CrossRef CAS.
- S. Agarwal, A. Greiner and J. H. Wendorff, Functional materials by electrospinning of polymers, Prog. Polym. Sci., 2013, 38, 963–991 CrossRef CAS.
- A. Brochocka and K. Majchrzycka, Technology for the production of bioactive melt-blown filtration materials applied to respiratory protective devices, Fibres Text. East. Eur., 2009, 17, 92–98 CAS.
- K. R. Aadil, S. I. Mussatto and H. Jha, Synthesis and characterization of silver nanoparticles loaded poly(vinyl alcohol)-lignin electrospun nanofibers and their antimicrobial activity, Int. J. Biol. Macromol., 2018, 120, 763–767 CrossRef CAS.
- ASTM, ASTM F2101 - 01 Standard Test Method for Evaluating the Bacterial Filtration Efficiency (BFE) of Medical Face Mask Materials, Using a Biological Aerosol of Staphylococcus aureus, 2019, https://compass.astm.org/Standards/HISTORICAL/F2101-01.htm.
- M. G. Baldry, The antimicrobial properties of magnesium monoperoxyphthalate hexahydrate, J. Appl. Bacteriol., 1984, 57, 499–503 CrossRef CAS.
- J. L. Sagripanti, P. L. Goering and A. Lamanna, Interaction of copper with DNA and antagonism by other metals, Toxicol. Appl. Pharmacol., 1991, 110, 477–485 CrossRef CAS.
- N. P. Klochko, K. S. Klepikova, D. O. Zhadan, V. R. Kopach, S. M. Chernyavskaya and S. I. Petrushenko, et al., Thermoelectric textile with fibers coated by copper iodide thin films, Thin Solid Films, 2020, 704, 138026 CrossRef CAS.
- EN 14683:2005, Surgical masks - Requirements and test methods, European Nation standards, 2005https://standards.iteh.ai/catalog/standards/cen/12acf007-918b-4825-9217-4b4a96938dcf/en-14683-2005 Search PubMed.
- ASTM F1862/F1862M - 17, Standard test method for resistance of medical face masks to penetration by synthetic blood (Horizontal Projection of Fixed Volume at a Known, Velocity), 2017https://www.astm.org/Standards/F1862.htm Search PubMed.
- M. Hashmi, S. Ullah and I. S. Kim, Copper oxide (CuO) loaded polyacrylonitrile (PAN) nanofiber membranes for antimicrobial breath mask applications, Curr. Res. Biotechnol., 2019, 1, 1–10 CrossRef.
- ASTM E96/E96M - 16, Standard test methods for water vapor transmission of materials, https://www.astm.org/Standards/E96 Search PubMed.
- AATCC, https://members.aatcc.org/store/tm100/513/.
- ASTM F2100 - 20, Standard specification for performance of materials used in medical face masks, https://www.astm.org/Standards/F2100.htm.
- European Committee for Standardization, CEN - Technical Bodies, https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT,FSP_ORG_ID:32928,6062&cs=1FC98AD34A5EE26A0CB5A6155ED4D6E5E Search PubMed.
- M. Charnley, M. Textor and C. Acikgoz, Designed polymer structures with antifouling–antimicrobial properties, React. Funct. Polym., 2011, 71(3), 329–334 CrossRef CAS https://linkinghub.elsevier.com/retrieve/pii/S1381514810001872.
- K.-S. Huang, C.-H. Yang, S.-L. Huang, C.-Y. Chen, Y.-Y. Lu and Y.-S. Lin, Recent advances in antimicrobial polymers: A mini-review, Int. J. Mol. Sci., 2016, 17, 1578 CrossRef.
- M. R. Badrossamay and G. Sun, Preparation of rechargeable biocidal polypropylene by reactive extrusion with diallylamino triazine, Eur. Polym. J., 2008, 44, 733–742 CrossRef CAS.
- A. Jain, L. S. Duvvuri, S. Farah, N. Beyth, A. J. Domb and W. Khan, Antimicrobial polymers, Adv. Healthcare Mater., 2014, 3, 1969–1985 CrossRef CAS.
- S. D. Worley, D. E. Williams and R. A. Crawford, Halamine water disinfectants, Crit. Rev. Environ. Control, 1988, 18, 133–175 CrossRef CAS.
- R. Nayak and R. Padhye, 12 - Antimicrobial finishes for textiles, in Functional Finishes for Textiles, ed. R. Paul, Woodhead Publishing, 2015, pp. 361–85, Woodhead Publishing Series in Textiles, http://www.sciencedirect.com/science/article/pii/B9780857098399500121 Search PubMed.
- L.-C. Xu and C. A. Siedlecki, Antibacterial polyurethanes, in Advances in Polyurethane Biomaterials, ed. S. L. Cooper and J. Guan, Woodhead Publishing, 2016, pp. 247–284, http://www.sciencedirect.com/science/article/pii/B9780081006146000093 Search PubMed.
- C. Yao, X. Li, K. G. Neoh, Z. Shi and E. T. Kang, Antibacterial activities of surface modified electrospun poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) fibrous membranes, Appl. Surf. Sci., 2009, 255, 3854–3858 CrossRef CAS.
- A. Carmona-Ribeiro and L. de Melo Carrasco, Cationic antimicrobial polymers and their assemblies, Int. J. Mol. Sci., 2013, 14, 9906–9946 CrossRef.
- S. P. Denyer, Mechanisms of action of antibacterial biocides, Int. Biodeterior. Biodegrad., 1995, 36, 227–245 CrossRef CAS.
- J. C. Tiller, C.-J. Liao, K. Lewis and A. M. Klibanov, Designing surfaces that kill bacteria on contact, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 5981–5985 CrossRef CAS.
- J. C. Tiller, S. B. Lee, K. Lewis and A. M. Klibanov, Polymer surfaces derivatized with poly(vinyl-N-hexylpyridinium) kill airborne and waterborne bacteria, Biotechnol. Bioeng., 2002, 79, 465–471 CrossRef CAS.
- K. Nakata, T. Tsuchido and Y. Matsumura, Antimicrobial cationic surfactant, cetyltrimethylammonium bromide, induces superoxide stress in Escherichia coli cells, J. Appl. Microbiol., 2011, 110, 568–579 CrossRef CAS.
- K. Majchrzycka, B. Gutarowska, A. Brochocka and B. Brycki, New filtering antimicrobial nonwovens with various carriers for biocides as respiratory protective materials against bioaerosol, Int. J. Occup. Saf. Ergon., 2012, 18, 375–385 CrossRef CAS.
- K. Majchrzycka, M. Okrasa, J. Szulc, B. Brycki and B. Gutarowska, Time-Dependent Antimicrobial Activity of Filtering Nonwovens with Gemini Surfactant-Based Biocides, Molecules, 2017, 22, 1620 CrossRef.
- ASTM E2721 - 10, Standard test method for evaluation of effectiveness of decontamination procedures for surfaces when challenged with droplets containing human pathogenic viruses, 2010 https://www.astm.org/DATABASE.CART/HISTORICAL/E2721-10.htm.
- A. Purohit, M. C. Kopferschmitt-Kubler, C. Moreau, E. Popin, M. Blaumeiser and G. Pauli, Quaternary ammonium compounds and occupational asthma, Int. Arch. Occup. Environ. Health, 2000, 73, 423–427 CrossRef CAS.
- S. E. Anderson, H. Shane, C. Long, E. Lukomska, B. J. Meade and N. B. Marshall, Evaluation of the irritancy and hypersensitivity potential following topical application of didecyldimethylammonium chloride, J. Immunotoxicol., 2016, 13, 557–566 CrossRef CAS.
- A. Varesano, C. Vineis, A. Aluigi, F. Rombaldoni, C. Tonetti and G. Mazzuchetti, Antibacterial efficacy of polypyrrole in textile applications, Fibers Polym., 2013, 14, 36–42 CrossRef CAS.
- K. H. Hong and G. Sun, Photoinduced antimicrobial polymer blends with benzophenone as a functional additive, J. Appl. Polym. Sci., 2009, 112, 2019–2026 CrossRef CAS.
- J. L. Fox, Antimicrobial peptides stage a comeback, Nat. Biotechnol., 2013, 31, 379–382 CrossRef CAS.
- G. J. Gabriel, A. Som, A. E. Madkour, T. Eren and G. N. Tew, Infectious disease: Connecting innate immunity to biocidal polymers, Mater. Sci. Eng., R, 2007, 57, 28 CrossRef.
- ASTM Compass, https://compass.astm.org/EDIT/html_annot.cgi?E2180+18.
- V. Geetha, A. Doss and A. P. A. Doss, Antimicrobial potential of Vitex trifolia Linn, Anc. Sci. Life, 2004, 23, 30–32 CAS.
- S. Esath Natheer, Evaluation of antibacterial activity of Morinda citrifolia, Vitex trifolia and Chromolaena odorata, Afr. J. Pharm. Pharmacol., 2012, 6(29), 10–14 Search PubMed.
- P. S. Negi and G. K. Jayaprakasha, Antioxidant and antibacterial activities of punica granatum peel extracts, J. Food Sci., 2003, 68, 1473–1477 CrossRef CAS.
- M. Haidari, M. Ali, S. Ward Casscells and M. Madjid, Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza virus and has a synergistic effect with oseltamivir, Phytomedicine, 2009, 16, 1127–1136 CrossRef CAS.
- P. Jadhav, N. Kapoor, B. Thomas, H. Lal and N. Kshirsagar, Antiviral potential of selected Indian medicinal (ayurvedic) plants against herpes simplex virus 1 and 2, N. Am. J. Med. Sci., 2012, 4, 641 CrossRef.
- N. D. Weber, D. O. Andersen, J. A. North, B. K. Murray, L. D. Lawson and B. G. Hughes, In vitro virucidal effects of Allium sativum (Garlic) extract and compounds, Planta Med., 1992, 58, 417–423 CrossRef CAS.
- L. P. Rees, S. F. Minney, N. T. Plummer, J. H. Slater and D. A. Skyrme, A quantitative assessment of the antimicrobial activity of garlic (Allium sativum), World J. Microbiol. Biotechnol., 1993, 9, 303–307 CrossRef CAS.
- N. Benkeblia, Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum), LWT--Food Sci. Technol., 2004, 37, 263–268 CrossRef CAS.
- A. Banso, Phytochemical and antibacterial investigation of bark extracts of Acacia nilotica, 2009, https://paper/Phytochemical-and-antibacterial-investigation-of-of-Banso/5ecd8a95cc2402e2c0bc3e53f0ae5eb3c291e46b Search PubMed.
- C. Wiart, K. Kumar, M. Y. Yusof, H. Hamimah, Z. M. Fauzi and M. Sulaiman, Antiviral properties of ent-labdene diterpenes of Andrographis paniculata nees, inhibitors of herpes simplex virus type 1, Phytother. Res., 2005, 19, 1069–1070 CrossRef CAS.
- S. Roy, K. Rao, C. Bhuvaneswari, A. Giri and L. N. Mangamoori, Phytochemical analysis of Andrographis paniculata extract and its antimicrobial activity, World J. Microbiol. Biotechnol., 2009, 26, 85 CrossRef.
- V. J. Galani, B. G. Patel and D. G. Rana, Sphaeranthus indicus Linn.: A phytopharmacological review, Int. J. Res. Ayurveda Pharm., 2010, 1, 247–253 CrossRef.
- W. Gu, W. Wang, X. Li, Y. Zhang, L. Wang and C. Yuan, et al., A novel isocoumarin with anti-influenza virus activity from Strobilanthes cusia, Fitoterapia, 2015, 107, 60–62 CrossRef CAS.
- Y.-C. Tsai, C.-L. Lee, H.-R. Yen, Y.-S. Chang, Y.-P. Lin and S.-H. Huang, et al., Antiviral action of tryptanthrin isolated from Strobilanthes cusia leaf against human coronavirus NL63, Biomolecules, 2020, 10, 366 CrossRef CAS.
- D. V. Zige, E. I. Ohimain and M. B. Nodu, Antibacterial activity of ethanol, crude and water extract of Chromolaena odorata leaves on S. typhi and E. coli, Greener J. Microbiol. Antimicrob., 2013, 1, 016–019 CrossRef.
- K. Saoo, H. Miki, M. Ohmori and W. D. Winters, Antiviral activity of Aloe extracts against Cytomegalovirus, Phytother. Res., 1996, 10, 348–350 CrossRef.
- K. Zandi, M. A. Zadeh, K. Sartavi and Z. Rastian, Antiviral activity of Aloe vera against herpes simplex virus type 2: An in vitro study, Afr. J. Biotechnol., 2007, 6(15), 1770–1773 CrossRef.
- R. Pandey and A. Mishra, Antibacterial activities of crude extract of Aloe barbadensis to clinically isolated bacterial pathogens, Appl. Biochem. Biotechnol., 2010, 160, 1356–1361 CrossRef CAS.
- M. Joshi, S. W. Ali and S. Rajendran, Antibacterial finishing of polyester/cotton blend fabrics using neem (Azadirachta indica): A natural bioactive agent, J. Appl. Polym. Sci., 2007, 106, 793–800 CrossRef CAS.
- M. Kubo, Y. Kimura, T. Odani, T. Tani and K. Namba, Studies on Scutellariae Radix, Planta Med., 1981, 43, 194–201 CrossRef CAS.
- C. Duan, S. Matsumura, N. Kariya, M. Nishimura and T. Shimono, In vitro antibacterial activities of Scutellaria baicalensis Georgi against cariogenic bacterial, Pediatr. Dent. J., 2007, 17, 58–64 CrossRef.
- S. Duong-Quy, Study of active compounds of Plectranthii amboinicii leaves growing in Dalat city, Vietnam and its antibacterial and antioxidant activities, World J. Pharm. Pharmaceut. Sci., 2017, 6(4), 77–94 Search PubMed.
- M. Catel-Ferreira, H. Tnani, C. Hellio, P. Cosette and L. Lebrun, Antiviral effects of polyphenols: Development of bio-based cleaning wipes and filters, J. Virol. Methods, 2015, 212, 1–7 CrossRef CAS.
- F.-S. Quan, I. Rubino, S.-H. Lee, B. Koch and H.-J. Choi, Universal and reusable virus deactivation system for respiratory protection, Sci. Rep., 2017, 7, 39956 CrossRef CAS.
- H.-J. Choi, D.-G. Yoo, B. J. Bondy, F.-S. Quan, R. W. Compans and S.-M. Kang, et al., Stability of influenza vaccine coated onto microneedles, Biomaterials, 2012, 33, 3756–3769 CrossRef CAS.
- Y. Fujimori, T. Nakayama and T. Sato, Antiviral agent, US20170086463A1, 2017, https://patents.google.com/patent/US20170086463A1/en.
- B. K. Heimbuch and J. D. Wander, Bioaerosol challenges to antimicrobial surface treatments: Enhanced Efficacy Against MS2 Coli Phage of Air Filter Media Coated With Polystyrene-4-Methyltrimethylammonium Triiodide, Appl. Res. Assoc. Inc., Tyndall AFB, FL, 2006, vol. 10 Search PubMed.
- W. Hu, C. Peng, W. Luo, M. Lv, X. Li and D. Li, et al., Graphene-based antibacterial paper, ACS Nano, 2010, 4, 4317–4323 CrossRef CAS.
- P. Kumar, P. Huo, R. Zhang and B. Liu, Antibacterial properties of graphene-based nanomaterials, Nanomaterials, 2019, 9(5:737), 1–32 Search PubMed.
- N. Noor, S. Mutalik, M. W. Younas, C. Y. Chan, S. Thakur and F. Wang, et al., Durable antimicrobial behaviour from silver-graphene coated medical textile composites, Polymers, 2019, 11, 2000 CrossRef CAS.
- J. Zhao, B. Deng, M. Lv, J. Li, Y. Zhang and H. Jiang, et al., Graphene oxide-based antibacterial cotton fabrics, Adv. Healthcare Mater., 2013, 2, 1259–1266 CrossRef CAS.
- F. Yaghoubidoust and E. Salimi, A Simple method for the preparation of antibacterial cotton fabrics by coating graphene oxide nanosheets, Fibers Polym., 2019, 20, 1155–1160 CrossRef CAS.
- M. S. Stan, I. C. Nica, M. Popa, M. C. Chifiriuc, O. Iordache and I. Dumitrescu, et al., Reduced graphene oxide/TiO2 nanocomposites coating of cotton fabrics with antibacterial and self-cleaning properties, J. Ind. Text., 2019, 49, 277–293 CrossRef CAS.
- A. Kumar, K. Sharma and A. R. Dixit, Role of graphene in biosensor and protective textile against viruses, Med. Hypotheses, 2020, 144, 110253 CrossRef CAS.
- J. Molina, Graphene-based fabrics and their applications: a review, RSC Adv., 2016, 6, 68261–68291 RSC.
- J. Lin, Z. Peng, Y. Liu, F. Ruiz-Zepeda, R. Ye and E. L. G. Samuel, et al., Laser-induced porous graphene films from commercial polymers, Nat. Commun., 2014, 5, 5714 CrossRef CAS.
- R. Ye, Y. Chyan, J. Zhang, Y. Li, X. Han and C. Kittrell, et al., Laser-induced graphene formation on wood, Adv. Mater., 2017, 29, 1702211 CrossRef.
- H. Zhong, Z. Zhu, J. Lin, C. F. Cheung, V. L. Lu and F. Yan, et al., Reusable and recyclable graphene masks with outstanding superhydrophobic and photothermal performances, ACS Nano, 2020, 14(5), 6213–6221 CrossRef CAS.
- L. Huang, S. Xu, Z. Wang, K. Xue, J. Su and Y. Song, et al., Self-reporting and photothermally enhanced rapid bacterial Killing on a laser-induced graphene mask, ACS Nano, 2020, 14, 12045–12053 CrossRef CAS.
- R. R. Bresee and U. A. Qureshi, Influence of processing conditions on melt blown web structure: Part 1 – DCD, Int. Nonwovens J., 2004, os-13, 1558925004os–13 Search PubMed.
- H. Kaoud, Tissue Regeneration, IntechOpen publisher, 2018, ISBN 978-1-78923-261-5 Search PubMed.
- L. E. Scriven, Physics and applications of dip coating and spin coating, MRS Proc., 1988, 121, 717 CrossRef CAS.
- D. R. Ceratti, B. Louis, X. Paquez, M. Faustini and D. Grosso, A new dip coating method to obtain large-surface coatings with a minimum of solution, Adv. Mater., 2015, 27, 4958–4962 CrossRef CAS.
- D. Park, J. Wang and A. M. Klibanov, One-step, painting-like coating procedures to make surfaces highly and permanently bactericidal, Biotechnol. Prog., 2006, 22, 584–589 CrossRef CAS.
- H. Singh, T. S. Sidhu and S. B. S. Kalsi, Cold spray technology: future of coating deposition processes, Frat. ed Integrita Strutt., 2012, 6, 69–84 Search PubMed.
- W. JlC and P. P. Tsai, Cotton-comfortable face masks with protective finishes and electret filter media for safety from microbial threats, Res. J. Text. Apparel, 2006, 10, 33–43 Search PubMed.
- I. M. Hutten, Processes for nonwoven filter media, in Handbook of Nonwoven Filter Media, ed. I. M. Hutten, Butterworth-Heinemann, Oxford, 2007, pp. 195–244, http://www.sciencedirect.com/science/article/pii/B9781856174411500202 Search PubMed.
- J. G. McCulloch, The history of the development of melt blowing technology, Int. Nonwovens J., 1999, os-8, 1558925099OS–80 Search PubMed.
- N. Hiremath and G. Bhat, Melt blown polymeric nanofibers for medical applications-an overview, Nanosci. Nanotechnol., 2015, 2, 1–9 Search PubMed.
- B. Gutarowska and A. Michalski, Antimicrobial activity of filtrating meltblown nonwoven with the addition of silver ions, Fibres Text. East. Eur., 2009, 3(74), 23–28 Search PubMed.
- J. Xue, T. Wu, Y. Dai and Y. Xia, Electrospinning and electrospun nanofibers: Methods, materials, and applications, Chem. Rev., 2019, 119, 5298–5415 CrossRef CAS.
- M. Zhu, J. Han, F. Wang, W. Shao, R. Xiong and Q. Zhang, et al., Electrospun nanofibers membranes for effective air filtration, Macromol. Mater. Eng., 2017, 302, 1600353 CrossRef.
- N. Bhardwaj and S. C. Kundu, Electrospinning: A fascinating fiber fabrication technique, Biotechnol. Adv., 2010, 28, 325–347 CrossRef CAS.
- Electrospun Nanofibers for Energy and Environmental Applications, ed. B. Ding and J. Yu, Springer Berlin Heidelberg, Berlin, Heidelberg, 2014, Nanostructure Science and Technology, http://link.springer.com/10.1007/978-3-642-54160-5 Search PubMed.
- M. Zahmatkeshan, M. Adel, S. Bahrami, F. Esmaeili, S. M. Rezayat, Y. Saeedi, et al., Polymer-Based Nanofibers: Preparation, Fabrication, and Applications, in Handbook of Nanofibers, A. Barhoum, M. Bechelany and A. S. H. Makhlouf, Springer International Publishing, Cham, 2019, pp. 215–261, https://doi.org/10.1007/978-3-319-53655-2_29 Search PubMed.
- R. Purwar, K. Sai Goutham and C. M. Srivastava, Electrospun Sericin/PVA/Clay nanofibrous mats for antimicrobial air filtration mask, Fibers Polym., 2016, 17, 1206–1216 CrossRef CAS.
- R. Bai, Q. Zhang, L. Li, P. Li, Y.-J. Wang and O. Simalou, et al., N-Halamine-containing electrospun fibers kill bacteria via a contact/release co-determined antibacterial pathway, ACS Appl. Mater. Interfaces, 2016, 8, 31530–31540 CrossRef CAS.
- A. K. Leichman, 7 Israeli mask and face shield solutions for coronavirus, ISRAEL21c, 2020, https://www.israel21c.org/7-israeli-mask-and-face-shield-solutions-for-coronavirus/ Search PubMed.
- Sonovia, Innovative active protection, 2020, https://sonoviatech.com/ Search PubMed.
- tradeKorea, Goldshield 3DN95 medical mask|tradekorea, tradeKorea, Global B2B Trade Website - Offer Global Business, Buyer Matching Services, 2020, https://www.tradekorea.com/product/detail/P756854/Goldshield-3DN95-medical-mask.html Search PubMed.
- Good research solutions LLC. silver shield Ag+, silver shield masks, 2020, https://www.silvershieldmasks.com/products/face-mask-black.
- Nonwovens Industry, Nexera launches first FDA-approved antimicrobial surgical respiratory mask, Nonwovens Industry Magazine- News, Markets & Analysis for the Nonwovens Industry, 2016https://www.nonwovens-industry.com/issues/2016-06-06/view_breaking-news/nexera-launches-first-fda-approved-antimicrobial-surgical-respiratory-mask/ Search PubMed.
- Copper3d. Hack the Pandemic – Copper 3D|Antibacterial 3D Printing, 2020, https://copper3d.com/hackthepandemic/ Search PubMed.
- Copper and Copper Clothing, Copper clothing copper infused face mask, 2020, https://www.copperclothing.com/product/copper-infused-face-mask/ Search PubMed.
- Noveko International Inc, Noveko receives the green light to market its new antimicrobial respirator model in Europe n.d., 2020, https://www.newswire.ca/news-releases/noveko-receives-the-green-light-to-market-its-new-antimicrobial-respiratormodel-in-europe-544931932.html Search PubMed.
- Scientific. FischerSafe, Life™ Disposable respirators with Triosyn™ antimicrobial protection, 2020, https://www.fishersci.com/shop/products/safe-life-disposable-respirators-triosyn-antimicrobial-protection-universal/19171016 Search PubMed.
- Infection Control Today, Safe life introduces N95 respirator with antimicrobial protection, Infection Control Today, 2009https://www.infectioncontroltoday.com/view/safe-life-introduces-n95-respirator-antimicrobial-protection Search PubMed.
- Nanosafe Solutions P. Ltd, Nanosafe Solutions P. Ltd.|India, 2020, https://nanosafesolutions.com/fight-against-covid-19.php Search PubMed.
- Erlanger, Union springs pharmaceuticals launches new disposable respirator with antimicrobial coating, n.d. EMS1, 2020, https://www.ems1.com/ems-products/ambulance-disposable-supplies/press-releases/union-springs-pharmaceuticals-launches-new-disposable-respirator-with-antimicrobial-coating-61XAN4zzmnecYQZj/ Search PubMed.
- R. Peleg. planarTECH and IDEATI launch graphene-enhanced antibacterial face masks|Graphene-Info, 2020, https://www.graphene-info.com/planartech-and-ideati-launch-graphene-enhanced-antibacterial-face-masks Search PubMed.
- Yanko Design, World's first transparent FDA registered, N99+ smart mask with UV-C sterilizing that shows-off your smile|Yanko Design, 2020, https://www.yankodesign.com/2020/06/27/worlds-first-transparent-fda-registered-n99-smart-mask-with-uv-c-sterilizing-that-shows-off-your-smile/ Search PubMed.
- T N, COVID-[Update], 19: First FDA-registered transparent N99+ mask with UV-C sterilizing now available|Tech Times, 2020https://www.techtimes.com/articles/250624/20200626/covid-19-update-first-fda-approved-transparent-n99-mask-with-uv-c-sterilizing-now-available.htm Search PubMed.
- Research and Markets, Global disposable face mask market (2020 to 2025) - Growth, trends and forecasts, GlobeNewswire News Room, 2020http://www.globenewswire.com/news-release/2020/06/15/2047842/0/en/Global-Disposable-Face-Mask-Market-2020-to-2025-Growth-Trends-and-Forecasts.html Search PubMed.
- Zion Market Research, Global N95 mask market set for rapid growth, to reach Value USD 1,890.3 Million by 2026, 2020https://www.zionmarketresearch.com/news/n95-mask-market Search PubMed.
- Grand View Research, Disposable face mask market size|Industry Report, 2020-2027, 2020, https://www.grandviewresearch.com/industry-analysis/disposable-face-masks-market Search PubMed.
- K. Bradsher and L. Alderman, The world needs masks. China makes them, but has been hoarding them, The New York Times, 2020, https://www.nytimes.com/2020/03/13/business/masks-china-coronavirus.html Search PubMed.
- C. Zhou, Coronavirus bubble bursts for China's low-end mask makers, South China Morning Post. 2020, https://www.scmp.com/economy/china-economy/article/3088810/coronavirus-wheels-come-chinas-mask-making-gravy-train-low Search PubMed.
- CISION, Global Face Mask Market Overview 2020-2025: Growth Analysis of N95, KN95, FFP2 & FFP3, P95 and R95 Masks Amid COVID-19, 2020, https://www.prnewswire.com/news-releases/global-face-mask-market-overview-2020-2025-growth-analysis-of-n95-kn95-ffp2--ffp3-p95-and-r95-masks-amid-covid-19-301101156.html.
- Research and Markets, Face mask market by type, material, nature, and distribution channel: Global Opportunity Analysis and Industry Forecast 2021-2027, 2020, https://www.researchandmarkets.com/reports/5125521/face-mask-market-by-type-material-nature-and Search PubMed.
- La Grande Traversee, Mask Blog, 2020, https://www.lagrandetraversee.ca/ Search PubMed.
- Fortune Business Insights, Breathable antimicrobial coatings market size, share, Industry Forecast 2026, 2020, https://www.fortunebusinessinsights.com/breathable-antimicrobial-coatings-market-102764 Search PubMed.
- H. Horvath, Buying antimicrobial face masks? Here’s what experts recommend, NBC News, 2020, https://www.nbcnews.com/shopping/apparel/best-antimicrobial-face-masks-n1231803 Search PubMed.
- J. C. Prata, A. L. P. Silva, T. R. Walker, A. C. Duarte and T. Rocha-Santos, COVID-19 Pandemic repercussions on the use and management of plastics, Environ. Sci. Technol., 2020, 54, 7760–7765 CrossRef CAS.
- L. Tenenbaum, The amount of plastic waste is surging because of the coronavirus pandemic, Forbes, n.d. 2020, https://www.forbes.com/sites/lauratenenbaum/2020/04/25/plastic-waste-during-the-time-of-covid-19/ Search PubMed.
- K. R. Vanapalli, H. B. Sharma, V. P. Ranjan, B. Samal, J. Bhattacharya and B. K. Dubey, et al., Challenges and strategies for effective plastic waste management during and post COVID-19 pandemic, Sci. Total Environ., 2020, 750, 141514 CrossRef.
- J. Katz, M. Sanger-Katz and K. Quealy, A detailed map of who is wearing masks in the U.S., The New York Times, 2020, https://www.nytimes.com/interactive/2020/07/17/upshot/coronavirus-face-mask-map.html Search PubMed.
- E. Beswick, Coronavirus: How the wearing of masks has exposed a divided Europe, Euronews, 2020, https://www.euronews.com/2020/07/14/coronavirus-how-the-wearing-of-face-masks-has-exposed-a-divided-europe Search PubMed.
- A. L. Allison, E. Ambrose-Dempster, T. Domenech Aparsi, M. Bawn, M. Casas Arredondo and C. Chau, et al., The environmental dangers of employing single-use face masks as part of a COVID-19 exit strategy, UCL Open Environ. Prepr., 2020 Search PubMed.
- S. Jones, The Great Mask Divide: Lessons from Asia, Asia Pacific Foundation of Canada, 2020, https://www.asiapacific.ca/publication/great-mask-divide-lessons-asia Search PubMed.
- A. Reddy, Delhi to Vijayawada, India has started dumping Covid-19 infected waste in public places, ThePrint, 2020, https://theprint.in/opinion/delhi-vijayawada-india-dumping-covid-19-infected-waste-public-places/496396/ Search PubMed.
- K. Samir Purkayastha, COVID-19: Rise in biomedical waste generation poses fresh challenge, The Federal, 2020, https://thefederal.com/news/covid-19-rise-in-biomedical-waste-generation-poses-fresh-challenge/ Search PubMed.
- B. Chatterjee, Mumbai's Covid-19 waste doubles in 3 months, treatment facility runs out of storage space, Hindustan Times, 2020, https://www.hindustantimes.com/india-news/city-s-covid-19-waste-doubles-in-3-months-treatment-facility-runs-out-of-storage-space/story-RB9z2efys8dlctlXXUxL0H.html Search PubMed.
- S. Ramteke and B. L. Sahu, Novel coronavirus disease 2019 (COVID-19) pandemic: considerations for the biomedical waste sector in India, Case Stud. Chem. Environ. Eng., 2020, 100029 CrossRef.
- M. Varmani, Bio-medical waste management during COVID-19, 2020, https://www.investindia.gov.in/team-india-blogs/bio-medical-waste-management-during-covid-19 Search PubMed.
- M. A. Zambrano-Monserrate, M. A. Ruano and L. Sanchez-Alcalde, Indirect effects of COVID-19 on the environment, Sci. Total Environ., 2020, 728, 138813 CrossRef CAS.
- J. Calma, The COVID-19 pandemic is generating tons of medical waste, The Verge, 2020, https://www.theverge.com/2020/3/26/21194647/the-covid-19-pandemic-is-generating-tons-of-medical-waste Search PubMed.
- I. Tiseo, COVID-19: Healthcare waste generation in Asia 2020, Statista, 2020, https://www.statista.com/statistics/1167512/healthcare-waste-generation-before-during-covid-19-asia-by-city/ Search PubMed.
- United Nations, Five things you should know about disposable masks and plastic pollution, UN News, 2020, https://news.un.org/en/story/2020/07/1069151 Search PubMed.
- T. A. Aragaw, Surgical face masks as a potential source for microplastic pollution in the COVID-19 scenario, Mar. Pollut. Bull., 2020, 159, 111517 CrossRef CAS.
- J. Lynch, The Plastic That Washes up on Hawaii’s beaches affects me personally, NIST, 2019, https://www.nist.gov/blogs/taking-measure/plastic-washes-hawaiis-beaches-affects-me-personally Search PubMed.
- United Nation Environment Programme, COVID-19 Waste management factsheets, UNEP - UN Environment Programme, 2020, http://www.unenvironment.org/resources/factsheet/covid-19-waste-management-factsheets Search PubMed.
- J. Chadwick, Face mask remnants and other microplastics found in the guts of sharks, Mail Online, 2020, https://www.dailymail.co.uk/sciencetech/article-8548139/Face-masks-remnants-guts-sharks.html Search PubMed.
- P. C. Ray, H. Yu and P. P. Fu, Toxicity and environmental risks of nanomaterials: Challenges and future needs, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2009, 27, 1–35 CrossRef CAS.
- M. H. Chua, W. Cheng, S. S. Goh, J. Kong, B. Li and J. Y. C. Lim, et al., Face masks in the New COVID-19 normal: Materials, testing, and perspectives, Research, 2020, 2020, 1–40 CrossRef.
- I. D. Posen, P. Jaramillo, A. E. Landis and W. M. Griffin, Greenhouse gas mitigation for U.S. plastics production: energy first, feedstocks later, Environ. Res. Lett., 2017, 12, 034024 CrossRef.
- I. D. Posen, P. Jaramillo and W. M. Griffin, Uncertainty in the life cycle greenhouse gas emissions from U.S. production of three biobased polymer families, Environ. Sci. Technol., 2016, 50, 2846–2858 CrossRef CAS.
- M. N. Moore, Do nanoparticles present ecotoxicological risks for the health of the aquatic environment?, Environ. Int., 2006, 32, 967–976 CrossRef CAS.
- D. T. Donia and M. Carbone, Fate of the nanoparticles in environmental cycles, Int. J. Environ. Sci. Technol., 2019, 16, 583–600 CrossRef CAS.
- T. Benn, B. Cavanagh, K. Hristovski, J. D. Posner and P. Westerhoff, The release of nanosilver from consumer products used in the home, J. Environ. Qual., 2010, 39, 1875–1882 CrossRef CAS.
- I. J. Yu, M. Gulumian, S. Shin, T. H. Yoon and V. Murashov, Occupational and environmental health effects of nanomaterials, BioMed Res. Int., 2015, 2015, e789312 Search PubMed.
- K. Hegde, R. Goswami, S. J. Sarma, V. D. Veeranki, S. K. Brar and R. Y. Surampalli, Environmental Hazards and Risks of Nanomaterials, ResearchGate, 2015, https://www.researchgate.net/publication/283788431_Environmental_Hazards_and_Risks_of_Nanomaterials Search PubMed.
- P. P. Fu, Q. Xia, H.-M. Hwang, P. C. Ray and H. Yu, Mechanisms of nanotoxicity: Generation of reactive oxygen species, J. Food Drug Anal., 2014, 22, 64–75 CrossRef CAS.
- D. R. Bickers and M. Athar, Oxidative stress in the pathogenesis of skin disease, J. Invest. Dermatol., 2006, 126, 2565–2575 CrossRef CAS.
Footnote |
| † Equally contributed. |
| This journal is © The Royal Society of Chemistry 2021 |