Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of new M-layer solid-solution 312 MAX phases (Ta1−xTix)3AlC2 (x = 0.4, 0.62, 0.75, 0.91 or 0.95), and their corresponding MXenes†
Maxwell T. P.
Rigby-Bell
 a,
Varun
Natu
b,
Maxim
Sokol
bc,
Daniel J.
Kelly
a,
David G.
Hopkinson
a,
Yichao
Zou
a,
James R. T.
Bird
a,
Varun
Natu
b,
Maxim
Sokol
bc,
Daniel J.
Kelly
a,
David G.
Hopkinson
a,
Yichao
Zou
a,
James R. T.
Bird
 a,
Lee J.
Evitts
d,
Matt
Smith
a,
Christopher P.
Race
a,
Philipp
Frankel
a,
Sarah J.
Haigh
a,
Lee J.
Evitts
d,
Matt
Smith
a,
Christopher P.
Race
a,
Philipp
Frankel
a,
Sarah J.
Haigh
 *a and
Michel W.
Barsoum
*a and
Michel W.
Barsoum
 *b
*b
aDepartment of Materials, University of Manchester, Manchester, M1 3BB, UK. E-mail: sarah.haigh@manchester.ac.uk
bDepartment of Materials Science & Engineering, Drexel University, Philadelphia, PA 19104, USA. E-mail: barsoumw@drexel.edu
cDepartment of Materials Science and Engineering, Tel Aviv University, Ramat Aviv 6997801, Israel
dNuclear Futures Institute, Bangor University, Gwynedd, LL57 2DG, UK
First published on 13th January 2021
Abstract
Quaternary MAX phases, (Ta1−xTix)3AlC2 (x = 0.4, 0.62, 0.75, 0.91 or 0.95), have been synthesised via pressureless sintering of TaC, TiC, Ti and Al powders. Via chemical etching of the Al layers, (Ta0.38Ti0.62)3C2Tz – a new MXene, has also been synthesised. All materials contain an M-layer solid solution of Ta and Ti, with a variable Ta concentration, paving the way for the synthesis of a range of alloyed (Ta,Ti)3C2Tz MXenes with tuneable compositions for a wide range of potential applications.
The MAX phases are a class of hexagonal nano-layered carbides and nitrides with general formula Mn+1AXn, where n = 1, 2, 3, etc. and referred to by their stoichiometry – for example M2AX as ‘211’, M3AX2 as ‘312’ and so on. Whilst the ‘M’ is generally an early transition metal, ‘A’ an A-group element and ‘X’ either carbon or nitrogen, all three sites may consist of more than one element in either solid solution or ordered form (such as the MI, MII ordered ‘o-MAX’ phases).1 This vast chemical diversity results in a wealth of material structures and properties, with more than 155 phases known to date – a number that likely represents a small proportion of the material possibilities. The MAX phases have attracted attention due to their interesting mix of ceramic and metallic properties. Like ceramics, some MAX phases are elastically rigid (Young's modulus > 300 GPa),2 strong at high temperatures,3 lightweight (<4.5 g cm−3) and creep and oxidation resistant.4,5 Like metals, MAX phases have shown excellent electrical and thermal conductivity,6,7 machinability,8 thermal shock resistance8 and even damage tolerance.9 Recently, the interest in MAX phase materials has increased dramatically because they are the feedstock for their two-dimensional derivatives, MXenes.10 MXenes are typically obtained via etching of the MAX phase ‘A-layer’ and subsequent chemical delamination of the two-dimensional ‘MX-layers’. They have the general formula Mn+1XnTz, where Tz refers to the MX-layer surface terminations – usually –OH, –O, and/or –F.11,12 MXenes have shown potential for use in a large range of applications, including photo- and electro-catalysis,13,14 energy storage and conversion,15,16 the development of novel hybrid nanocomposites,17,18 biosensors,19 water purification,20 electromagnetic interference shielding,21 lubrication,22 and conductive inks.23,24
The discovery of new MAX phases and, by extension, new MXenes with different/improved properties can thus be highly valuable. Here we report on the successful synthesis of a range of 312 MAX phase materials, where M is a solid solution of Ta and Ti with variable Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ta ratios, A = Al and X = C. The work builds on previous experimental reports of a 211 MAX phase with similar elemental components – (Ta1−xTix)2AlC (0 < x < 1),25 and the pure M-layer 312 MAX phases M3AlC2 (M = Ta or Ti).26,27 Our experimental realisation of the (Ta1−xTix)3AlC2 (0 < x < 1) system was motivated by recent theoretical predictions demonstrating the phase stability of TaTi2AlC2 and Ta2TiAlC2 by Dahlqvist and Rosen.28 We further demonstrate successful exfoliation of (Ta0.38Ti0.62)3AlC2 to generate a new MXene composition (Ta0.38Ti0.62)3C2Tx.
Ta ratios, A = Al and X = C. The work builds on previous experimental reports of a 211 MAX phase with similar elemental components – (Ta1−xTix)2AlC (0 < x < 1),25 and the pure M-layer 312 MAX phases M3AlC2 (M = Ta or Ti).26,27 Our experimental realisation of the (Ta1−xTix)3AlC2 (0 < x < 1) system was motivated by recent theoretical predictions demonstrating the phase stability of TaTi2AlC2 and Ta2TiAlC2 by Dahlqvist and Rosen.28 We further demonstrate successful exfoliation of (Ta0.38Ti0.62)3AlC2 to generate a new MXene composition (Ta0.38Ti0.62)3C2Tx.
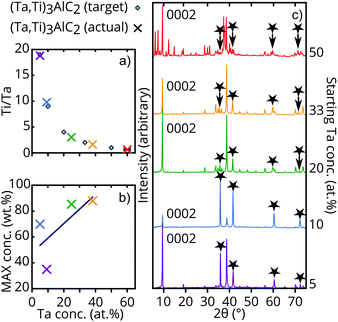
Synthesis of the (Ta1−xTix)3AlC2 quaternary, with nominal starting compositions of x = 0.5, 0.66, 0.8, 0.90 and 0.95, was investigated starting from TaC, TiC, Ti and Al powders (Tables S1 and S2, ESI†). Powder mixtures were uniaxially cold-pressed at 250 MPa before being heated to 1600 °C for 8 h in a pressure-less flowing Ar tube furnace. Powder X-ray diffraction (XRD) analysis showed each sample contained peaks consistent with a hexagonal P63/mmc symmetry 312 MAX phase (Fig. 1c), with unit cell volumes ranging from 153.29 ± 0.03 Å3 (153.29(3) Å3)‡ to 156.0(1) Å3 for (Ta0.09Ti0.91)3AlC2 and (Ta0.6Ti0.4)3AlC2 respectively (Table S3, ESI†). A fairly linear increase in cell volume with increasing Ta at% is seen, indicating increasing substitution of the Ti M-site with Ta (atomic radius of 1.45 Å compared to 1.40 Å).29 This puts the cell volumes in the expected range between the reported value of 151.8(2) Å3 for Ti3AlC2, and the value of 158.73(1) Å3 reported for Ta3AlC2,27,30 as expected (Fig. S3, ESI†). The highest phase purity of better than 85 wt% was obtained for Rietveld refined compositions of (Ta0.25Ti0.75)3Al0.77C2 and (Ta0.38Ti0.62)3Al0.81C2 (Fig. 1 and Table S2, ESI†).
The best fitting of the XRD data was achieved with the Al layer arranged in the α-312 stacking configuration for (Ta0.25Ti0.75)3Al0.77C2, (Ta0.38Ti0.62)3Al0.81C2 and (Ta0.6Ti0.4)3AlC2, with (Ta0.05Ti2.95)3AlC2 and (Ta0.09Ti0.91)3AlC2 arranged in the β-312 configuration (see Table S3, ESI†). For most of the samples, the major impurity peaks were those belonging to cubic (Ta,Ti)Cx (x ≤ 2) and small quantities of Al2O3 and TiAl2 (for discussion, see ‘XRD’, ESI†). The sample with the highest starting Ta content (50 at%), however, produced a multitude of additional phases, including at least two that have not been identified. As such, structural refinement used a combination of the Rietveld and Pawley methods, that indicated the presence of the expected 312 MAX phase as well as up to five further MAX phases (‘XRD’ and Fig. S4, ESI†).31 The lower purity and presence of these extra phases at the highest Ta concentration suggests a Ta solubility limit in the Ti M-layers.
Energy dispersive X-ray spectroscopy (EDS) implemented within a scanning electron microscope (SEM) was also used for characterisation. EDS provided mean Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ta ratios in the primary phase regions that decreased with increasing Ta concentrations in the starting mixtures (Fig. 1a). In particular, the sample with intended formula (Ta1/3Ti2/3)3AlC2 produced a Ti
Ta ratios in the primary phase regions that decreased with increasing Ta concentrations in the starting mixtures (Fig. 1a). In particular, the sample with intended formula (Ta1/3Ti2/3)3AlC2 produced a Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ta ratio of 1.62
Ta ratio of 1.62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 compared with the expected 2
1 compared with the expected 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 from the starting mixture (Table S2, ESI†). This is due to the formation of Ti-rich (Ta,Ti)Cx (x ≤ 2) impurities during synthesis (see ‘XRD’, ESI†). Both SEM- and scanning transmission electron microscope (STEM) EDS quantitative analysis indicated a sub-stoichiometric Al content in (Ta0.25Ti0.75)3Al0.77C2 and (Ta0.38Ti0.62)3Al0.81C2. Synthesis trials with increased nominal starting Al stoichiometries as high as 1.6 resulted in a decrease in MAX phase purity and increasing quantities of Al2O3 and TiAl2. This suggests that the reduced Al occupancy is a thermodynamic effect rather than being due to a deficiency of Al in the starting mixture.
1 from the starting mixture (Table S2, ESI†). This is due to the formation of Ti-rich (Ta,Ti)Cx (x ≤ 2) impurities during synthesis (see ‘XRD’, ESI†). Both SEM- and scanning transmission electron microscope (STEM) EDS quantitative analysis indicated a sub-stoichiometric Al content in (Ta0.25Ti0.75)3Al0.77C2 and (Ta0.38Ti0.62)3Al0.81C2. Synthesis trials with increased nominal starting Al stoichiometries as high as 1.6 resulted in a decrease in MAX phase purity and increasing quantities of Al2O3 and TiAl2. This suggests that the reduced Al occupancy is a thermodynamic effect rather than being due to a deficiency of Al in the starting mixture.
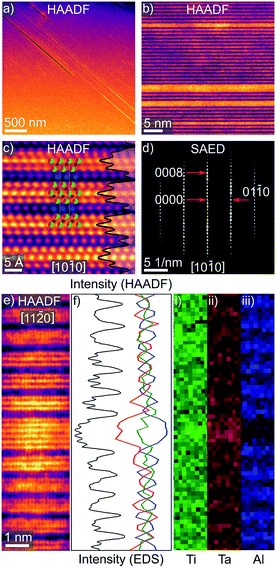
The characteristic 312 MAX phase layered structure of (Ta0.38Ti0.62)3Al0.81C2 can be seen in the high angle annular dark field (HAADF) STEM micrographs in Fig. 2c and e, aligned with the [10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] and [11
0] and [11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0] zone axes, respectively. The positions of Al in Fig. 2c are consistent with the β-312 stacking configuration, in contrast to the XRD data, which is likely the result of an α–β transformation during TEM sample preparation, as reported by one of us previously.9 The HAADF STEM data also reveals that the central metal layers (MII) have visibly higher HAADF intensities than the metal layers that sandwich it (MI) (see Fig. S1, ESI for diagram†), indicative of a higher atomic number and hence a higher Ta concentration. This is in-line with the XRD data, in which MI and MII Ta site occupancies converged to 0.340(6) and 0.466(7), respectively (Table S2, ESI†). This preferential elemental enrichment is distinct from the full out-of-plane ordering seen in quaternary 312 o-MAX phases with a 2
0] zone axes, respectively. The positions of Al in Fig. 2c are consistent with the β-312 stacking configuration, in contrast to the XRD data, which is likely the result of an α–β transformation during TEM sample preparation, as reported by one of us previously.9 The HAADF STEM data also reveals that the central metal layers (MII) have visibly higher HAADF intensities than the metal layers that sandwich it (MI) (see Fig. S1, ESI for diagram†), indicative of a higher atomic number and hence a higher Ta concentration. This is in-line with the XRD data, in which MI and MII Ta site occupancies converged to 0.340(6) and 0.466(7), respectively (Table S2, ESI†). This preferential elemental enrichment is distinct from the full out-of-plane ordering seen in quaternary 312 o-MAX phases with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 M-element starting ratio.1 Nonetheless, similar preferential enrichment of the centre MII layer has been observed in 312 M-layer solid solution MAX phases such as (Cr,V)3AlC2.1,32 Lattice parameters obtained from selected area electron diffraction (SAED) patterns, like in Fig. 2d, of a = 3.01(2) Å and c = 18.59(2) Å, are also in good agreement with bulk lattice parameters from XRD (a = 3.0981(1) Å and c = 18.6140(7) Å, see Table S3, ESI†). It should be stressed at this point that the pristine MAX phase layered structure is occasionally interrupted by stacking faults of varying thickness that, at times, penetrate entire crystallites (Fig. 2a, b and e). Structurally, these defects can be thought of as either few-layer ternary carbide impurities, or as MX layers in the MAX phase matrix with an unexpected number of M-layers – such as the 6 shown in Fig. 2e, compared with the expected 3.
1 M-element starting ratio.1 Nonetheless, similar preferential enrichment of the centre MII layer has been observed in 312 M-layer solid solution MAX phases such as (Cr,V)3AlC2.1,32 Lattice parameters obtained from selected area electron diffraction (SAED) patterns, like in Fig. 2d, of a = 3.01(2) Å and c = 18.59(2) Å, are also in good agreement with bulk lattice parameters from XRD (a = 3.0981(1) Å and c = 18.6140(7) Å, see Table S3, ESI†). It should be stressed at this point that the pristine MAX phase layered structure is occasionally interrupted by stacking faults of varying thickness that, at times, penetrate entire crystallites (Fig. 2a, b and e). Structurally, these defects can be thought of as either few-layer ternary carbide impurities, or as MX layers in the MAX phase matrix with an unexpected number of M-layers – such as the 6 shown in Fig. 2e, compared with the expected 3.
High resolution EDS scans, performed over several regions on (Ta0.38Ti0.62)3Al0.81C2 grains, show the expected elemental segregation of Ti, Ta and Al layers, as seen in Fig. 2f(i, ii, and iii), respectively. Quantitative STEM-EDS analysis provided Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ta ratios, excluding stacking faults, from 1.6–1.9
Ta ratios, excluding stacking faults, from 1.6–1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, with a mean value of 1.7(1)
1, with a mean value of 1.7(1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is close to the 1.62(2)
1, which is close to the 1.62(2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 obtained from SEM-EDS. Note that electron channelling effects and the limited spatial resolution prevent absolute quantification of the relative Ti
1 obtained from SEM-EDS. Note that electron channelling effects and the limited spatial resolution prevent absolute quantification of the relative Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ta concentrations in MIIversus MI sites (see ‘Electron microscopy’, ESI for methods†).
Ta concentrations in MIIversus MI sites (see ‘Electron microscopy’, ESI for methods†).
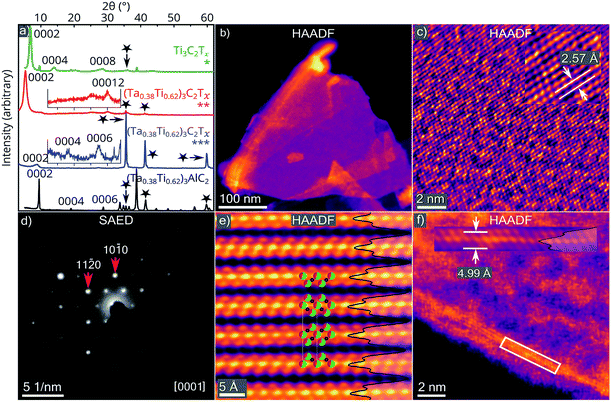
To demonstrate the potential to produce solid solution Ta/Ti MXenes, we used an in situ HF etching process at 20 °C for 12 h, similar to that performed by Ghidiu et al.33 (see ‘Synthesis’, ESI†). Etching of the (Ta0.38Ti0.62)3Al0.81C2 sample produced the expected (Ta0.38Ti0.62)3C2Tz MXene (Fig. 3). Characteristic multilayer (ML) flakes were observed following vacuum drying of the MXene suspension (Fig. 3b), with SEM-EDS analysis revealing a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ti
1 Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ta ratio over micrometre sized areas – including contributions from Ti-rich (Ta,Ti)Cx impurities (similar to the parent MAX phase sample). The etching resulted in a c-lattice parameter increase from 18.6140(7) Å to 19.7(1) Å (Fig. 3a). After sonication, this increased dramatically to 34.9(4) Å, indicating full intercalation of the MX-layers. This suggests a similar formation mechanism to that proposed for producing Ti3C2Tz MXene from the parent Ti3AlC2 MAX phase, where Al is replaced by terminating species in the etchant such as –OH, –F, or
Ta ratio over micrometre sized areas – including contributions from Ti-rich (Ta,Ti)Cx impurities (similar to the parent MAX phase sample). The etching resulted in a c-lattice parameter increase from 18.6140(7) Å to 19.7(1) Å (Fig. 3a). After sonication, this increased dramatically to 34.9(4) Å, indicating full intercalation of the MX-layers. This suggests a similar formation mechanism to that proposed for producing Ti3C2Tz MXene from the parent Ti3AlC2 MAX phase, where Al is replaced by terminating species in the etchant such as –OH, –F, or ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, followed by intercalation of the MX-layers by Li+, allowing for full delamination via sonication.10,15,34 The lower intensity of (Ta,Ti)Cx peaks in the XRD of the delaminated MXene compared to the parent MAX phase (Fig. 3a) suggests an improvement in the purity of the sample (better than the 87.9(2) at% of the parent MAX phase) achieved via sonication and centrifugation.
O, followed by intercalation of the MX-layers by Li+, allowing for full delamination via sonication.10,15,34 The lower intensity of (Ta,Ti)Cx peaks in the XRD of the delaminated MXene compared to the parent MAX phase (Fig. 3a) suggests an improvement in the purity of the sample (better than the 87.9(2) at% of the parent MAX phase) achieved via sonication and centrifugation.
STEM imaging and diffraction was further used to investigate the MXene structure. The pre-delamination (Ta0.38Ti0.62)3C2Tz ML produced lattice parameters of a = 3.14(3) Å and c = 11.8(1) Å, with a being similar to the parent MAX phase and c reduced following the removal of the Al layer. SAED performed on a fully exfoliated monolayer (Ta0.38Ti0.62)3C2Tz flake was indexed using a hexagonal basis (Fig. 3d), and produced an a-lattice parameter of 2.97(3) Å, which is in agreement with the value of 2.98(3) Å obtained from fast Fourier transform of the high resolution STEM data (e.g.Fig. 3e), and slightly less than the value of 3.0981(1) Å in the parent phase. The monolayer sheet thickness was estimated from HAADF-STEM analysis of the curled-up edge of a single flake as 4.9(5) Å (Fig. 3c) – close to the MX-layer (4.85(2) Å) in the parent phase. Furthermore, the HAADF contrast suggests Ta enrichment in the MII layer relative to the MI layer, as also observed in the parent MAX phase.
Conclusions
In summary, a new quaternary (Ta1−xTix)3AlC2 phase system has been synthesised with a variable Ta![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti ratio (x = 0.4, 0.62, 0.75, 0.91 or 0.95) and up to 87.9(2) at% purity. The ‘M’ sites exist as a solid solution of Ta and Ti, with a higher concentration of Ta in the central MII layers. Experimental results suggest a mean Ta M-layer concentration limit between 38 and 60 at%, beyond which the formation of several alternative MAX phases is favoured, and thus a significant reduction in phase purity. The (Ta0.38Ti0.62)3Al0.81C2 MAX phase was used to synthesise a new solid solution MXene – (Ta0.38Ti0.62)3C2Tz – via chemical etching, with the synthesis pathway likely to be similar to the unalloyed Ti3C2Tx MXene. It is proposed that this approach can be used to synthesise a range of alloyed (Ta,Ti)3C2Tx MXenes, with compositions that can be optimised for a wide range of potential applications.
Ti ratio (x = 0.4, 0.62, 0.75, 0.91 or 0.95) and up to 87.9(2) at% purity. The ‘M’ sites exist as a solid solution of Ta and Ti, with a higher concentration of Ta in the central MII layers. Experimental results suggest a mean Ta M-layer concentration limit between 38 and 60 at%, beyond which the formation of several alternative MAX phases is favoured, and thus a significant reduction in phase purity. The (Ta0.38Ti0.62)3Al0.81C2 MAX phase was used to synthesise a new solid solution MXene – (Ta0.38Ti0.62)3C2Tz – via chemical etching, with the synthesis pathway likely to be similar to the unalloyed Ti3C2Tx MXene. It is proposed that this approach can be used to synthesise a range of alloyed (Ta,Ti)3C2Tx MXenes, with compositions that can be optimised for a wide range of potential applications.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by funding from the Engineering and Physical Sciences Research Council Fusion CDT programme and grants [EP/L01663X/1, EP/M010619/1, EP/S021531/1, EP/P009050/1]; Henry Royce Institute for Advanced Materials [EP/R00661X/1, EP/S019367/1, EP/P025021/1, EP/P025498/1]; the Graphene NoWNano CDT programme [EP/L01548X/1], H2020 under the European Research Council Starter grant EvoluTEM (715502); the Sêr Cymru II programme funded through the Welsh European Funding Office (WEFO) under the European Development Fund (ERDF), NSF DMR 1740795 and the Department of Materials XRD Facility.Notes and references
- M. Sokol, V. Natu, S. Kota and M. W. Barsoum, Trends Chem., 2019, 1–14 Search PubMed.
- T. Lapauw, A. K. Swarnakar, B. Tunca, K. Lambrinou and J. Vleugels, Int. J. Refract. Met. Hard Mater., 2018, 72, 51–55 CrossRef CAS.
- M. W. Barsoum and M. Radovic, Annu. Rev. Mater. Res., 2011, 41, 195–227 CrossRef CAS.
- D. J. Tallman, On the Potential of MAX phases for Nuclear Applications, Drexel University, 2015 Search PubMed.
- G. M. Song, Y. T. Pei, W. G. Sloof, S. B. Li, J. T. M. De Hosson and S. van der Zwaag, Scr. Mater., 2008, 58, 13–16 CrossRef CAS.
- X. H. Wang and Y. C. Zhou, J. Mater. Sci. Technol., 2010, 26, 385–416 CrossRef CAS.
- M. W. Barsoum, T. El-Raghy, C. J. Rawn, W. D. Porter, H. Wang, E. A. Payzant and C. R. Hubbard, J. Phys. Chem. Solids, 1999, 60, 429–439 CrossRef CAS.
- M. W. Barsoum and T. El-Raghy, J. Am. Ceram. Soc., 1996, 79, 1953–1956 CrossRef CAS.
- M. W. Barsoum, MAX Phases: Properties of Machinable Ternary Carbides and Nitrides, 2013 Search PubMed.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- M. Naguib, O. Mashtalir, J. Carle, V. Presser, J. Lu, L. Hultman, Y. Gogotsi and M. W. Barsoum, ACS Nano, 2012, 6, 1322–1331 CrossRef CAS PubMed.
- C. Shi, M. Beidaghi, M. Naguib, O. Mashtalir, Y. Gogotsi and S. J. L. Billinge, Phys. Rev. Lett., 2013, 112, 1–5 Search PubMed.
- J. Ran, G. Gao, F. T. Li, T. Y. Ma, A. Du and S. Z. Qiao, Nat. Commun., 2017, 8, 13907 CrossRef CAS PubMed.
- H. Lin, X. Wang, L. Yu, Y. Chen and J. Shi, Nano Lett., 2017, 17, 384–391 CrossRef CAS PubMed.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS.
- X. Zhang, Z. Zhang and Z. Zhou, J. Energy Chem., 2018, 27, 73–85 CrossRef.
- Z. Ling, C. E. Ren, M. Q. Zhao, J. Yang, J. M. Giammarco, J. Qiu, M. W. Barsoum and Y. Gogotsi, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 16676–16681 CrossRef CAS PubMed.
- M. Carey and M. W. Barsoum, Mater. Today Adv., 2021, 9, 100120 CrossRef CAS.
- L. Lenka, T. Bertoka, E. Dosekova, A. Holazova, D. Paprckova, A. Vikartovska, V. Sasinkova, J. Filipb, P. Kasak, M. Jerigova, D. Velic, K. A. Mahmoude and J. Tkaca, J. Phys. Chem. C, 2017, 235, 471–479 Search PubMed.
- Q. Peng, J. Guo, Q. Zhang, J. Xiang, B. Liu, A. Zhou, R. Liu and Y. Tian, J. Am. Chem. Soc., 2014, 136, 4113–4116 CrossRef CAS PubMed.
- F. Shahzad, M. Alhabeb, C. B. Hatter, B. Anasori, S. M. Hong, C. M. Koo and Y. Gogotsi, Science, 2016, 353, 1137–1140 CrossRef CAS PubMed.
- X. Zhang, M. Xue, X. Yang, Z. Wang, G. Luo, Z. Huang, X. Sui and C. Li, RSC Adv., 2015, 5, 2762–2767 RSC.
- W. Yang, J. Yang, J. J. Byun, F. P. Moissinac, J. Xu, S. J. Haigh, M. Domingos, M. A. Bissett, R. A. W. Dryfe and S. Barg, Adv. Mater., 2019, 31, 1–8 Search PubMed.
- J. Orangi, F. Hamade, V. A. Davis and M. Beidaghi, ACS Nano, 2020, 14, 640–650 CrossRef CAS PubMed.
- S. Sridharan and H. Nowotny, Zeitschrift fuer Met. Res. Adv. Tech., 1983, 74, 468–472 CAS.
- N. V. Tzenov and M. W. Barsoum, J. Am. Ceram. Soc., 2000, 83, 825–832 CrossRef CAS.
- J. Etzkorn, M. Ade and H. Hillebrecht, Inorg. Chem., 2007, 46, 1410–1418 CrossRef CAS PubMed.
- M. Dahlqvist and J. Rosen, Nanoscale, 2020, 12, 785–794 RSC.
- J. C. Slater, J. Chem. Phys., 1964, 41, 3199–3204 CrossRef CAS.
- G. P. Bei, V. Gauthier-Brunet, C. Tromas and S. Dubois, J. Am. Ceram. Soc., 2012, 95, 102–107 CrossRef CAS.
- G. S. Pawley, J. Appl. Crystallogr., 1981, 14, 357–361 CrossRef CAS.
- E. N. Caspi, P. Chartier, F. Porcher, F. Damay and T. Cabioc'h, Mater. Res. Lett., 2015, 3, 100–106 CrossRef.
- M. Ghidiu, M. R. Lukatskaya, M. Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2015, 516, 78–81 CrossRef PubMed.
- L. Verger, V. Natu, M. Carey and M. W. Barsoum, Trends Chem., 2019, 1, 656–669 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Computational and experimental details; XRD and electron microscopy characterisations. CCDC 2036624. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra09761f |
| ‡ Herein this error notation will be used, in which the number in parentheses is the numerical value of the standard uncertainty referred to the corresponding last digit of the quoted result. |
| This journal is © The Royal Society of Chemistry 2021 |