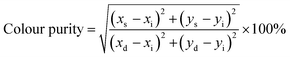Open Access Article
Open Access ArticleCoronene diimide-based ‘bowl’ nanostructures as red emitters for the analysis of latent fingerprints and metal ion detection†
Prabhpreet Singh * and
Poonam Sharma
* and
Poonam Sharma
Department of Chemistry, UGC Centre for Advanced Studies, Guru Nanak Dev University, Amritsar 143 005, India. E-mail: prabhpreet.chem@gndu.ac.in; Tel: +91-84271-01534
First published on 2nd February 2021
Abstract
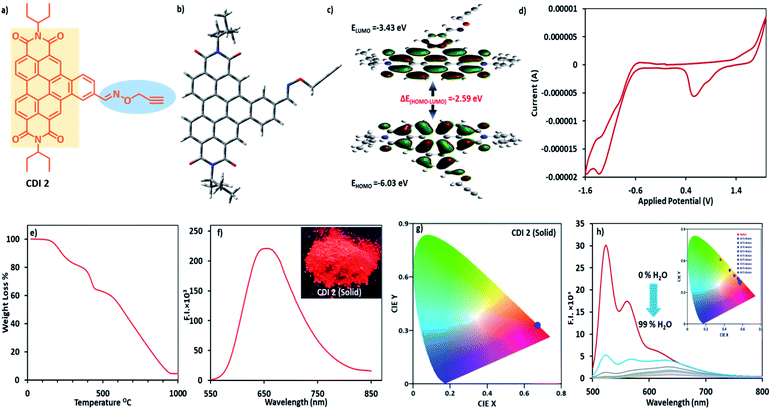
We report an NIR-based photoluminescent material, namely benzo-coronene diimide (CDI 2), and its use in the visualization of latent fingerprints (LFPs) and in the metal ion detection in an aqueous medium. CDI 2 exhibited nano-sized interlinked fibre structures forming ‘bowl’ shaped nanoarchitectures as red emitters with the Commission Internationale de l'Eclairage (CIE) coordinates (x, y) of (0.67, 0.33) with 100% colour purity in the solid state. CDI 2 was confirmed to be the potential candidate for the analysis of LFPs and the detection of Pd2+/Cu2+ in an aqueous medium.
Nowadays, latent (invisible) fingerprints are utilized as a personal identification mark in numerous instances to decrypt information during criminal and forensic investigations, to access computers and equipments utilized for administrative purposes, to provide aid in health and medical facilities, to mark the attendance of employees in government and private institutes and to monitor the entry/exit in security rooms owing to national interest.1,2 Each and every individual, even the identical twins, have a unique pattern of friction ridges formed from the epidermis of the fingertips.3 Different imaging techniques are used to visualize the LFPs; however, low resolution, destruction of the fingerprints during the analysis and low sensitivity of these techniques reduce the effectiveness of the LFPs.4–6 The power-dusting technique, which involves the use of NIR-photoluminescent materials, emerged as a new advanced approach to develop the latent fingerprints.7,8
The availability of fluorescent red emitters is relatively limited in comparison with green and blue emitters for their application in organic light emitting diodes (OLEDs) due to their colour impurities.9 According to previous literature, red OLEDs are available, which are based on either organometallic complexes or organic materials, such as dicyanomethylene, polyarene and chromene dopants.10a,b Therefore, the focus of the current research is in developing new red emitters for LFPs and metal ion sensing.11a,b Blue, green and red emitters are equally essential for the generation of white light. However, research over the past decade demonstrated high external quantum efficiencies of green and red emitters with a high colour purity and brightness compared with those of the blue triplet emitters. The characteristics of saturated red emission, spectral stability and stable electroluminescence efficiency of red emitters at high brightness and current enable them to be used as an emitting species in OLEDs. Herein, we report on benzocoronene12,13 (CDI 2), which exhibited a highly conjugated π-system with emission extended to the red region (red emitters).14,15 CDI 2 (Fig. 1a) exhibited nano-sized interlinked fibre structures forming a ‘bowl’ like nanoarchitecture and was used to lift the LFPs developed on glass, metal, or aluminium surfaces. The CIE 1931 (RGB) coordinates16 demonstrated a 100% colour purity in the solid state and on LFPs. CDI 2 could be a potential candidate for the detection of Pd2+/Pd0 in water.
The preparation of the building block commenced with the Suzuki coupling reaction of 1-bromo-N,N′-di-(3-pentyl)-perylene 3,4,9,10-tetracarboxylic diimide with 4-formylphenylboronic acid, which was then subjected to condensation with hydroxylamine hydrochloride (Schiff-base condensation). Subsequent propargylation with propargyl bromide provided PDI 1 in good yield.11b CDI 2 was subsequently obtained through the photocyclization of PDI 1 in CH3CN (96% yield). The chemical structure of CDI 2 was elucidated explicitly via 1D NMR (1H and 13C), 2D NMR (COSEY) and FTIR techniques (Fig. S1a–e†). CDI 2 exhibited solubility in common organic solvents. The thermal stability of CDI 2 was measured via the thermogravimetric analysis (TGA). CDI 2 showed an initial mass loss of ∼35% in the temperature range of 170–450 °C. This mass loss corresponds to the removal of propargyl and oxime moieties from CDI 2. The decomposition temperature (Td, 60% weight loss) of CDI 2 was found to be 550 °C (Fig. 1e).
The computational study was performed at the B3LYP/6-31G* basis level using the Gaussian 09 software. The contour picture of HOMO/LUMO revealed that the electron density of the HOMO localized predominately on the π-conjugated benzocoronene and oxygen heteroatom of the oxime moiety, while that of LUMO was mainly distributed on the benzocoronene part. The calculated HOMO and LUMO energy levels were −6.03 and −3.43 eV, respectively. The band gap energy in CDI 2 was 2.59 eV (Fig. 1b, c, S2† and 3a).
In cyclic voltammetry, CDI 2 exhibited an onset reduction (Ered) and weak oxidation potential (Eox) values of 0.56 V and 1.39 V, respectively. The HOMO and LUMO energy levels calculated using the formula ELUMO = −[Ered(onset) + 4.4] eV and EHOMO = −[Eox(onset) + 4.4] eV from experimentally determined Eox and Ered values were −5.80 eV and −3.43 eV, respectively, which were in line with the HOMO–LUMO energies calculated via the density functional theory (Fig. 1d). The onset reduction peaks at −1.39 V and −1.6 V could be assigned to the second and third reductions of CDI 2 characteristic for the π-conjugated system.
The introduction of more planar groups into CDI 2 shifted the solid-state emission (powder form and thin film) to the red region with a λem of 660 nm (Fig. 1f) and a photoluminescence quantum yield of 3.89%. The emission wavelength of CDI 2 was further complimented by considering the CIE-1931 RGB colour coordinates. The colour purity17 of CDI 2 in the powder form was calculated using the following equation:
The red photoluminescence of CDI 2 encouraged us to investigate the possibility of their AIE properties. The AIE feature of CDI 2 was evaluated by measuring its photoluminescence spectra in a binary mixture of DMSO–H2O with different water fractions (fw). The emission maxima of CDI 2 exhibited a red shift from 530 to 640 nm with an increase in fw, revealing the formation of nanoaggregates. When fw was increased to 20%, the ratiometric (I640 nm/I530 nm) photoluminescence intensity of CDI 2 gradually changed. Afterwards, large ratiometric changes (∼14-fold) were observed for fw up to 80% (Fig. 1h). Accordingly, we prepared a solution of CDI 2 in DMSO for comparison. As expected, the photoluminescence and the UV-vis spectra of CDI 2 recorded in DMSO showed an emission wavelength only at 530 nm and an absorption peak at 474/508 nm (Fig. S5 and S11†). In DMSO, the Franck–Condon progression value A0–0/A0–1 ≈ 1.34 indicated a monomer state of CDIs, whereas a value of A0–0/A0–1 of ≤0.7 in a 90% water–DMSO mixture indicated an aggregated state of PDIs (Table S1†).
Complimenting the UV-vis and the fluorescence changes on a supramolecular level, we performed an AFM imaging of thin films prepared on a glass surface using a 10 μM solution of CDI 2 in DMSO and H2O–DMSO (fw = 50% and 90%) (Fig. 2a–d). In DMSO, the formation of oval shape aggregates with a diameter in the range of 240–300 nm over a large surface area was observed (Fig. S6a†). In a 50% H2O–DMSO binary mixture, the oval aggregates deproliferated into smaller structures and an invariant background of oval and sphere aggregates was detected and their diameters slightly decreased to 90–200 nm (Fig. S6b and c†).
Interestingly, in a 90% H2O–DMSO mixture, the changes in the structures occurred, and we detected a nano-sized interlinked fibre arranged in such a fashion that it formed a ‘bowl’ like nano-architecture with a central cavity inside. The invariant clusters of fibres detected on the surface were 70–110 nm in diameter (Fig. 2c and d). The hollow cavity in the centre exhibited a diameter of 138 nm and a depth of ∼2–3 nm (Fig. S6d–f†). We further recorded the dynamic light scattering data for these nanoaggregates in H2O–DMSO binary mixtures (Fig. 2e–g). In DMSO, CDI 2 displayed a diameter in the range of 190–530 nm with Z-average and polydispersity index (PDI) values of 353 nm and 0.24, respectively. In the 50% H2O–DMSO mixture, the size of the aggregates lay in two different sizes, i.e. 50–120 nm and 220–531 nm (PDI = 0.29). In the 90% H2O–DMSO mixture, the size of the nanoaggregates decreased to 80–165 nm, which was in concordance with the AFM data.
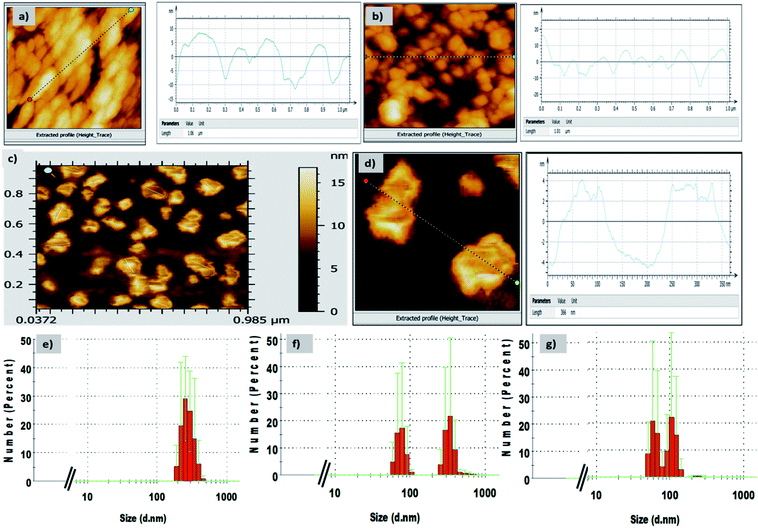
The use of near IR-based fluorescent materials in the powder-dusting method for the visualization of LFPs was a simple and non-destructive tool and did not require rigorous post-treatment method (Fig. 3). We applied our CDI 2 for the development of LFPs on porous and non-porous surfaces, such as ceramic floor tiles, glass, metals, and aluminium foils (Fig. 3a). In all cases, we obtained good quality LFPs to extract information of the ridge pattern (Ist level) and minutae details, such as ridge termination, bifurcation, core, island, lake, and bridge (2nd level) (Fig. 3c). The photoluminescence contrast between the papillary ridges and furrows was analysed by examining the cross-sectional view. The gray value varied significantly over the line/imaginary white line extended on LFPs, thereby clearly showing a discrimination between the ridges and furrows in terms of fluorescence contrast in LFPs (Fig. 3b). The solid-state emission of CDI 2 was also recorded by directly placing the fingerprint stamped substrate in the spectrofluorometer. The solid-state emission spectrum showed high emission intensity at 660 nm in accordance with the emission maximum of CDI 2 in solution form. The solid-state emission wavelength of CDI 2 was further complimented by considering the CIE-1931 RGB colour coordinates. The CIE-1931 RGB colour coordinates (0.67, 0.33) of CDI 2 on developed LFPs showed 100% colour purity (Fig. 4).
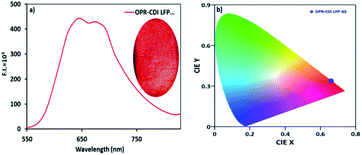 | ||
| Fig. 4 (a) Emission spectrum of LFP developed with the CDI 2 powder over a glass slide and (b) CIE-1931 plots corresponding to emission data of (a). | ||
Given that CDI 2 possessed good emission properties in the solution as well as it contained an oxime group coupled with a propargyl group, CDI 2 provided heteroatom binding sites, such as O and N. The sensing behaviour of CDI 2 in the DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HEPES buffer (1
HEPES buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, pH = 7.2) towards numerous metal cations was examined. The fluorescence intensity of CDI 2 (5 μM) at 640 nm exhibited no detectable changes, whereas the quenching of the fluorescence intensity was observed for Cu2+ ions and Pd0/Pd2+ species (Fig. S7†). Later, the detailed response of CDI 2 towards Cu2+ ions and Pd0/Pd2+ species in a 90% aqueous medium was investigated. CDI 2 (5 μM) exhibited an emission band at 640 nm in the DMSO
9, pH = 7.2) towards numerous metal cations was examined. The fluorescence intensity of CDI 2 (5 μM) at 640 nm exhibited no detectable changes, whereas the quenching of the fluorescence intensity was observed for Cu2+ ions and Pd0/Pd2+ species (Fig. S7†). Later, the detailed response of CDI 2 towards Cu2+ ions and Pd0/Pd2+ species in a 90% aqueous medium was investigated. CDI 2 (5 μM) exhibited an emission band at 640 nm in the DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HEPES buffer (1
HEPES buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, pH = 7.2, 10−2 M) upon excitation at 450 nm. Upon addition of a Pd0/Pd2+ solution (25 μM for Pd0/50 μM for Pd2+) to CDI 2 in a 90% buffered medium, the emission intensity at 640 nm was quenched. The Stern–Volmer constant (KSV) values calculated for Pd0 and Pd2+ were 1.74 × 106 M−1 and 1.79 × 106 M−1, respectively. The fluorescence response of CDI 2 towards Pd2+/Pd0 exhibited a linear relationship (Fig. S8 and S9;† R2 = 0.98 for Pd0/Pd2+) between the concentration of Pd0/Pd2+ (0–1 × 10−7 M for Pd0 and 0–1.5 × 10−7 M for Pd2+) and the I/I0 ratio of the fluorescence intensity. The lowest detection limits (LOD) calculated for CDI 2 towards Pd0 and Pd2+ species were 86.1 nM and 83.9 nM, respectively (Fig. S8 and S9†). Similarly, CDI 2 (5 μM) could also detect Cu2+ ion in the DMSO
9, pH = 7.2, 10−2 M) upon excitation at 450 nm. Upon addition of a Pd0/Pd2+ solution (25 μM for Pd0/50 μM for Pd2+) to CDI 2 in a 90% buffered medium, the emission intensity at 640 nm was quenched. The Stern–Volmer constant (KSV) values calculated for Pd0 and Pd2+ were 1.74 × 106 M−1 and 1.79 × 106 M−1, respectively. The fluorescence response of CDI 2 towards Pd2+/Pd0 exhibited a linear relationship (Fig. S8 and S9;† R2 = 0.98 for Pd0/Pd2+) between the concentration of Pd0/Pd2+ (0–1 × 10−7 M for Pd0 and 0–1.5 × 10−7 M for Pd2+) and the I/I0 ratio of the fluorescence intensity. The lowest detection limits (LOD) calculated for CDI 2 towards Pd0 and Pd2+ species were 86.1 nM and 83.9 nM, respectively (Fig. S8 and S9†). Similarly, CDI 2 (5 μM) could also detect Cu2+ ion in the DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HEPES buffer (1
HEPES buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, pH = 7.2, 10−2 M) (Fig. S10†). The Cu2+ ions tended to bind to the heteroatom binding sites, such as O and N of CDI 2. The Stern–Volmer constant (KSV) value for Cu2+ was found to be 1.2 × 106 M−1. The quenching of the fluorescence intensity showed a linear correlation of I/I0 ratio at 640 nm vs. Cu2+ concentration (0–1 × 10−7 M) with a good correlation (R2 = 0.9912) and a minimal detection limit of 124 nM.
9, pH = 7.2, 10−2 M) (Fig. S10†). The Cu2+ ions tended to bind to the heteroatom binding sites, such as O and N of CDI 2. The Stern–Volmer constant (KSV) value for Cu2+ was found to be 1.2 × 106 M−1. The quenching of the fluorescence intensity showed a linear correlation of I/I0 ratio at 640 nm vs. Cu2+ concentration (0–1 × 10−7 M) with a good correlation (R2 = 0.9912) and a minimal detection limit of 124 nM.
In conclusion, CDI 2 based ‘bowl’ nanostructures could be a potential candidate for use as red emitters with a 100% colour purity for OLED applications. CDI 2 was best for the analysis of latent fingerprints and the detection of important metal ions in a >90% aqueous medium by following the fluorescence approach.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We gratefully acknowledge the financial support from the Council of Scientific and Industrial Research, India (grant no. 02(0267)/16/EMR-II). P. Sharma is thankful to the Council of Scientific and Industrial Research, India for Senior Research Fellowship (grant no. 09/254(0303)/2020-EMR-I).Notes and references
- A. Bécue, Anal. Methods, 2016, 8, 7983–8003 RSC.
- Q. H. Wei, M. Q. Zhang, B. Ogorevc and X. J. Zhang, Analyst, 2016, 141, 6172–6189 RSC.
- P. Rastogi and K. R. Pillai, J. Indian Acad. Forensic Med., 2010, 32, 11–14 Search PubMed.
- (a) J. C. Li, X. J. Zhu, M. Xue, W. Feng, R. L. Ma and F. Y. Li, Inorg. Chem., 2016, 55, 10278–10283 CrossRef CAS; (b) C. L. Chen, Y. Yu, C. G. Li, D. Liu, H. Huang, C. Liang, Y. Lou, Y. Han, Z. Shi and S. H. Feng, Small, 2017, 13, 1702305 CrossRef.
- (a) H. Singh, R. Sharma, G. Bhargava, S. Kumar and P. Singh, New J. Chem., 2018, 42, 12900–12907 RSC; (b) P. Singh, H. Singh, R. Sharma, G. Bhargava and S. Kumar, J. Mater. Chem. C, 2016, 4, 11180–11189 RSC.
- M. Srinivasa, G. R. Vijayakumar, K. M. Mahadevan, H. Nagabhushana and H. S. Bhojya Naik, J. Sci., 2017, 2, 156–164 Search PubMed.
- G. S. Sodhi and J. Kaur, Forensic Sci. Int., 2001, 120, 172–176 CrossRef CAS.
- A. Baride, G. Sigdel, W. M. Cross, J. J. Kellar and P. S. May, ACS Appl. Nano Mater., 2019, 7, 4518–4527 CrossRef.
- N. Thejokalyani and S. J. Dhoble, Renew. Sust. Energ. Rev., 2014, 32, 448–467 CrossRef CAS.
- (a) H. Xiang, J. Cheng, X. Ma, X. Zhou and J. J. Chruma, Chem. Soc. Rev., 2013, 42, 6128–6185 RSC; (b) K. H. Lee, M. H. Park, B. M. Seo, J. H. Seo, Y. K. Kim and S. S. Yoon, Mol. Cryst. Liq. Cryst., 2011, 550, 260–269 CrossRef CAS.
- (a) P. Singh, J. Photochem. Photobiol., A, 2020, 403, 112824 CrossRef CAS; (b) P. Sharma, S. Kaur, S. Kaur and P. Singh, Photochem. Photobiol. Sci., 2020, 19, 504–514 RSC.
- (a) Q. Shi, E. S. Andreansky, S. R. Marder and S. B. Blakey, J. Org. Chem., 2017, 82, 10139–10148 CrossRef CAS; (b) M. Yoshida, H. Sakai, K. Ohkubo, S. Fukuzumi and T. Hasobe, J. Phys. Chem. C, 2018, 122, 13333–13346 CrossRef CAS.
- C. Lutke Eversloh, C. Li and K. Mullen, Org. Lett., 2011, 13, 4148–4150 CrossRef.
- U. Rohr, P. Schlichting, A. Böhm, M. Gross, K. Meerholz, C. Bräuchle and K. Müllen, Angew. Chem., Int. Ed., 1998, 37, 1434–1437 CrossRef CAS.
- K. Kumar, H. Singh, V. Vanita, R. Singh, K. B. Joshi, G. Bhargava, S. Kumar and P. Singh, Sens. Actuators, B, 2019, 283, 651–658 CrossRef CAS.
- C. Wyman, P.-P. Sloan and P. Shirley, Journal of Computer Graphics Techniques, 2013, 2, 1–11 Search PubMed.
- Y.-F. Wu, Y.-T. Nien, Y.-J. Wang and I.-G. Chen, J. Am. Ceram. Soc., 2012, 95, 1360–1366 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic characterization data, additional spectroscopic and microscopic data of CDI 2. See DOI: 10.1039/d0ra09607e |
| This journal is © The Royal Society of Chemistry 2021 |