Open Access Article
Open Access ArticleFullerene–porphyrin hybrid nanoparticles that generate activated oxygen by photoirradiation†
Kouta Sugikawa *,
Kosuke Masuda,
Kentaro Kozawa,
Riku Kawasaki
*,
Kosuke Masuda,
Kentaro Kozawa,
Riku Kawasaki and
Atsushi Ikeda
and
Atsushi Ikeda *
*
Applied Chemistry Program, Graduate School of Advanced Science and Engineering, Hiroshima University, Higashi-Hiroshima 739-8527, Japan. E-mail: sugikawa@hiroshima-u.ac.jp; aikeda@hiroshima-u.ac.jp
First published on 5th January 2021
Abstract
The preparation of water-dispersible hybrid nanoparticles comprising fullerene and porphyrin from cyclodextrin complexes is described. In the presence of polyethylene glycol, C60 fullerene and porphyrin were expelled from the cyclodextrin cavity to form fullerene–porphyrin hybrid nanoparticles in water. The fullerene–porphyrin hybrid nanoparticles exhibit improved singlet oxygen generation ability under photoirradiation compared with that of C60 nanoparticles.
Water-dispersible colloidal fullerene assemblies, referred to as fullerene nanoparticles (NPs), have recently received increasing attention.1–3 Fullerene NPs are negatively charged and can be dispersed in water in the absence of any solubilizer. Fullerene NPs demonstrate promise within biological and medical applications, as radical scavengers and photosensitizers for photodynamic therapy. To further extend the applications of fullerene NPs, additional hybridization with desired functional molecules is required. Porphyrin and associated derivatives are highly promising candidates for hybridization with fullerenes to increase photoactivity.4 Numerous studies on the complexation of fullerenes with porphyrin molecules using synthetic organic chemistry5–7 or supramolecular chemistry8–10 have been reported. Although fullerene NPs have been intensively studied over the last decade, no reliable method to achieve the hybridization of porphyrins with fullerene NPs has been proposed.
Poly(ethylene glycol) monomethyl ether (PEG) was recently observed to accelerate the decomposition of fullerene C60–γ-CD complexes in water, which leads to the rapid aggregation of C60 to form water-dispersible C60 NPs.11 In this method, C60–γ-CD complexes can exist as stable isolated molecules in water, enabling the precise size control and step-wise growth of C60 NPs.12,13 Herein, the preparation of hybrid NPs comprising C60 and hydrophobic porphyrin molecules are reported. C60–γ-CD and porphyrin-trimethyl-β-cyclodextrin (por–TMe-β-CD) complexes are mixed in water in the presence of PEG. Both complexes decompose through the interaction of PEG with the CDs, leading to the formation of C60–porphyrin hybrid NPs (denoted as C60–por NPs). The C60–por NPs are negatively charged and easily disperse in water. Additionally, the ability of C60–por NPs to generate activated oxygen is also evaluated.
The C60–γ-CD complex14,15 and 1–TMe-β-CD complex (Fig. 1)16–18 were prepared according to a previously described procedure (see the ESI† for details). The 1H NMR spectrum of the mixed solution comprising the C60–γ-CD complex and PEG after 1 h incubation at 80 °C shows that the peaks attributed to the C60–γ-CD complexes completely disappeared (Fig. S1†). Hence, the 1H NMR data confirm the decomposition of the C60–γ-CD complexes and the formation of water-dispersible C60 NPs, as previously reported.11–13 The effect of PEG on 1–TMe-β-CD complexes was also investigated by 1H-NMR as shown in Fig. S2.† After incubating the mixed solution of 1–TMe-β-CD complex and PEG ([1] = 0.1 mM, [PEG] = 5.0 g L−1) for 1 h at room temperature, peaks attributed to the 1–TMe-β-CD complex were still evident at 4.97 ppm and above 7.7 ppm (Fig. S2(i)†). Hence, PEG has no influence upon the 1–TMe-β-CD structure at room temperature. Conversely, after incubating for 1 h at 80 °C, a dark purple precipitate formed and the aforementioned 1H NMR peaks completely disappeared (Fig. S2(ii)†). In the absence of PEG, the 1–TMe-β-CD complex was stable in water both at room temperature (Fig. S2(iii)†) and 80 °C (Fig. S2(iv)†). These results suggest a decomposition route of 1–TMe-β-CD by interaction with PEG at 80 °C, with a concomitant formation of non-dispersible large aggregates.
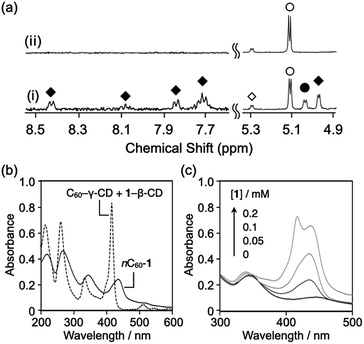
To obtain hybrid NPs comprising C60 and 1 (C60–1 NPs), PEG (Mw = 2000) was added to an aqueous solution containing C60–γ-CD and 1–TMe-β-CD complexes ([C60] = [1] = 0.1 mM, [PEG] = 5.0 g L−1), which were thereafter incubated at room temperature or 80 °C. The 1H NMR spectrum of the mixed solution at room temperature shows peaks attributed to γ-CD in the C60–γ-CD complex at 5.03 ppm, TMe-β-CD in the 1–TMe-β-CD complex at 4.97 ppm, and 1 in the 1–TMe-β-CD complex in the region of 7.6–8.5 ppm (Fig. 2a(i)). The data indicate that PEG fails to induce the decomposition of the C60–γ-CD and 1–TMe-β-CD complexes at room temperature. Conversely, after the mixture was heated at 80 °C for 1 h, the peaks attributed to the C60–γ-CD and 1–TMe-β-CD complexes completely disappeared (Fig. 2a(ii)). Hence, C60–γ-CD and 1–TMe-β-CD were decomposed at 80 °C, in the presence of PEG. The solution after being subjected to heat treatment at 80 °C for 1 h, was dark purple in the absence of any precipitate. The hydrodynamic diameter and ζ-potential of the reacted solution were 125 nm (polydispersity index = 0.21) and −20.2 mV, respectively. Water dispersible fullerene NPs typically exhibit negative ζ-potentials, the origin of which still requires elucidating.19,20 Hence, the formation of water-dispersible nano-composites, C60–1 NPs, is suggested.
The por–TMe-β-CD complexes using 2–6 (Fig. 1), were also prepared adopting the same procedure as that for the 1–TMe-β-CD complex. Each por–TMe-β-CD complex solution was mixed with C60–γ-CD and PEG ([C60] = [por] = 0.1 mM, [PEG] = 5.0 g L−1). The 1H NMR spectrum of each individual mixed solution after being incubated for 1 h at 80 °C, is shown in Fig. S3.† In the 1H NMR spectrum of the mixture comprising C60–γ-CD and 2–TMe-β-CD, the peaks attributed to these complexes at 5.03, 4.98, and 7.60–8.50 ppm, completely disappeared after being subjected to incubation for 1 h at 80 °C, without precipitation (Fig. S3a†). Similar changes in the 1H NMR spectrum of the mixture comprising C60–γ-CD and 3–TMe-β-CD complexes are observed, as shown in Fig. S3b.† The data suggest that the 2–TMe-β-CD and 3–TMe-β-CD complexes can be decomposed in a similar manner as the C60–γ-CD complexes, and imply the formation of C60–2 and C60–3 NPs.
The 1H NMR spectra of the mixed solutions comprising C60–γ-CD and 4, 5, or 6–TMe-β-CD complexes failed to show peaks attributed to the C60–γ-CD complex, and peaks associated with the respective por–TMe-β-CD complexes were observed after incubation for 1 h at 80 °C (Fig. S3c–e,† respectively). Hence, the data suggest that the C60–γ-CD complex decomposed in the presence of PEG, and the 4, 5, and 6–TMe-β-CD complexes were observed to be stable without decomposition at 80 °C. There have been reports suggesting the strong interaction of water-soluble tetraphenyl porphyrins with TMe-β-CDs.21,22 Polar substituents prompt the penetration of the polarized porphyrin rims into the TMe-β-CD cavity. Porphyrins 4–6 possess polar substituents, which are suggested to enable the formation of stable TMe-β-CD complexes. Furthermore, the size of the β-CD cavity is sufficiently narrow to prevent any strong interaction with PEG.23 Thus, PEG-induced decomposition of the 4, 5, and 6–TMe-β-CD complexes is not possible.
The absorption behavior of C60–1 NPs was investigated using ultraviolet-visible (UV/Vis) spectroscopy. In the UV/Vis spectra, the characteristic peak of solvated C60–γ-CD, at 333 nm shifted to 344 nm after heating at 80 °C for 1 h (Fig. 2b). An additional broad absorption at 400–550 nm is also apparent, which is characteristic of solid-state crystalline C60 and arises from the electronic interactions between adjacent C60 molecules.24,25 The characteristic peak of the solvated 1–TMe-β-CD complex at 415 nm, shifted to 432 nm, with induced broadening after being subjected to heat treatment at 80 °C for 1 h (Fig. 2b). In the absence of C60–γ-CD complexes, the characteristic absorption peak attributed to the solvated 1–TMe-β-CD complex completely disappeared after heating for 1 h at 80 °C, in the presence of PEG (Fig. S4†). The data show that 1, when expelled from the TMe-β-CD cavities, forms non-dispersible precipitates in the absence of C60. For 1 dispelled from the TMe-β-CD cavities to be stably dispersed in water, formation of co-aggregates with C60 may be a prerequisite. C60–2 and C60–3 NPs also show similar UV-Vis absorption spectra after being subjected to heating at 80 °C for 1 h, as shown in Fig. S5a and b,† respectively.
To further elucidate the composite formation of C60 and 1, the influence of 1 concentration on C60–1 NP formation was investigated by UV/Vis spectroscopy (Fig. 2c). The intensity of the absorption peak at 432 nm increased as a function of 1 concentration from 0.05 to 0.1 mM. Conversely, the absorption peak at 345 nm, derived from the formation of fullerene NPs, shifted to 338 nm, with increasing concentration of 1. This absorption peak derives from the fullerene nanoparticle size, and as the size decreased (i.e., the NPs became smaller), the peak blue-shifted.11 The results suggest that in the presence of 1, the fullerene interaction might be disturbed, or smaller C60 NPs might form. For C60–1 NPs formulated with 0.2 mM of 1 ([C60] = 0.1 mM, [1] = 0.2 mM), the absorption peak derived from the Soret band of 1 split into two peaks (Fig. 2c). The absorption peak at the shorter wavelength of 415 nm is consistent with that of the 1–TMe-β-CD complex. The absorption peak at the longer wavelength of 431 nm is almost consistent with the absorption peaks in the UV/Vis spectra of C60–1 NPs fabricated with 0.05 and 0.1 mM of 1. The findings indicate that in the sample comprising 0.2 mM of 1, a portion of the 1–TMe-β-CD complexes remained in the complex state after heating for 1 h at 80 °C, in the presence of PEG. The absorption peak at 338 nm, which reflects the state of fullerene NPs, is similar to that of C60–1 NPs fabricated with 0.1 mM of 1. When C60 and 1 form co-aggregated NPs, the ratio of 1 to C60 is thought to be limited to ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
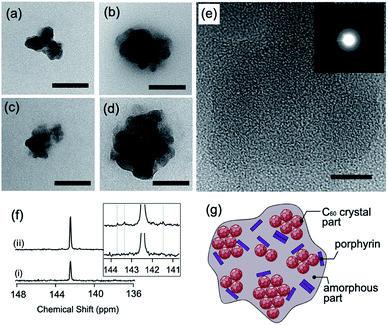
Morphological observations of the hybrid NPs were also undertaken. In the absence of the por–TMe-β-CD complex, C60 NPs possessing fairly monodisperse size distributions were observed (Fig. S6a†). The average diameter of the individual NPs, determined from the transmission electron microscopy (TEM) images, is 82 nm. C60 NPs have been previously reported to exhibit lattice fringes and diffraction patterns, which suggests that C60 NPs maintain the face-centered cubic (fcc) crystalline structure.11 C60–1 NPs prepared with 0.05 mM C60 and 0.1 mM 1, possessed irregular shapes (Fig. 3a and b, respectively). The average diameter of C60–1 NPs, determined by TEM, is 119 nm (Fig. 3a and S6b†), demonstrating the larger C60–1 NP size than that of the C60 NPs (82 nm). Increasing the concentration of 1 to 0.1 mM results in the average diameter of C60–1 NPs to increase to 131 nm (Fig. 3b and S6c†). Similar morphology is observed from the TEM micrographs of C60–2 and C60–3 NPs having average diameters of 109 nm and 144 nm, respectively (Fig. 3c and d, respectively). A lower PEG molecular weight or a lower reaction temperature during C60 NP formation via C60–γ-CD complexes have been reported to induce an increase in the diameter of the C60 NPs.11,12 Thus, the findings suggest that slower reaction conditions result in less nucleation and a larger NP formation. Porphyrin 3 possesses a methoxy substituent at the para position of the phenyl group and is more polar than 1 or 2. Previous reports have demonstrated that the higher the polarity of the phenyl group, the more stable the complex with TMe-β-CD,21,22 which indicates that 3–TMe-β-CD is more stable than 1– or 2–TMe-β-CD in water. Thus, the aforementioned decomposition, which results from the interaction with PEG, is slower in 3 with a concomitant increase in the NP size.
In the high-resolution TEM micrograph (Fig. 3e), the C60–1 NPs only exhibited partial lattice fringes, and hence did not show clear diffraction patterns compared with the C60 NPs (inset in Fig. 3e).11 The findings demonstrate the highly amorphous nature of the C60–1 NPs, and that the C60 crystal structure was only retained in part. 13C NMR spectra also provide important information about the structure of the C60–1 NPs. A characteristic C60 cluster signal at 142.4 ppm was detected in both C60 NPs and C60–1 NPs (Fig. 3f).26 The C60–1 NP dispersions also exhibited several small new signals at 141.5, 143.3, and 143.7 ppm, as shown in Fig. 3f(ii). When a fullerene and a porphyrin molecule form a stable complex in solution, the C60 signal shifts depending on the interaction type between the fullerene and the porphyrin molecule.27 Thus, the porphyrin molecule interacted with the aggregate of C60 in C60–1, as illustrated in Fig. 3g.
Some porphyrin molecules can form a co-crystal with fullerene C60.28 To form a crystal structure, not only the interaction between molecules but also the relationship with the solvent, such as gradually changing the polarity of the solvent or removing the solvent, are important. In our system, porphyrin molecules that are pseudo-dissolved by TMe-β-CDs are added to water, which is a poor solvent for porphyrins, through the interaction of PEG with TMe-β-CDs. The porphyrin molecule should immediately aggregate and have difficulty forming a crystal structure. Furthermore, because water is also a poor solvent for fullerene C60, C60 also immediately aggregates in water. Thus, it should be extremely difficult for C60 and porphyrin molecules to regularly associate to form a co-crystal structure.
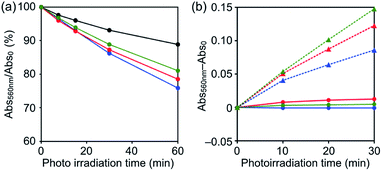
The concentration of singlet oxygen molecules (1O2, Type-II energy transfer pathway) generated by photoirradiation was measured according to a chemical method using 9,10-anthracenediyl-bis(methylene) dimalonic acid (ABDA)15,29 as a marker to determine the biological activities of C60 NPs, C60–1 NPs, C60–2 NPs, and C60–3 NPs. The absorption of ABDA at the absorption maximum (380 nm) was monitored as a function of irradiation time ([C60] = 0.1 mM, [por] = 0 or 0.1 mM). Under visible-light irradiation at wavelengths > 620 nm, C60–1 NPs, C60–2 NPs, and C60–3 NPs generated higher levels of 1O2 than C60 (Fig. 4a). These results show that the 1O2 photoproduction abilities of the C60–por NPs were higher than that of the C60 NPs. There are no significant differences in the 1O2 photoproduction abilities of C60–1 NPs, C60–2 NPs, and C60–3 NPs, which suggests that the structure of the porphyrin has an insignificant influence on the ability of the hybrid NPs. The generation of formazan, via the reduction of nitroblue tetrazolium (NBT) by oxygen radicals (O2˙−), is observed as an increase of absorption intensity at 560 nm.30 The reduction of NBT by O2˙− was scarcely detected in solutions containing C60–1 NPs, C60–2 NPs, and C60–3 NPs under photoirradiation, even though formazan was readily detected in the positive control sample in the presence of reduced nicotinamide adenine dinucleotide (NADH) (Fig. 4b). The results suggest that the reactive oxygen species produced by C60–1 NPs, C60–2 NPs, and C60–3 NPs are predominantly 1O2 generated by a Type-II reaction.18
In summary, the preparation of hybrid C60–porphyrin NPs was achieved via a guest exchange reaction comprising porphyrin CD complexes and C60. Seven C60–por NP derivatives with various moieties were prepared. CD porphyrin complexes possessing phenyl and methoxyphenyl moieties were decomposed in the presence of PEG at the same time as C60–γ-CD complexes and formed NPs with C60. Porphyrins containing a hydrophilic moiety form stable complexes with TMe-β-CD and fail to co-aggregate with C60. The C60–por NPs are negatively charged and are easily dispersed and stable in water. The 1O2 generation ability of C60–por NPs under photoirradiation (>620 nm) is greater than that of C60 NPs. The findings herein demonstrate a new method to fabricate fullerene–porphyrin composite materials, which provides a route to highly functional fullerene-based materials.
Conflicts of interest
The authors declare no conflicts of interests.Acknowledgements
This work was supported by the Japan Society for the Promotion of Science, KAKENHI (Grant No. JP16H04133 and JP19K15523).Notes and references
- G. V. Andrievsky, V. K. Klochkov, A. B. Bordyuh and G. I. Dovbeshko, Chem. Phys. Lett., 2002, 364, 8–17 CrossRef CAS.
- K. J. Moor, S. D. Snow and J. H. Kim, Environ. Sci. Technol., 2015, 49, 5990–5998 CrossRef CAS.
- N. O. McHedlov-Petrossyan, Chem. Rev., 2013, 113, 5149–5193 CrossRef CAS.
- D. M. Guldi, Chem. Soc. Rev., 2002, 31, 22–36 RSC.
- H. Imahori, K. Tamaki, D. M. Guldi, C. P. Luo, M. Fujitsuka, O. Ito, Y. Sakata and S. Fukuzumi, J. Am. Chem. Soc., 2001, 123, 2607–2617 CrossRef CAS.
- C. O. Obondi, G. N. Lim and F. D'Souza, J. Phys. Chem. C, 2015, 119, 176–185 CrossRef CAS.
- S. M. Rezayat, S. V. S. Boushehri, B. Salmanian, A. H. Omidvari, S. Tarighat, S. Esmaeili, S. Sarkar, N. Amirshahi, R. N. Alyautdin, M. A. Orlova, I. V. Trushkov, A. L. Buchachenko, K. C. Liu and D. A. Kuznetsov, Eur. J. Med. Chem., 2009, 44, 1554–1569 CrossRef CAS.
- M. Ayabe, A. Ikeda, Y. Kubo, M. Takeuchi and S. Shinkai, Angew. Chem., Int. Ed., 2002, 41, 2790–2792 CrossRef CAS.
- P. D. W. Boyd and C. A. Reed, Acc. Chem. Res., 2005, 38, 235–242 CrossRef CAS.
- F. D'Souza and O. Ito, Coord. Chem. Rev., 2005, 249, 1410–1422 CrossRef.
- K. Sugikawa, K. Kozawa, M. Ueda and A. Ikeda, RSC Adv., 2016, 6, 74696–74699 RSC.
- K. Sugikawa, K. Kozawa, M. Ueda and A. Ikeda, Chem.–Eur. J., 2017, 23, 13704–13710 CrossRef CAS.
- K. Sugikawa, Y. Inoue, K. Kozawa and A. Ikeda, ChemNanoMat, 2018, 4, 682–687 CrossRef CAS.
- K. Sugikawa, A. Kubo and A. Ikeda, Chem. Lett., 2016, 45, 60–62 CrossRef CAS.
- Y. Doi, A. Ikeda, M. Akiyama, M. Nagano, T. Shigematsu, T. Ogawa, T. Takeya and T. Nagasaki, Chem.–Eur. J., 2008, 14, 8892–8897 CrossRef CAS.
- A. Ikeda, S. Hino, T. Mae, Y. Tsuchiya, K. Sugikawa, M. Tsukamoto, K. Yasuhara, H. Shigeto, H. Funabashi, A. Kuroda and M. Akiyama, RSC Adv., 2015, 5, 105279–105287 RSC.
- Y. Tsuchiya, T. Shiraki, T. Matsumoto, K. Sugikawa, K. Sada, A. Yamano and S. Shinkai, Chem.–Eur. J., 2012, 18, 456–465 CrossRef CAS.
- T. Nakaya, B. Horiguchi, S. Hino, K. Sugikawa, H. Funabashi, A. Kuroda and A. Ikeda, Photochem. Photobiol. Sci., 2019, 18, 459–466 RSC.
- R. G. Alargova, S. Deguchi and K. Tsujii, J. Am. Chem. Soc., 2001, 123, 10460–10467 CrossRef CAS.
- J. Brant, H. Lecoanet, M. Hotze and M. Wiesner, Environ. Sci. Technol., 2005, 39, 6343–6351 CrossRef CAS.
- K. Kano, R. Nishiyabu, T. Asada and Y. Kuroda, J. Am. Chem. Soc., 2002, 124, 9937–9944 CrossRef CAS.
- T. CaroFiglio, R. Fornasier, V. Lucchini, C. Rosso and U. Tonellato, Tetrahedron Lett., 1996, 37, 8019–8022 CrossRef CAS.
- A. Harada, Coord. Chem. Rev., 1996, 148, 115–133 CrossRef CAS.
- Y. Ishibashi, M. Arinishi, T. Katayama, H. Miyasaka and T. Asahi, Chem. Lett., 2012, 41, 1104–1106 CrossRef CAS.
- X. Chang and P. J. Vikesland, Environ. Sci. Technol., 2011, 45, 9967–9974 CrossRef CAS.
- C. S. Yannoni, R. D. Johnson, G. Meijer, D. S. Bethune and J. R. Salem, J. Phys. Chem., 1991, 95, 9–10 CrossRef CAS.
- D. Y. Sun, F. S. Tham, C. A. Reed, L. Chaker and P. D. W. Boyd, J. Am. Chem. Soc., 2002, 124, 6604–6612 CrossRef CAS.
- P. D. W. Boyd, M. C. Hodgson, C. E. F. Rickard, A. G. Oliver, L. Chaker, P. J. Brothers, R. D. Bolskar, F. S. Tham and C. A. Reed, J. Am. Chem. Soc., 1999, 121, 10487–10495 CrossRef CAS.
- B. A. Lindig, M. A. J. Rodgers and A. P. Schaap, J. Am. Chem. Soc., 1980, 102, 5590–5593 CrossRef CAS.
- I. Nakanishi, S. Fukuzumi, T. Konishi, K. Ohkubo, M. Fujitsuka, O. Ito and N. Miyata, J. Phys. Chem. B, 2002, 106, 2372–2380 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and Fig. S1–S6. See DOI: 10.1039/d0ra09387d |
| This journal is © The Royal Society of Chemistry 2021 |