Open Access Article
Open Access ArticleRecent advancement in the synthesis of diverse spiro-indeno[1,2-b]quinoxalines: a review
Ruby Singh *,
Diksha Bhardwaj and
Munna Ram Saini
*,
Diksha Bhardwaj and
Munna Ram Saini
Department of Chemistry, School of Basic Sciences, Jaipur National University, Jaipur, Rajasthan, India. E-mail: drrubychem@yahoo.com
First published on 25th January 2021
Abstract
The nitrogen-containing indeno[1,2-b]quinoxaline ring is a privileged structurally fused active system and has notable applications in various fields of chemistry. For the past several years, it has been recognized as an important building block in organic synthesis. This review summarizes the reactions of indeno[1,2-b]quinoxalinone as a key construction block for producing a variety of indeno[1,2-b]quinoxaline-based spiro-heterocyclic frameworks by means of a wide range of chemical reactions. Most of the reactions described here are multicomponent ones with readily available starting materials that produce complex molecular spiro architectures. Some of the synthesized spiro-indenoquinoxalines exhibit interesting biological activities and have promise for the generation of new drug candidates. Spiro compounds in which two cyclic rings are fused at a common carbon atom are promising interesting skeletal systems in drug discovery due to their unique conformational features, structural complexity and rigidity. The present review aims to highlight the reactions, synthetic strategies and pharmaceutical applications of diverse spiro-indeno[1,2-b]quinoxalines to date.
1. Introduction
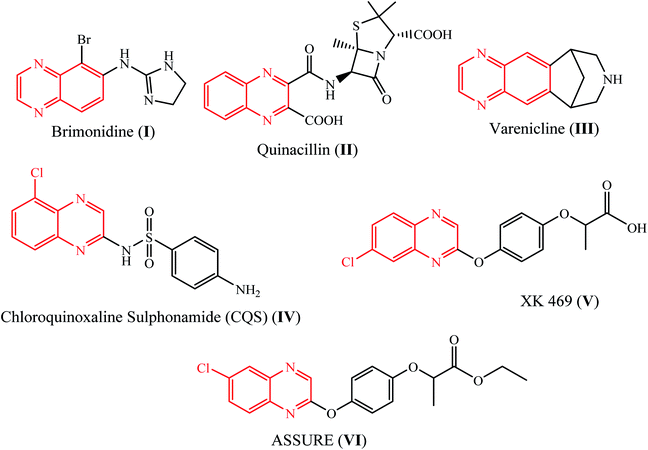
Nitrogen-containing heterocyclic compounds occupy a prominent place among organic compounds due to their key role in drug discovery and significant impact on the pharmaceutical industry. The quinoxaline skeleton is a central structure of drugs including brimonidine (I), quinacillin (II), and varenicline (III), which are well known as therapeutic treatments for glaucoma and to aid in the cessation of smoking (Fig. 1).1 The quinoxaline drugs CQS (chloroquinoxaline sulfonamide) (IV) and XK469 (V) have been observed to have anticancer activity against solid tumors. (2-Quinoxalinyloxy)phenoxypropanoic acid derivatives, such as Assure (VI), are well known to act as herbicides (Fig. 1).2 The quinoxaline structural motif is also found in several natural products and antibiotics such as echinomycin, leromycin, and actinomycin,3 which are known to inhibit the growth of Gram-positive bacteria. Compounds with a quinoxaline core are used as allosteric dual Akt1 and Akt2 inhibitors,4 human cytomegalovirus polymerase inhibitors,5 Src-family kinase p56Lck inhibitors,6 SRPK-1 inhibitors,7 and monoamine oxidase A inhibitors.8Indeno[1,2-b]quinoxalinones derived by the reaction of ninhydrin and substituted 1,2-phenylenediamines have potential pharmaceutical applications with anti-cancer,9 c-Jun N-terminal kinase inhibitory,10 anti-inflammatory, antinociceptive,11 antiproliferative,12 etc., activities. Additionally, indeno[1,2-b]quinoxalines show potential as acid corrosion inhibitors for mild steel surfaces.13
Spiro compounds have received special attention in medicinal chemistry because the presence of spiro carbon provides rigidity to the structure, and their ability to elaborate along well defined vectors.14,15 Indeno[1,2-b]quinoxalinone is a heterocyclic ketone used as privileged scaffold in construction of various spiro polycyclic frameworks like spiro-pyran, spiro-pyrrolidine/pyrrolizidine, spiro-β-lactam, spiro-indolizine, and spiro-furan derivatives with pharmaceutical importance. A literature survey reveals no published review article on the synthesis of spiro-indenoquinoxalines and this review therefore gives an overview on the synthesis of spiro-indenoquinoxalines constructed to date.
2. Synthesis of indeno[1,2-b]quinoxalinone
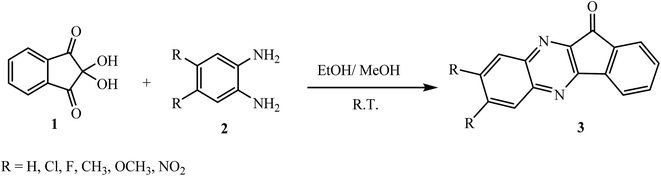
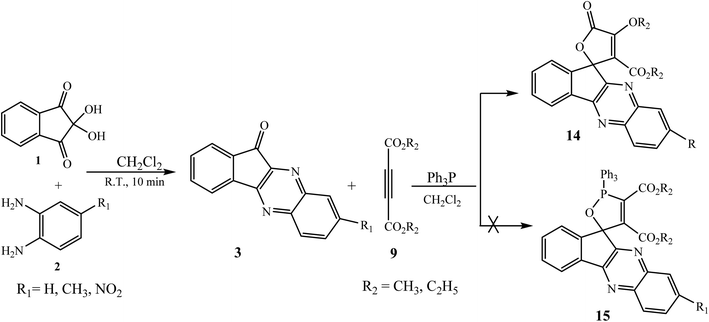
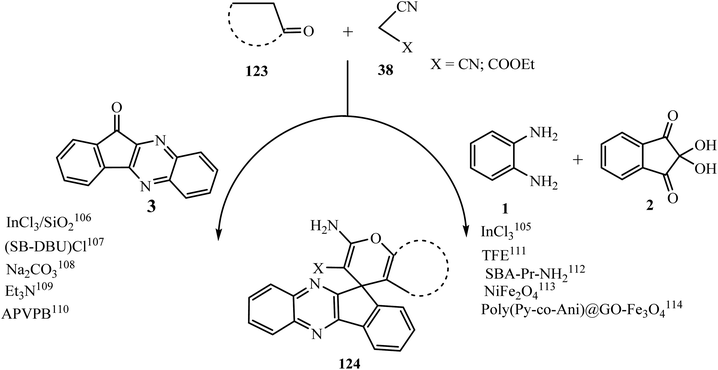
The most widely used method for the synthesis of indeno[1,2-b]quinoxalinone 3 involves the condensation reaction of ninhydrin 1 with 1,2-phenylenediamines 2 in EtOH/MeOH at room temperature.16 Under similar conditions, various substituted indeno[1,2-b]quinoxalinones 3 were prepared from the corresponding substituted 1,2-phenylenediamines in good yields (Scheme 1). This one-step route has emerged as a valuable method due to its mild reaction conditions, high atom economy, and non-toxicity as a convenient approach towards green chemistry.3. Generation of a three-membered ring on the indeno[1,2-b]quinoxaline moiety
3.1. Synthesis of [indeno[1,2-b]quinoxalin-11,1′-cyclopropane]
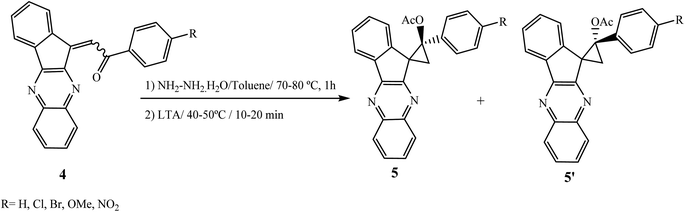
Cyclopropane ring-containing compounds are structural parts of various synthetic and naturally occurring compounds and exhibit anti-viral, anti-tumor, antibiotic and other potent activities.17–20 Shaabanzadeh et al.21 have reported the synthesis of two diastereoisomers of a new spiro-indenoquinoxaline system 2′-acetyloxy-2′-phenylspiro[indeno[1,2-b]quinoxalin-11,1′-cyclopropane] 5 and 5′ in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio from the reaction of chalcones of indeno[1,2-b]quinoxaline 4 with hydrazine hydrate in the presence of solid lead(IV) tetraacetate (LTA) at 85 °C (Scheme 2). Chalcones have previously been prepared by the reaction of indenoquinoxalinone 3 with acetophenones in the presence of dimethylamine as a base catalyst in acetic acid and HCl.22
1 ratio from the reaction of chalcones of indeno[1,2-b]quinoxaline 4 with hydrazine hydrate in the presence of solid lead(IV) tetraacetate (LTA) at 85 °C (Scheme 2). Chalcones have previously been prepared by the reaction of indenoquinoxalinone 3 with acetophenones in the presence of dimethylamine as a base catalyst in acetic acid and HCl.22
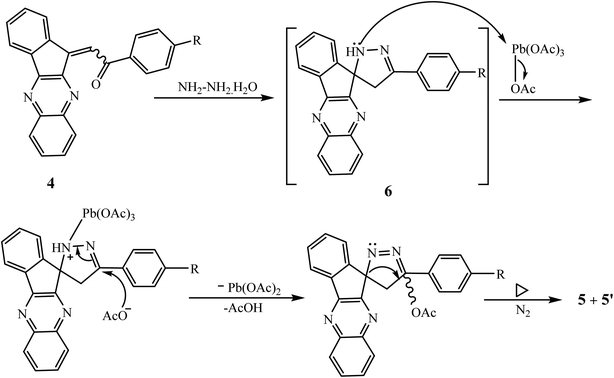
A plausible mechanism for the synthesis of the presented spiro system involves the intermediate spiro-indenoquinoxaline-pyrazoline 6 formed in situ by the reaction of 4 and hydrazine hydrate, which subsequently react with LTA to afford the desired product 2′-acetyloxy-2′-phenylspiro[indeno[1,2-b]quinoxalin-11,1′-cyclopropane] 5 and its diastereoisomer 5′ (Scheme 3).
Later on, authors also accomplished this reaction in another solvent, ortho-xylene, and observed the same diastereoselective ratio of 5 and 5′.23 Their chemical structures were fully optimized at the B3LYP/6-311+G(d,p) level of theory using the Gaussian 03W program package. Previously, the same research group also reported the synthesis of spiro-indenoquinoxaline-pyrazolines 6, separately by the reaction of chalcones 4 and hydrazine hydrate.24
4. Generation of a four-membered ring on indeno[1,2-b]quinoxaline
4.1. Synthesis of spiro[azetidine-2,11′-indeno[1,2-b]quinoxalin]-4-one
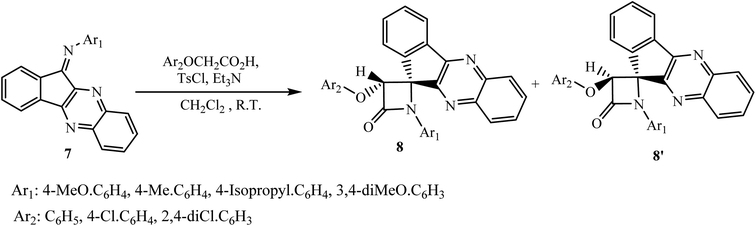
β-Lactam ring is a very important structural unit present in important antibiotics like penicillins, cephalosporins and carbapenems.25 Jarrahpour and coworkers26 have designed and finished the synthesis of novel spiro-β-lactams bearing indeno[1,2-b]quinoxaline hybrid system 8 via a modified Staudinger reaction in a short reaction time with high yields. The construction of spiro-β-lactam system 8 involves the [2 + 2] cycloaddition reaction between N-phenyl-11H-indeno[1,2-b]quinoxalin-11-imine derivatives 7 and various phenoxyacetic acid derivatives in the presence of triethylamine and p-toluenesulfonyl chloride (TsCl) at room temperature using CH2Cl2 as a solvent. The resultant spiro-β-lactams have been obtained in two diastereomeric forms 8 and 8′ in equal amount. Further enhancement of the diastereoselectivity of the reaction was studied, in anhydrous CH2Cl2 at low temperature, −10 or −83 °C, or in toluene at various temperatures but satisfactory results were not observed (Scheme 4).5. Generation of a five-membered ring on indeno[1,2-b]quinoxaline
5.1. Synthesis of spiro-furan indenoquinoxaline derivatives
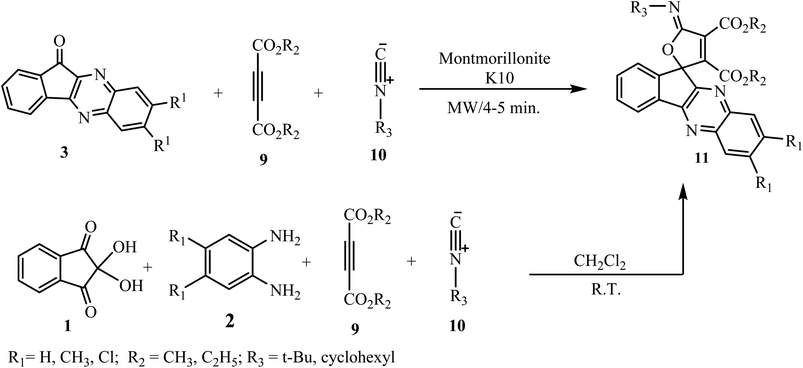
γ-Spirolactones are the subject of great attention because of their consequence as aldestrone inhibitors.27 By considering this fact, various types of γ-spirolactones of pharmacological interest have been derived by researchers. Azizian and co-workers28 have reported the synthesis of γ-spiroiminolactones 11 via a three-component condensation of indenoquinoxalin-11-ones 3, dialkylacetylenedicarboxylates 9 and isocyanides 10 under microwave irradiation using montmorillonite KSF as a solid support in a shorter reaction time and in good yields in comparison to conventional synthesis (Scheme 5).Later on, Mahdavinia and co-workers29 modified the method by reducing the steps and they synthesized spirofuran-indenoquinoxaline 11 via four-component reaction of ninhydrin 1, benzene-1,2-diammine 2, tert-butyl isocyanide 10, and dialkylacetylenedicarboxylates 9 in dry CH2Cl2 at room temperature in 8 h. In this process the intermediate indenoquinoxalinones 3 were generated in situ from the reaction of ninhydrin 1 and benzene-1,2-diamines 2 (Scheme 5).
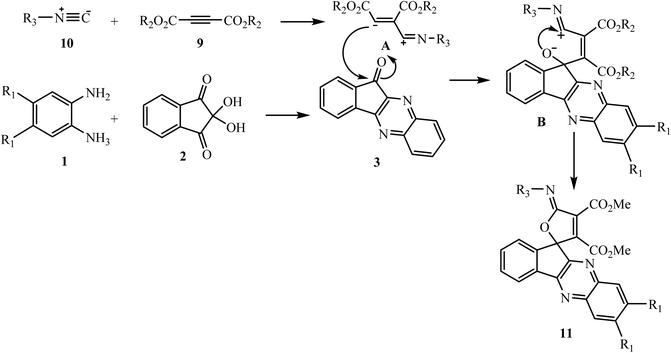
In the cited report the authors discussed the detailed mechanism for formation of adduct 11. Initially, the formation of a zwitterionic intermediate A occurs by the reaction of isocyanide 10 and acetylenedicarboxylate 9, which further attacks the carbonyl group of indenoquinoxalinone 3 (formed by interaction between ninhydrin 1 and benzene-1,2-diamine 2) to form a dipole intermediate C, and after that cyclization leads to spirocyclic adduct (Scheme 6).
The structure and arrangement of functional groups in spiro systems can be fully characterized by single-crystal X-ray analysis. Various isocyanides 10 and acetylenedicarboxylates 9 have been condensed with different substituted o-phenylenediamine 1 and ninhydrin 2 to afford the corresponding desired spirofuran-indenoquinoxaline derivatives 11 in excellent yields.
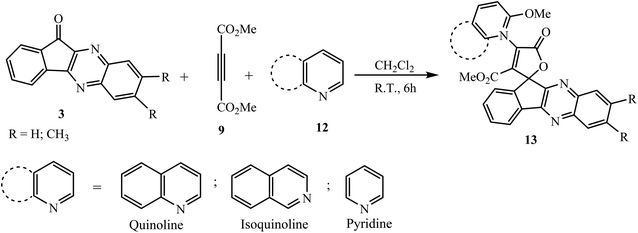
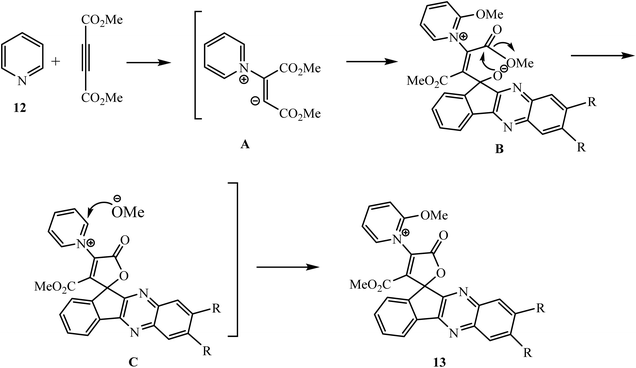
Mosslemin and co-workers have developed a new and efficient protocol for the present four-component reaction using imidazolium-based ionic liquid [bmin]BF4 as a catalyst and solvent at ambient temperature.30 Maghsoodlou et al.31 demonstrated a convenient, efficient, one-pot approach for the synthesis of N-heterocycle-substituted spiroindenoquinoxaline-lactones 13 via the three-component reaction of 11H-indeno[1,2-b]quinoxalin-11-one 3, DMAD 9 and N-heterocyclic compounds 12 such as pyridine, quinoline and isoquinoline using CH2Cl2 as a solvent (Scheme 7).
The mechanism involves the generation of 1,3-dipolar intermediate A via the reaction of DMAD and pyridine 12, which further react with the carbonyl carbon of indeno[1,2-b]quinoxalin-11-one 3 by nucleophilic attack, and subsequent cyclization and attack of the methoxy anion generate the spirolactone 13 (Scheme 8).
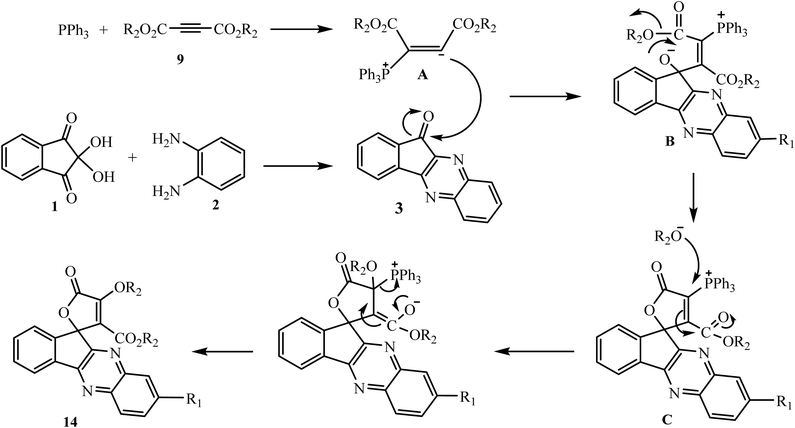
In ongoing research in this area, Maghsoodlou et al. have investigated a domino reaction between ninhydrin 1, benzene-1,2-diammine 2, dialkylethynedicarboxylates 9 and triphenylphosphine (Ph3P) to synthesize the expected desired product spiro-1,2-oxaphosphorus 15. However, the results showed the successful formation of unexpected product spirofuran-indenoquinoxaline derivatives 14 instead of the formation of 15 (Scheme 9).32
In this reaction Ph3P acts as catalyst, and initially 1,3-dipolar intermediate A is formed by the interaction of dialkylethynedicarboxylates 9 with Ph3P and subsequently A attacks the carbonyl carbon of 3 leading to the formation of zwitterionic intermediate B, which on further cyclization gives intermediate C and is then converted into desired spirocyclic products 14 in excellent yields (Scheme 10).
The one-pot domino strategy has several advantages, such as mild and neutral conditions, high atom economy with excellent yield, good functional group tolerance, and no need for chromatographic purification.
5.2. Synthesis of spiro-pyrrolidine/pyrrolizine/pyrrolothiazole-indenoquinoxalines
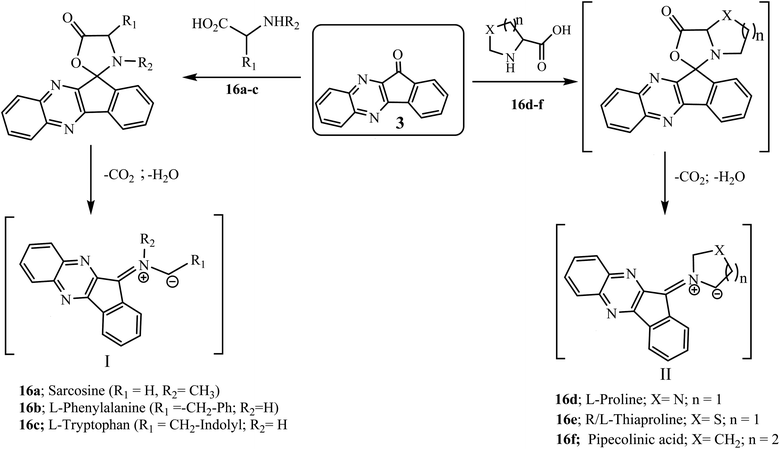
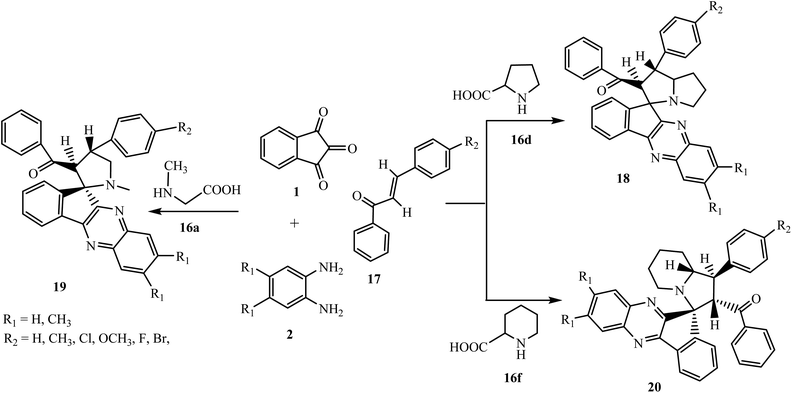
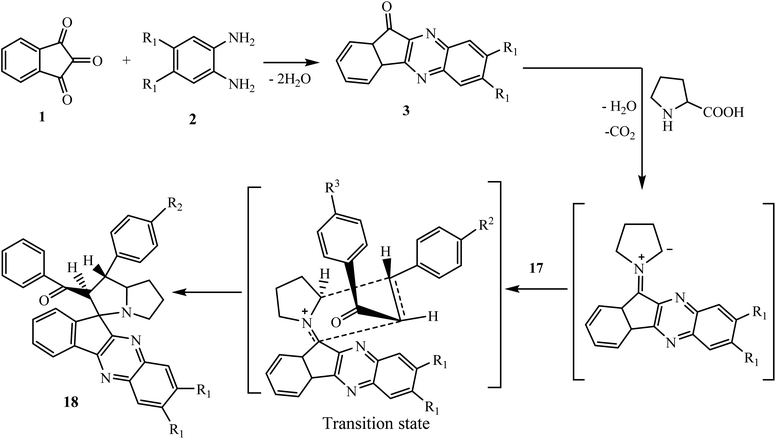
The 1,3-dipolar cycloaddition reaction has been described as the single most important method for the construction of heterocyclic five-member ring compounds in organic chemistry.33,34 The reaction of azomethine ylides with dipolarophiles offers an effective approach for the construction of mono- and di-spiropyrrolidine, pyrrolizidine and pyrrolothiazole derivatives that are present in many biologically active compounds.35–38 Azomethine ylides are an important and well documented class of 1,3-dipoles generated in situ from the decarboxylative condensation of carbonyl compounds with α-amino acids. The subsequent addition of azomethine ylides with appropriate dipolarophiles leads to the construction of these significant bioactive spiro compounds. Various azomethine ylide type I and II in a [3 + 2] cycloaddition reaction can be generated in situ from the decarboxylative condensation of carbonyl compounds, i.e., indeno[1,2-b]quinoxalin-11-one 3, with α-amino acids such as sarcosine 16a, L-phenylalanine 16b, L-tryptophan 16c, L-proline 16d, R/L-thiaproline 16e, and pipecolinic acid 16f (Scheme 11).5.2.1.1. Using α,β-unsaturated carbonyl compounds as a dipolarophile. Mohammadizadeh and co-workers39 investigated a four-component 1,3-dipolar reaction of ninhydrin 1, 1,2-phenylenediamine 2, substituted chalcones 17 and α-amino acid L-proline 16d in ethanol to fabricate the novel spiro[indeno[1,2-b]quinoxaline-11,3′-pyrrolizine] scaffold 18 stereoselectively (Scheme 12). After that Jadidi and co-workers expanded the work and reported the synthesis of new cycloadducts spiro[indeno[1,2-b]quinoxaline-11,2′-pyrrolidines] 19 and spiroindenoquinoxaline-indolizidines 20 using α-amino acid sarcosine 16a40 and pipecolinic acid 16f,41 respectively, in place of L-proline 16d.
A reasonable mechanism for the construction of spiro[indeno[1,2-b]quinoxaline-11,3′-pyrrolizine] 18 is shown in Scheme 13. The mechanism involves the in situ generation of intermediate indenoquinoxalinone 3, which subsequently reacts with L-proline 16d to produce 1,3-dipolar azomethine ylide type II. Finally, azomethine ylide interacts with the most electrophilic β-carbon of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of dipolarophile chalcone 17 selectively from the less hindered side to afford endo-cycloadduct 18 in good yields with excellent regio- and diastereoselectivity without the formation of other possible diastereoisomers. Similarly, the azomethine ylide generated in situ from the reaction of sarcosine 16a or pipecolinic acid 16f with indenoquinoxalinone 3 reacts with chalcone 17 to afford single isomers of spiro[indeno[1,2-b]quinoxaline-11,2′-pyrrolidines] 19 and spiroindenoquinoxaline-indolizidine 20, respectively.
C bond of dipolarophile chalcone 17 selectively from the less hindered side to afford endo-cycloadduct 18 in good yields with excellent regio- and diastereoselectivity without the formation of other possible diastereoisomers. Similarly, the azomethine ylide generated in situ from the reaction of sarcosine 16a or pipecolinic acid 16f with indenoquinoxalinone 3 reacts with chalcone 17 to afford single isomers of spiro[indeno[1,2-b]quinoxaline-11,2′-pyrrolidines] 19 and spiroindenoquinoxaline-indolizidine 20, respectively.
 | ||
| Scheme 13 Regio- and diastereoselective route for synthesis of spiro[indeno[1,2-b]quinoxaline-11,3′-pyrrolizine] 18. | ||
Sosnovskikh and co-workers42 have accomplished the synthesis of new derivatives of spiropyrrolizidines 18 and spiropyrrolidine-indenoquinoxalines 19 in an endo- and diastereoselective manner using arylideneacetones as a dipolarophile.
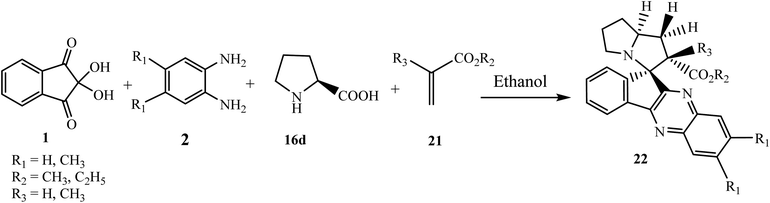
Mohammadizadeh et al.43 reported the synthesis of novel alkylspiro[indeno[1,2-b]quinoxaline-11,3′-pyrrolizine]-2′-carboxylates 22 using acrylic acid 21 as a dipolarophile via the four-component reaction of ninhydrin 1, o-phenylenediamine 2, L-proline 16d and acrylic acid 21 in ethanol at reflux temperature (Scheme 14). The stereochemistry of the product was confirmed by 1H nuclear Overhauser effect spectroscopy (NOESY).
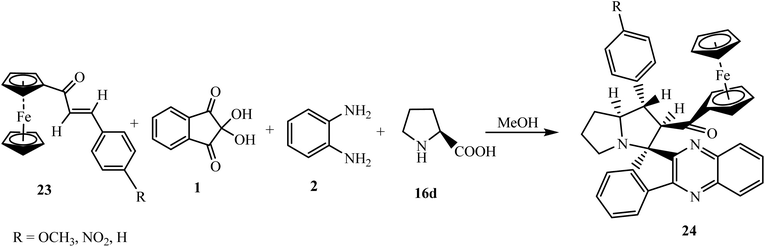
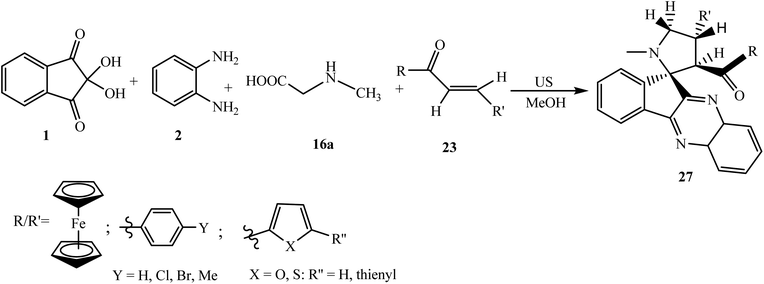
5.2.1.2. Using ferrocene grafted α,β-unsaturated carbonyls as a dipolarophile. Ferrocene derivatives have attracted worldwide attention over other organometallics due to their sandwich structure, synthetic versatility, thermal and photochemical stability, and biological activity.44 Hence, there has been a renewed interest in the synthesis of ferrocene-based heterocyclic frameworks in organic chemistry. Raghunathan et al.45 described the synthesis of highly functionalized and ferrocene grafted spiroindenoquinoxaline-pyrrolizidines 24 via the one-pot four-component reaction of ninhydrin 1, 1,2-phenylenediamine 2, and L-proline 16d with various ferrocene-derived chalcone dipolarophiles 23 using methanol as a reaction medium (Scheme 15).
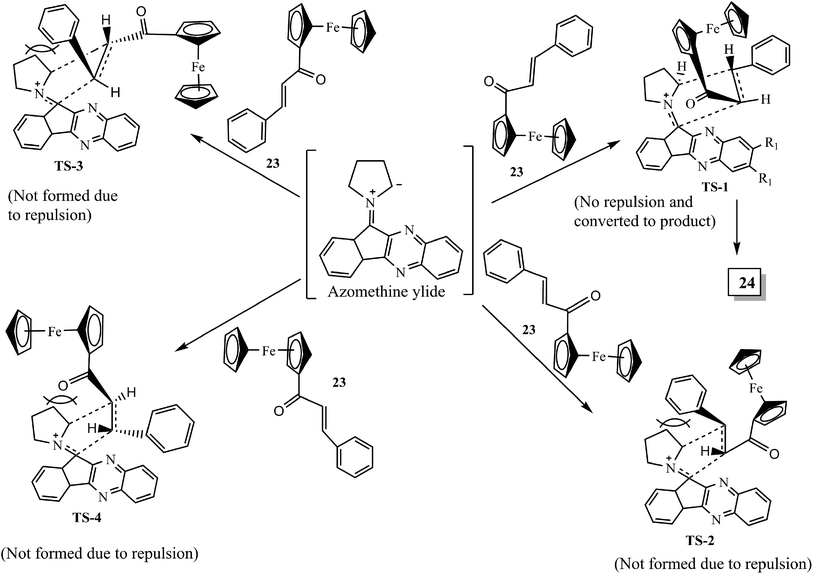
A plausible mechanism for the formation of ferrocene grafted spiroindenoquinoxaline-pyrrolizidines 24 is shown in Scheme 16. The mechanism involves the in situ formation of azomethine ylide, which subsequently interacts with ferrocene-derived chalcones 23 leading to the formation of product 24. The authors demonstrated all of the possible transition states TS-1 to TS-4 that can be formed during the interaction of dipolarophile 23 and azomethine ylide II regio- and stereochemically. However, due to the lower steric hindrance in exo-TS-1, the reaction proceeds via exo-TS-1 leading to the formation of single isomer spirocycloadduct 24 with regio- and diastereoselectivity. The stereochemical assignments of cycloadducts have been confirmed by nuclear Overhauser effect (NOE) studies and single-crystal X-ray analysis.
To further expand the work, 1-ferrocenyl-3-furylprop-2-ene-1-one/1-ferrocenyl-3-thienylprop-2-ene-1-one/1-ferrocenyl-3-pyridylprop-2-ene-1-one and 1,3-diferrocenylprop-2-ene-1-one 25 as dipolarophiles have also been used to synthesize the corresponding spiroindenoquinoxaline-pyrrolizidines 26 (Scheme 17).
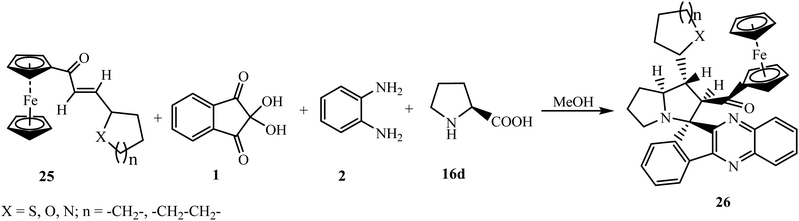 | ||
| Scheme 17 Synthesis of heterocyclic ring-containing ferrocene-grafted spiroindenoquinoxaline-pyrrolizidines 26. | ||
Later on in 2013, Raghunathan et al.46 expanded the scope of the reaction by employing sarcosine 16a in place of L-proline 16d to build highly substituted novel ferrocene grafted spiroindenoquinoxaline-pyrrolidines 27 of biological significance under ultrasound irradiation in shorter time and with better yield compared to the conventional method (Scheme 18). Two sets of single-crystal X-ray data of spiroindenoquinoxaline-pyrrolidines 27 have also been reported.47,48 The observed regio- and diastereoselectivity was found to be similar to that of the spiro adduct 24.
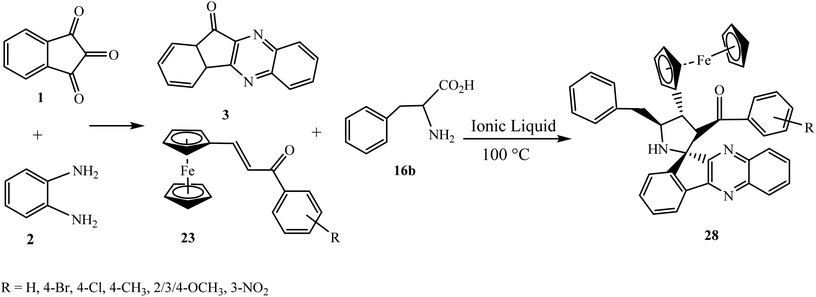
Recently, Arumugam et al.49 fabricated a new spiropyrrolidine grafted ferrocene heterocycle 28 by the four-component reaction of ninhydrin 1, o-phenylenediamine 2, L-phenylalanine 16b and ferrocenyl chalcones 23 utilizing an ionic liquid, 1-butyl-3-methylimidazoliumbromide, affording excellent yields.
The in situ generated azomethine ylide type I derived from a combination of ninhydrin 1 and o-phenylenediamine 2 along with L-phenylalanine 16b reacts efficiently with ferrocenyl chalcone 23 in [bmim]Br affording spiro cycloadduct 28, regioselectively. The ionic liquid [bmim]Br plays an important role in accelerating this cycloaddition reaction sequence in a sustainable fashion (Scheme 19).
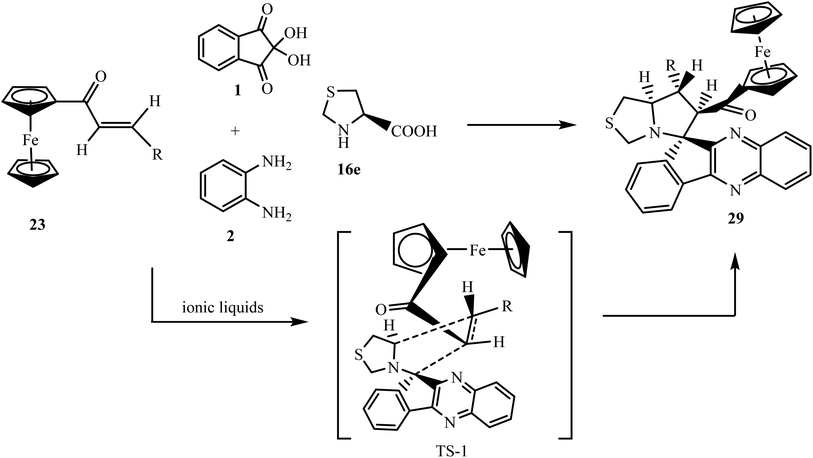
Raghunathan and coworkers50 have introduced a new amino acid, (R)-thiazolidine-4-carboxylic acid 16e, to accomplish new ferrocene grafted spiro-indenoquinoxaline-pyrrolo[1,2-c]thiazole 29 using a new ionic liquid, N-(1-acroloyl)-N-(4-cyclopentyl)piperazinium dihydrogen phosphate, as a reaction medium and accelerator. Azomethine ylide type II generated in situ from the condensation of indenoquinoxline-11-one 3 and (R)-thiazolidine-4-carboxylic acid 16e interacts with ferrocene derived dipolarophile 23 via endo-transition state TS-1 to exclusively form spiro adduct 29. The endo-transition state TS-1 minimizes the steric interaction between the phenyl ring of dipolarophile 23 and CH2 of the thiazolidine ring proximate to the quinoxaline ring of azomethine ylide (Scheme 20).
5.2.1.3. Using nitrostyrenes as a dipolarophile. A literature survey reveals that azomethine ylides type I derived from open-ring α-amino acids like sarcosine 16a and cyclic carbonyl compounds usually react with their least substituted terminal atom interacting with the most electrophilic carbon of electron-deficient alkenes regioselectively (Fig. 2a). In the case of cyclic azomethine ylides type II, where the nitrogen atom is a part of pyrrolidine/thiazolidene or piperidine ring, related regioselectivity of the reaction was observed for dipolarophiles such as acrylates, chalcones, and CX3-nitroalkenes (Fig. 2b).
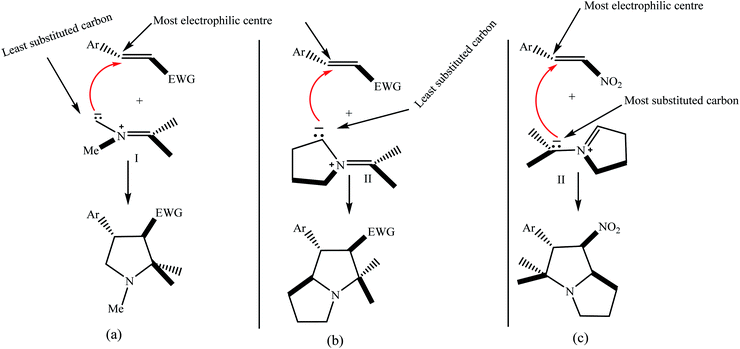 | ||
| Fig. 2 (a–c) Representations of the regioselective attack of azomethine ylide types I and II with dipolarophiles. | ||
However, in the case of dipolarophiles trans-β-nitrostyrenes, the practical regioselectivity was dissimilar and the most electrophilic carbon of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of the dipolarophile attached to the more substituted end of the 1,3-dipole (Fig. 2c).
C bond of the dipolarophile attached to the more substituted end of the 1,3-dipole (Fig. 2c).
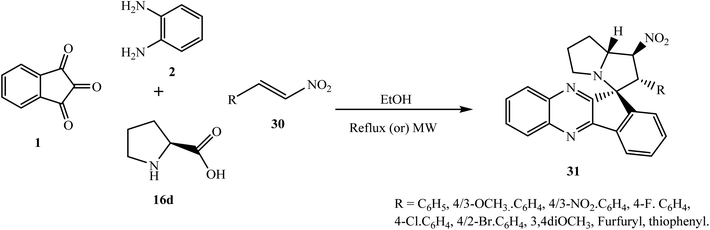
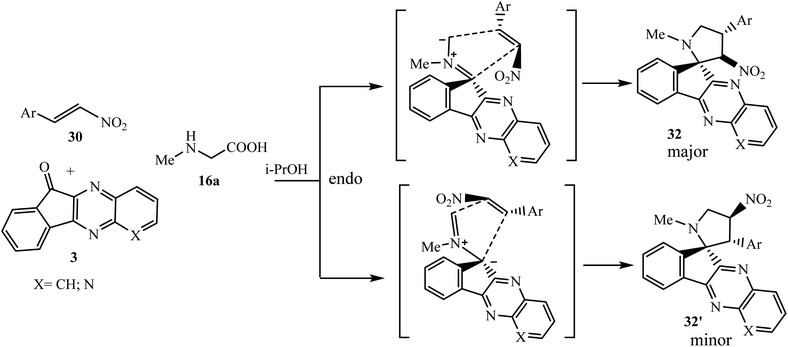
Due to the unique and unpredictable reactivity of β-nitrostyrenes, Trivedi et al.51 have investigated a four-component [3 + 2] cycloaddition reaction of ninhydrin 1, o-phenylenediamine 2, L-proline 16d and trans-β-nitrostyrenes 30 under microwave irradiation and classical conditions to synthesize densely functionalized heterocyclic scaffold spiro[indeno[1,2-b]quinoxaline-11,3′-pyrrolizine] 31 regio- and diastereoselectively (Scheme 21).
Mechanistically, reaction of 1,3-dipolar azomethine ylide generated from indenoquinoxalinone 3 and L-proline 16d with dipolarophiles β-nitrostyrenes 30 can proceed via two paths A and B regioselectively, to afford two regioisomers 31 and 31′. In the present case, the reaction proceeded via an unusual path and the more substituted electron-rich carbon of azomethine ylide type II attacks exclusively the most electrophilic center of the dipolarophile leading to the formation of only single regioisomer 31 through path A. Furthermore, this reaction is also diastereoselective, and in path A the addition of azomethine ylide with β-nitrostyrene 30 can proceed in two ways (i) exo and (ii) endo with respect to NO2 group, but due to less hindrance and thermodynamic stability, only single endo product 31 was formed and no trace of exo-isomer 31′′ was detected (Scheme 22).
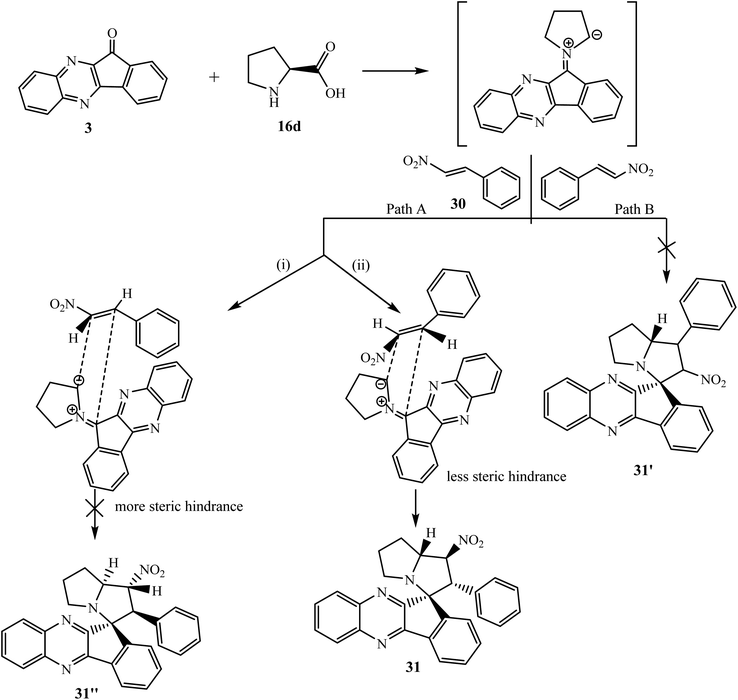 | ||
| Scheme 22 Mechanism for the synthesis of spiroindenoquinoxalines 31 regio- and diastereoselectively. | ||
The regio- and stereochemistry of the product was determined based on single-crystal X-ray analysis. β-Nitrostyrenes with methyl, methoxy, nitro and halogen functions were reacted smoothly in the presented cycloaddition reaction affording corresponding spiro adducts 31. Additionally, all the synthesized spiro[indeno[1,2-b]quinoxaline-11,3′-pyrrolizine] 31 were screened for AChE inhibitory activity and 6 out of 21 compounds exhibited significant activity in the low micromolar IC50 range.
Earlier reports mentioned that the presence of an OH group in the reacting alkenes influences the regioselectivity of the reaction, due to the possibility of intermolecular hydrogen bond formation, which tends to stabilize the transition state leading to a selective regioisomer.52 Considering this, Sosnovskikh et al.53 investigated the [3 + 2] cycloaddition reaction with hydroxyl-substituted β-nitrostyrenes 30 along with other substituents using 2-propanol as a solvent medium. In the majority of cases, spiro-nitropyrrolizidines 31 were formed as a single isomer. The single-crystal X-ray structure analysis of 31 justified the formation of endo cycloadduct with a trans relationship of the quinoxaline moiety and nitro group, as reported by Trivedi and co-workers.51 The presence of a phenolic hydroxyl group in β-nitrostyrene did not interfere in this reaction and the interaction of β-nitrostyrene with azomethine ylide generated from indenoquinoxalinone 3 and L-proline 16d proceeded via the attack of the more electrophilic center of the alkene with the less available atom of the dipole.
In further elaboration of this work, a three-component reaction was investigated with sarcosine 16a in place of L-proline 16d under similar conditions, and two regioisomers of spiro-indenoquinoxaline-nitropyrrolidines 32 as the main isomer and 32′ as the minor isomer were obtained with decreased regioselectivity. The reaction proceeded successfully with β-nitrostyrenes with phenyl and methoxy and chlorine substituents on the aryl ring, but failed to occur with OH-substituted β-nitrostyrenes.
The single-crystal X-ray characterization of 32 clearly indicates a different regiomeric nature from spiro-nitropyrrolizine 31 due to the faster attack of the more electrophilic α-carbon atom of β-nitrostyrenes 30 with the more readily available less substituted terminal atom of the ylide through an endo-transition state. Similarly to spiro-nitropyrrolizidines 31, the trans configuration of the starting β-nitrostyrene was also conserved in spiro-nitropyrrolidines 32 (Scheme 23).
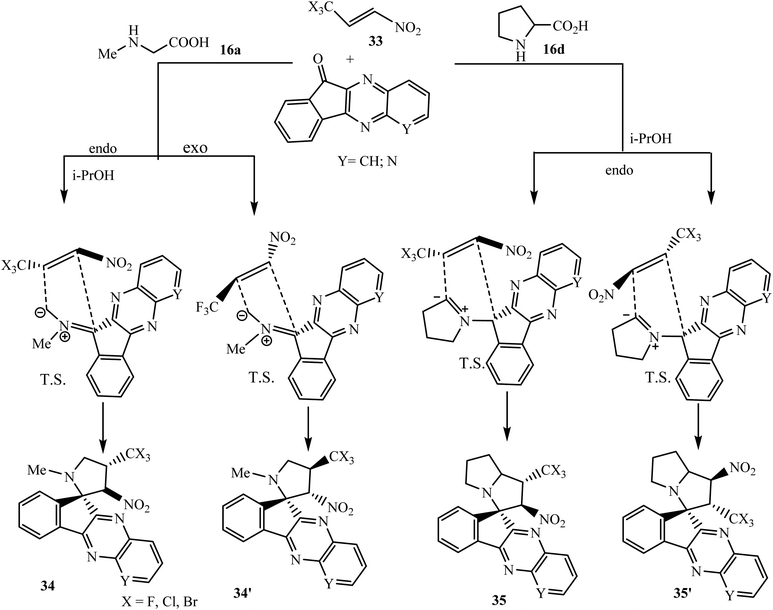
The introduction of trihalomethyl groups, particularly CF3 groups, in organic molecules not only enhances their therapeutic index, but sometimes influences the regio- and stereochemistry of compounds.54 In this connection, Sosnovskikh et al.55 have described the [3 + 2] cycloaddition reaction of (E)-3,3,3-trihalogeno-1-nitropropenes 33 with azomethine ylides type I and II to synthesize a number of trihalomethylated spiroindenoquinoxaline-pyrrolidines 34 and spiroindenoquinoxaline-pyrrolizidines 35 regio- and diastereoselectively in isopropanol solvent. Azomethine ylides derived from indenoquinoxalinones 3 and sarcosine 16a react with 3,3,3-trichloro-1-nitropropene 33 giving only the endo regioisomer 34 with the NO2 group at C-3′ and the CX3 group at C-4′ exclusively according to a general mechanism and interaction occurs between the more electrophilic alkene atom and the more nucleophilic and accessible terminal ylide atom. The alternative exo regioisomer does not form due to the steric repulsion of the bulky CCl3 group with the π-system of the quinoxaline ring. However, in the case of 3,3,3-trifluoro-1-nitropropene 33, a small quantity of exo isomer 34′ was detected with major endo isomer 34. Hence, the selectivity of CCl3-alkene is better than CF3-alkene, and CBr3-alkene is found not to be suitable due to the formation of unidentifiable mixture of products.
Similarly, dipolarophile nitroalkenes 33 having CF3 and CCl3 groups react with azomethine ylides type II generated from indenoquinoxalinones (Y = CH, N) and L-proline 16d to produce endo-adducts spiroindenoquinoxaline-pyrrolizidines 35 with similar regio- and diastereoselectivity as observed in product 34. However, in the case of the ylide derived from indenoquinoxalinone 3 (X = CH), the appearance of a small amount of regioisomers 35′ (4–6% according to the 1H NMR data) was observed in the crude product. Regio- and stereochemistry of spiropyrrolidines 34 and spiropyrrolizidines 35 were clearly confirmed by single-crystal X-ray analysis of the representative compounds (Scheme 24).
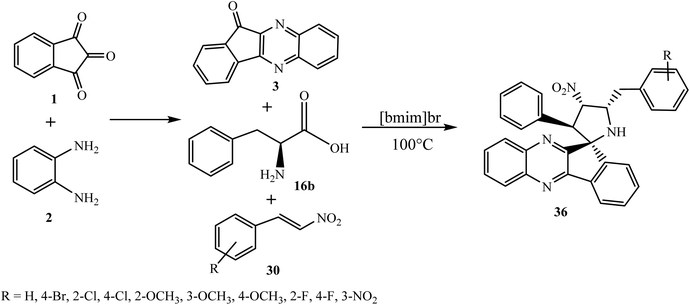
Recently, Arumugam and co-workers56 constructed a novel series of structurally interesting spiropyrrolidine grafted quinoxaline heterocyclic hybrids 36 in ionic liquid using β-nitrostyrenes 30 as dipolarophiles, and a new 1,3-dipole component azomethine ylide, generated in situ from indenoquinoxalinone 3 and L-phenylalanine 16b in good yield (Scheme 25).
The observed regio- and diastereoselectivity of synthesized spiro hybrid 36 is comparable to that of spiro compound 31 (Scheme 22), where the more substituted electron-rich carbon of the azomethine ylide exclusively attacks the most electrophilic center of dipolarophile 30 leading to the formation of only single regioisomer 36 to minimize the repulsion and stabilize the transition state.
The reaction provided three new bonds and four contiguous stereocenters with full diastereomeric control. The synthesized spiro heterocyclic hybrids 36 have been evaluated for their in vitro anti-bacterial activity against Mycobacterium tuberculosis H37Rv by microplate alamar blue assay (MABA). In vitro activity of these spiro heterocyclic hybrids revealed that the compounds with m-nitro, p-bromo and o-chloro substituents on the aryl ring displayed potent activity against Mtb and exhibited MIC values, but a nitro group on the phenyl ring was found to give the most active candidate among the other analogues of the series and leads to an activity similar to that of the standard drug ethambutol.
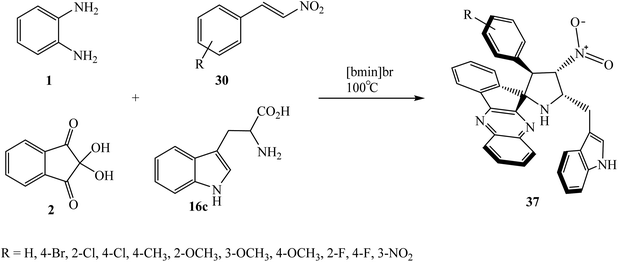
Recently, Arumugam et al.57 successfully synthesized novel spiro[indeno[1,2-b]quinoxaline-pyrrolidine] hybrids 37 regio- and stereoselectively, comprising spiropyrrolidine, indenoquinoxaline and indole structural units, in excellent yields. In this 1,3-dipolar cycloaddition reaction, a new class of azomethine ylide is generated in situ from indenoquinoxalinone 3 and L-tryptophan 16c and reacts with various substituted β-nitrostyrenes 30 affording the spiro heterocyclic hybrids 37 with similar regio- and stereoselectivity as observed in the case of spiro adduct 36 (Scheme 26). The ring system thus creates two C–C and three C–N bonds and four adjacent stereogenic carbons, one of which is quaternary, and the reaction proceeds with full diastereomeric control. All of the synthesized compounds were evaluated for their in vitro activity against Mycobacterium tuberculosis H37Rv using MABA. Interestingly, the compound bearing a 2-fluoro substituent on the aryl ring displayed an equipotent activity (MIC of 1.56 mg mL−1) to that of ethambutol against Mycobacterium tuberculosis H37Rv.
5.2.1.4. Using nitrile-substituted Knoevenagel adducts as a dipolarophile. Li et al. in 201158 successfully accomplished the synthesis of new spiro indenoquinoxaline-pyrrolidine derivatives 40 via the five-component tandem reaction of ninhydrin 1, 1,2-phenylenediamine 2, sarcosine 16a, malononitrile or cyanoacetic ester 38 and aldehydes 39 in a one-pot operation for the first time. This was a great achievement in a Huisgen reaction, in which the dipole azomethine ylide and dipolarophile were both evaluated in situ and converted into spiro cycloadduct 40 with high regio- and diastereoselectivity. The optimized reaction conditions for this cycloaddition reaction were using ethanol as a solvent and a temperature of 100 °C to obtain a maximum yield of the product (Scheme 27).
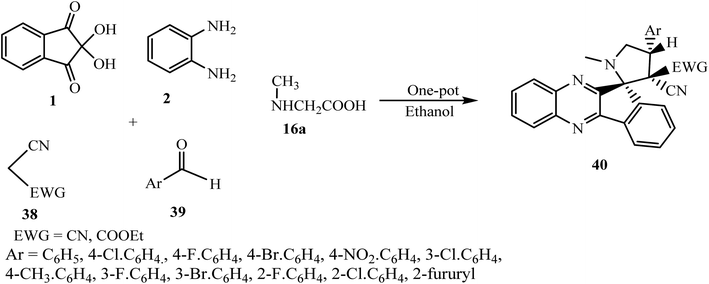 | ||
| Scheme 27 Five-component synthesis of nitrile-substituted spiro indenoquinoxaline-pyrrolidine derivatives 40. | ||
Mechanistically, in this reaction sarcosine pays a dual role as a catalyst as well as a substrate. The dipolarophile Knoevenagel adducts A are formed by the Knoevenagel condensation of malononitrile or cyanoacetic ester 38 and aldehydes 39 in the presence of sarcosine 16a, showing catalytic behavior (Scheme 28). The Knoevenagel adducts A then react with azomethine ylide formed by indenoquinoxalinone 3 and 16a regioselectively, as well as diastereoselectively, leading to the formation of only single product 40 without any trace of other possible regio- and diastereoisomers.
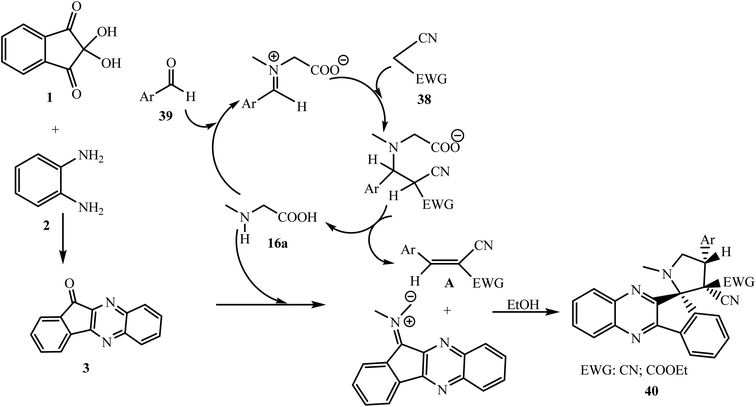 | ||
| Scheme 28 Plausible mechanism of the synthesis of spiro-pyrrolidines 40 via a five-component reaction. | ||
The addition of dipolarophile Knoevenagel adducts A to azomethine ylide proceeds in the usual way where the less substituted end of the ylide and β-carbon of alkene-nitrile (Knoevenagel adducts) A interact with each other in an exo manner and the aryl ring of alkene-nitrile A and indenoquinoxaline rings are present in a trans orientation.
Later on, this reaction was studied by Velikorodov et al.59 to synthesize highly functionalized spiro[indeno[1,2-b]quinoxaline-11,2′-pyrrolidine] derivative 40 via the five-component condensation of ninhydrin 1, o-phenylenediamine 2, sarcosine 16a, malononitrile/ethylcyanoacetate 38 and 4-formylphenyl N-phenylcarbamate in refluxing EtOH conditions with [bmin]Br.
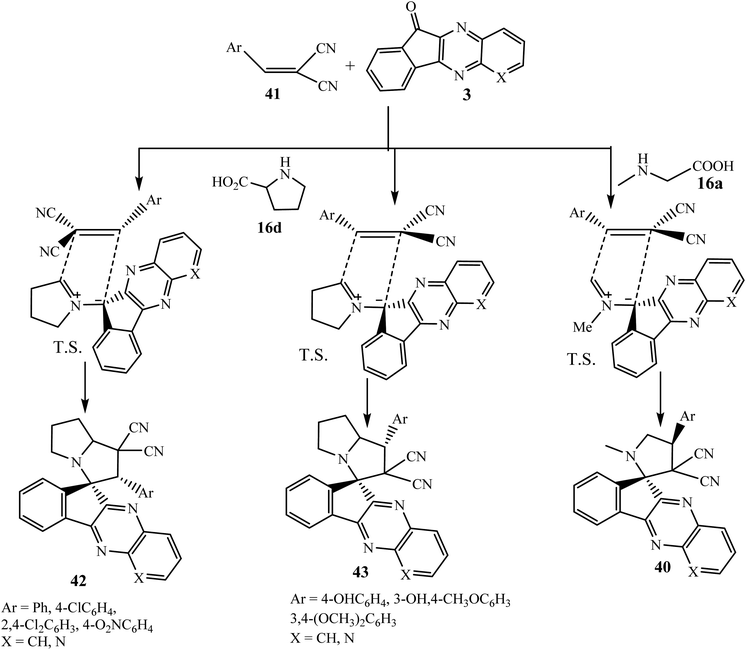
After that, Korotaev et al.60 investigated the 1,3-dipolar cycloaddition of azomethine ylides generated in situ from L-proline 16d and indenoquinoxalinones 3 with previously synthesized dipolarophile arylidene malononitriles 41 using isopropanol as a solvent, to generate new hybrid spiro[indeno[1,2-b]quinoxaline-11,3′-pyrrolizine]-1,1′(2′H)-dicarbonitriles 42/43 (Scheme 29).
The regioselectivity of the process is very interesting and depends upon the nature of substituents present on the aromatic ring of dipolarophile molecule 41. The three-component reaction of dinitriles 41 with an unsubstituted benzene ring or electron-withdrawing substituents (Cl, NO2), L-proline 16d and quinoxalinones 3 (X = CH, N) produced spiropyrrolizidines 42 (56–86% yields) with unexpected regioselectivity. Here, the electrophilic β-C atom of dipolarophile 41 attacks at the more substituted terminus of the ylide, an unusual direction that was previously observed only in β-nitrostyrenes 30. However, when the benzene ring of dinitriles 41 is substituted with one or two electron-donating substituents (OH, OCH3), the standard direction was observed where the interaction of the less substituted terminal atom of the ylide and the β-C atom of the alkene was observed to produce regioisomer 43 in 60–88% yields. However, in a few cases different results were obtained.
To further elaborate, the present three-component reaction has also been studied with azomethine ylide derived from indenoquinoxalinone 3 and sarcosine 16a. This reaction was found to fail with dinitriles 41 substituted with electron-donating groups and only succeeded with dinitriles 41 containing electron-withdrawing substituents to produce spiroindenoquinoxaline-pyrrolidines 40 through interaction of the electrophilic site (the β-C atom) with the less substituted terminus of the dipole to produce regioisomer 40 exclusively with a trans configuration of the bulkiest substituents, as observed by Li et al.58 (Scheme 29).
Novel indole-appended spiroindenoquinoxaline-pyrrolidines/pyrrolizidines 46/47 were derived by Zhu et al.61 through the five-component reaction of ninhydrin 1, o-phenylenediamine 2, amino acids 16a/16d, 3-cyanoacetyl indoles 44 and aryl aldehydes 39 in EtOH. The Knoevenagel product 45 generated from 3-cyanoacetyl indoles 44 and aryl aldehydes 39 acts as a dipolarophile (Scheme 30). Notably, the utilization of primary amino acids such as glycine or phenylalanine in this reaction did not afford the target product.
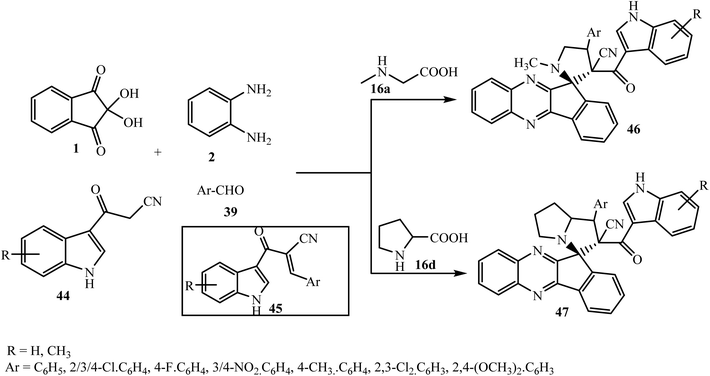 | ||
| Scheme 30 Five-component synthesis of indole-substituted spiroindenoquinoxaline-pyrrolidines/pyrrolizidines 46/47. | ||
A reasonable mechanism of the reaction is shown in Scheme 31. The formation of the regio- and diastereoisomer 46 via path B is more favorable due to the presence of a secondary orbital interaction (SOI) that takes place between the carbonyl group of dipolarophile 45 and the azomethine ylide.
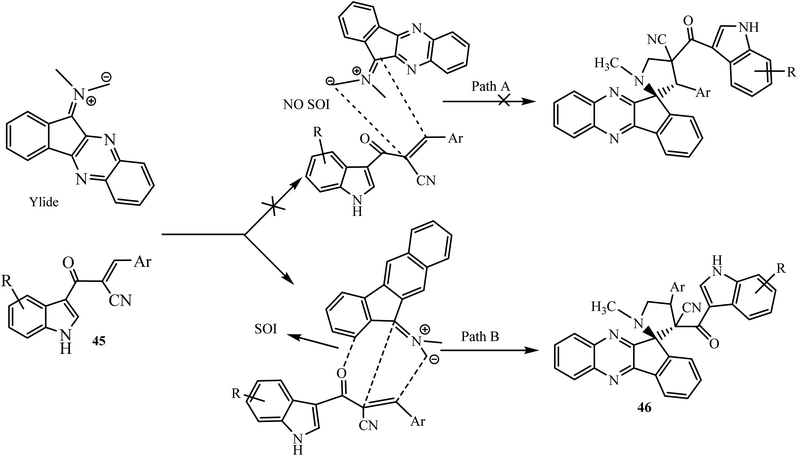 | ||
| Scheme 31 Proposed mechanisms for the selective synthesis of spiroindenoquinoxaline-pyrrolidines 46. | ||
This type of SOI is not possible in path A. Hence, the reaction proceeds via path B to lead to the exclusive formation of regio- and diastereoselective spiropyrrolidines as products. The selective formation of spiropyrrolidines 46 and spiropyrrolizidines 47 in this reaction is also confirmed by single-crystal X-ray studies of representative compounds.
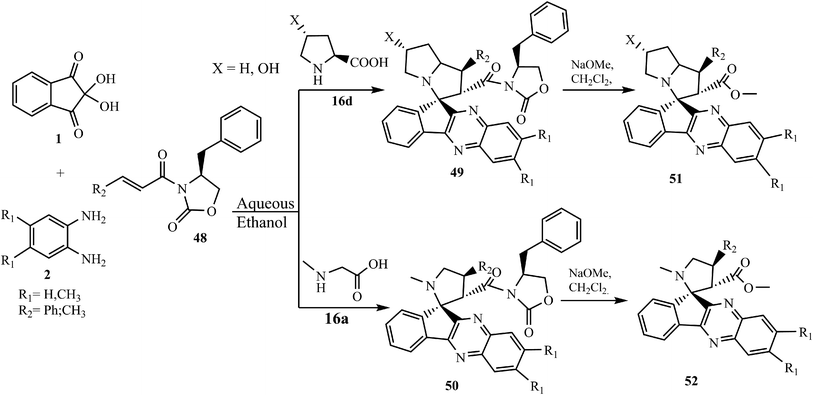
5.2.1.5. Using heterocyclic ring-containing dipolarophiles. Jadidi et al.62 described an efficient, four-component process for the construction of novel oxazolidinone-containing spiroindenoquinoxaline-pyrrolidines 49 and spiroindenoquinoxaline-pyrrolizidines 50 with high regio-, diastereo- (up to 96 dr), and enantioselectivity (up to 99% ee), using optically active cinnamoyl-crotonoyl oxazolidinone 48 as dipolarophile. Revealing the mechanism was based on the assignment of the absolute configuration of the cycloadducts and using quantum mechanical calculations. The regio- and stereoselectivity of spiroindenoquinoxaline-pyrrolidines 49 and spiroindenoquinoxaline-pyrrolizidines 50 was explained in terms of transition state stabilities and global and local reactivity indices of the substrates. The chiral auxiliary was removed easily from spiro compounds 49 and 50 in the presence of sodium methoxide in CH2Cl2 to afford the corresponding carboxylate derivatives 51 and 52, respectively (Scheme 32).
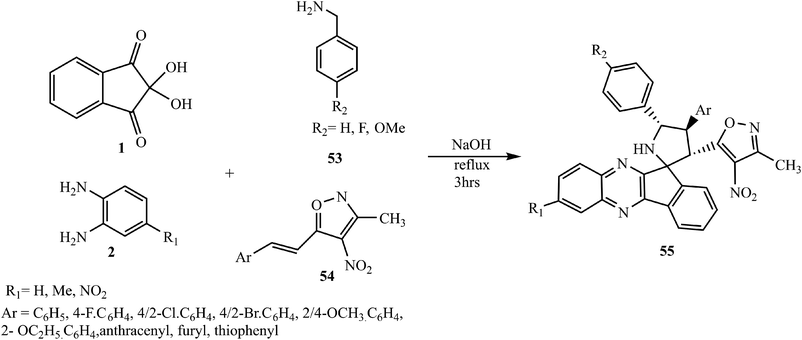
Recently, Chowhan et al.63 expanded the scope of the reaction and introduced a new dipole moiety using benzylamines 53 in place of α-amino acids and described an expedient synthesis of novel polyheterocyclic spiropyrrolidinylquinoxaline derivative 55 via the four-component [3 + 2] cycloaddition reaction of dipolarophile 3-methyl-4-nitro-5-alkenylisoxazoles 54, ninhydrin 1, o-phenylenediamine 2 and benzylamines 53 under convenient reaction conditions (Scheme 33). The striking feature of this work is the variability in the yield and diastereoselectivity of products 55, depending upon the nature and position of substitutions present on all partners of this reaction. In the case of 4-methyl-substituted o-phenylenediamine 2, an excellent yield of products was obtained, while no reaction occurred using 4-nitro-substituted o-phenylenediamine 2. The reactivity of halosubstituted styrenes (3-methyl-4-nitro-5-alkenylisoxazoles) 54 was found to be better than that of simple and alkoxy-substituted styrenes. Naphthalene-substituted dipolarophile afforded excellent yields, and in the case of heteroaromatic styrenes poor yield of product was obtained.
During the reaction, indeno[1,2-b]quinoxalinone 3 reacts with benzylamines 53 to form a dynamic isomeric intermediate ketimine (A) and aldimine (B) after 1,3-hydride shift and its equilibrium structure (C). This intermediate (C) acts like azomethine ylides generated by α-amino acids and undergoes [3 + 2] cycloaddition with dipolarophile 3-methyl-4-nitro-5-alkenylisoxazoles 54 through two plausible paths (path a and path b). The formation of major endo isomer 55 over exo isomer 55′ is due to less steric hindrance and proximity of the 54 core with the dipolar region. However, in path b, the formation of possible regioisomer 55′′ was not detected (Scheme 34).
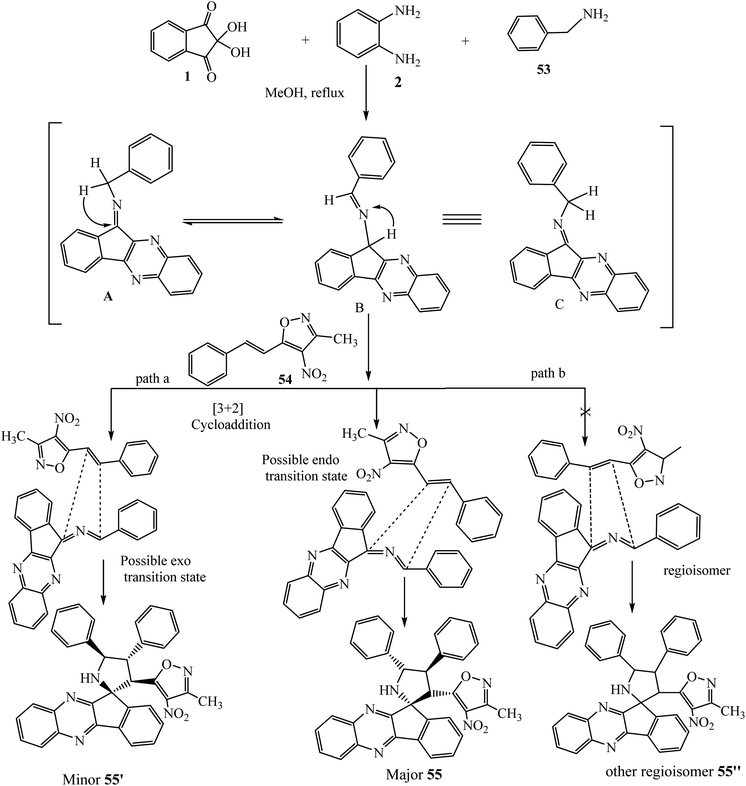 | ||
| Scheme 34 Plausible mechanism for the in situ generation of imines and their conversion to spiroindenoquinoxalines 55 and other possible isomers. | ||
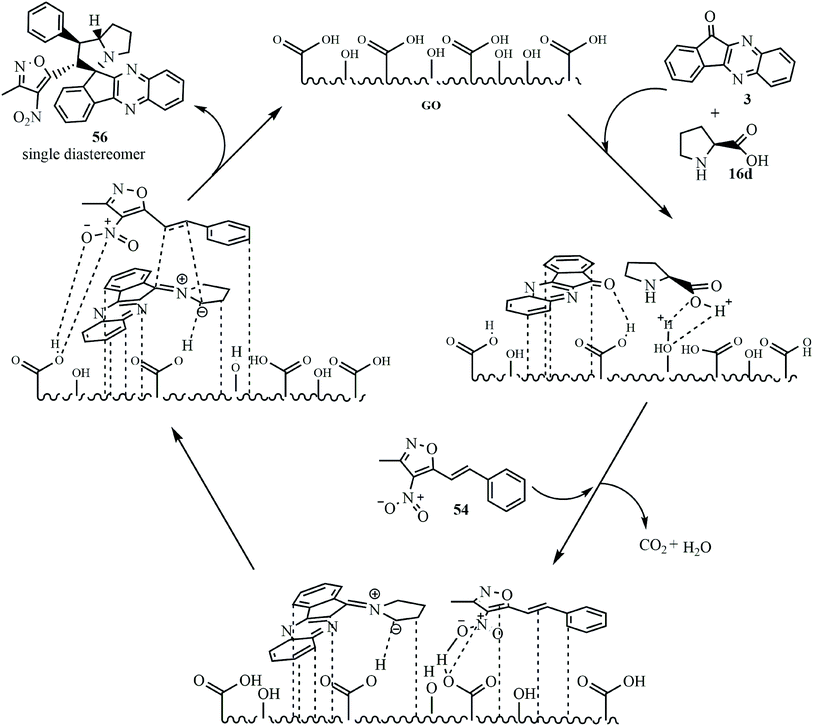
Reddy and Chowhan for the first time64 established a graphene oxide (GO)-catalyzed 1,3-dipolar cycloaddition reaction to achieve polyheterocyclic spiroindenoquinoxaline-pyrrolizidines 56 in good to excellent yields along with excellent regio- and diastereoselectivity. An ultra-low catalyst loading of 0.50 wt% was found to be efficient to catalyze the reaction in aqueous ethanolic solution. In this work, 3-methyl-4-nitro-5-alkenylisoxazoles 54 were used as a dipolarophile and azomethine ylides were derived from indenoquinoxalinone 3 and L-proline 16d (Scheme 35).
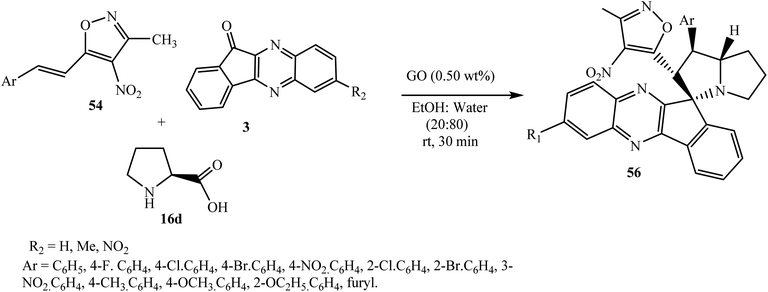 | ||
| Scheme 35 Graphene oxide-catalyzed synthesis of isoxazole-substituted spiroindenoquinoxaline-pyrrolizidines 56. | ||
GO has an acidic nature and catalyzed imine formation and decarboxylation to generate the corresponding azomethine ylide. The reactants are localized onto the surface of the catalyst due π-stacking and hydrogen bonding capability of GO and dipolarophile and ylide exclusively react only on one side to yield single diastereomer 56 as the product. The regioselectivity can be reasonably assumed to be due to possible additional interactions that arise between heteroatoms in the isoxazole motif (Scheme 36).
Recently, Khurana et al.65 extended this reaction and synthesized spiroindeno[1,2-b]quinoxaline-11,3′-pyrrolizines 56/thia-pyrrolizines 57 via the four-component domino reaction of reactants 1, 2, dipolarophile 54 and L-proline 16d/L-thioproline 16e with regio- and diastereoselectively (Scheme 37).
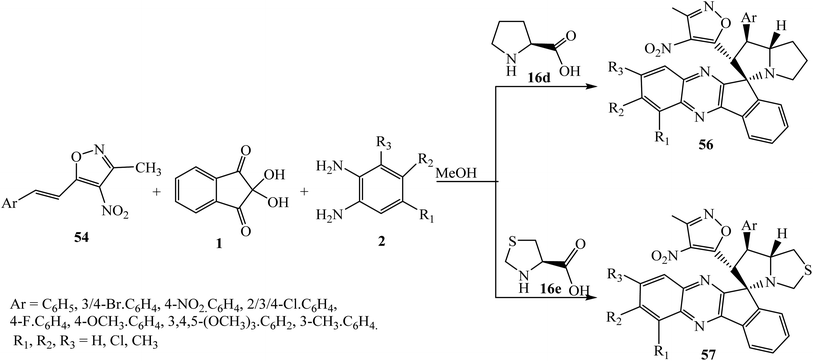 | ||
| Scheme 37 Synthesis of isoxazole-substituted spiroindenoquinoxaline-pyrrolizidines 56/thia-pyrrolizidines 57. | ||
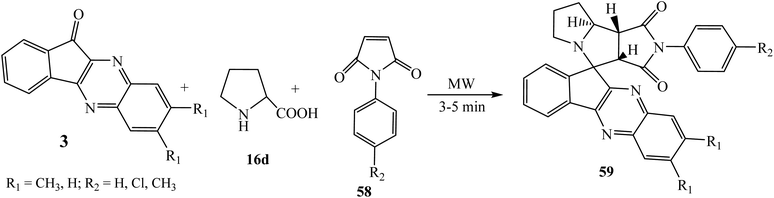
Azizian et al.66 studied the diastereoselective synthesis of novel spiropyrrolo fused pyrrolizidines 59 via a four-component 1,3-dipolar cycloaddition of azomethine ylide generated in situ from L-proline 16d and indenoquinoxalone 3 with dipolarophile N-arylmaleimides 58. The reaction was carried out under microwave irradiation using DMSO as a solvent to afford the corresponding spiroindenoquinoxaline-pyrrolizidines 59 with high diastereomeric excess (Scheme 38).
Nayak et al.67 reported the efficient and selective one-pot synthesis of highly substituted novel functionalized indenoquinoxalinone grafted spiropyrrolizine/spiropyrrolidine connected chromene-3-carbonitrile conjugates 61 and 62 via the 1,3-dipolar cycloaddition reaction of indenoquinoxalone 3, L-proline 16d/benzylamine 53 and dipolarophile chromene-3-carbonitrile 60 in ethanol under microwave irradiation and conventional conditions (Scheme 39).
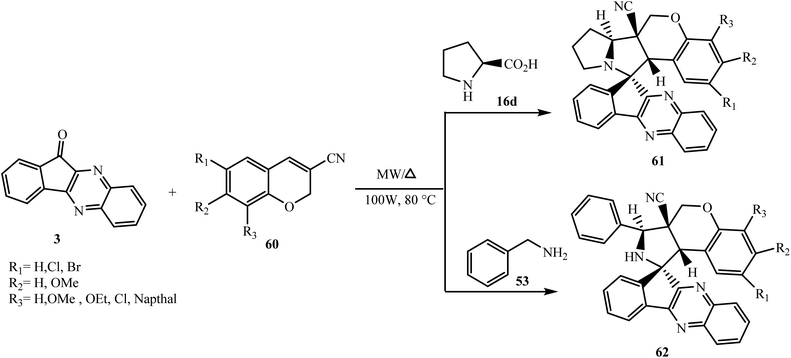 | ||
| Scheme 39 Synthesis of spiroindenoquinoxaline-pyrrolizidine/pyrrolidine fused chromene-3-carbonitriles 61/62. | ||
To test the generality of the reaction, a number of chromene-3-carbonitrile derivatives 60 with substituents such as methoxy, ethoxy, naphthyl, and halogen groups at different positions were used. In all cases, the reaction proceeded smoothly and only single diasteroisomers of spiroindenoquinoxaline-pyrrolizines 61/pyrrolidines 62 were obtained in good to excellent yields. Yields of products were variable with respect to the substituent and their positions on the aromatic ring of the 3-cyano-chromene 60. The reaction failed to occur with pipecolic acid 16f and L-phenylalanine 16b.
The dipolarophile chromene-3-carbonitriles 60 have been synthesized via the treatment of acrylonitrile with salicylaldehydes in the presence of DABCO under solvent-free conditions via an oxa-Michael–aldol reaction. The more substituted electron-rich side of the azomethine ylide reacts with the β-carbon of the dipolarophile at the endo side leading to the formation of highly functionalized regio- and diastereoselective molecular hybrids.
Recently, Nayak et al.68 have extended this work and reported the synthesis of spiroindenoquinoxaline-pyrrolizidines 64 and spiroindenoquinoxaline-pyrrolidines 65 via the 1,3-dipolar cycloaddition reaction of aryl-substituted 3-nitrochromens 63 as dipolarophile and L-proline 16d and L-phenylalanine 16b as α-amino acids to generate the corresponding azomethine ylide with indenoquinoxalinone 3 under microwave heating (Scheme 40).
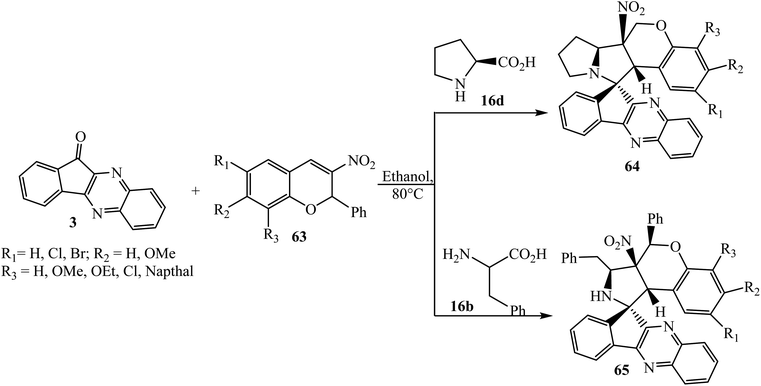 | ||
| Scheme 40 Synthesis of spiroindenoquinoxaline-pyrrolizidine/pyrrolidine fused 3-nitro-2H-chromenes 64/65. | ||
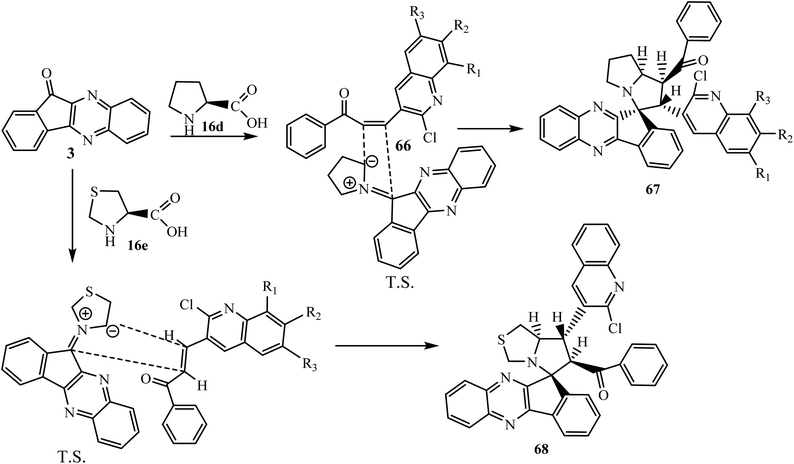
Nowadays, synthetic chemists are engaged in the synthesis of novel bioactive molecules for cancer treatment with low side effects. In this direction Rajendran et al.69 described a facile synthesis of new bioactive quinoline ring-containing spiroindenoquinoxaline-pyrrolizine hybrids 67 via four-component 1,3-dipolar cycloaddition reaction of ninhydrin 1, 1,2-phenylenediamine 2, L-proline 16d and quinolone-bearing chalcones 66. Parallel to this work, Rajendran et al.70 also synthesized spiroindenoquinoxaline-pyrrolothiazoles 68 through the 1,3-dipolar cycloaddition reaction of azomethine ylides generated in situ from indenoquinoxalinone 3 and thiazolidin-2-carboxylic acid 16e and quinolone-bearing dipolarophiles 66 (Scheme 41).
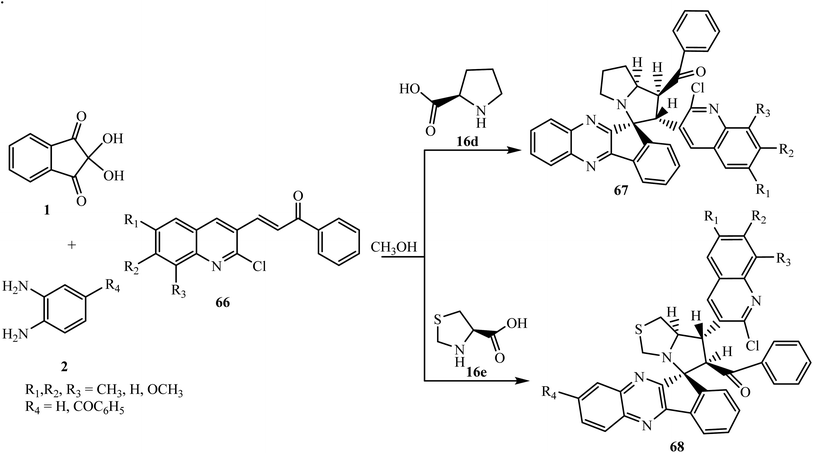 | ||
| Scheme 41 Synthesis of benzoyl-substituted spiroindenoquinoxaline-pyrrolizines 67/pyrrolothiazoles 68. | ||
The formation of both spiro compounds 67 and 68 proceeds via the initial generation of the corresponding azomethine ylides followed by the interaction with dipolarophiles 66 leading to the formation of single final products regio- and diastereoselectively. In the presented study, the authors reported the different regioselectivity of both spiro compounds 67 and 68 due to the different modes of interaction of azomethine ylides with dipolarophile 66. During the formation of spiro compound 67 the electrophilic β-C atom of dipolarophile quinoline attacks at the more substituted terminus of the ylide generated from L-proline 16d and indenoquinoxalinone 3 by means of an unusual direction. While, in the case of spiro compound 68 the β-C atom of the dipolarophile interacts with the less substituted terminus of the azomethine ylide generated from thiazolidin-2-carboxylic acid 16e and indenoquinoxalinone 3 via the usual pattern (Scheme 42).
5.2.1.6. Using carbohydrate-based dipolarophile. Carbohydrate-derived heterocycles have attracted special attention in medicinal chemistry due to their promising bioactivities.71,72 Chromenes are important classes of heterocycles because of their core being incorporated in a large variety of natural products and biologically active compounds.73,74
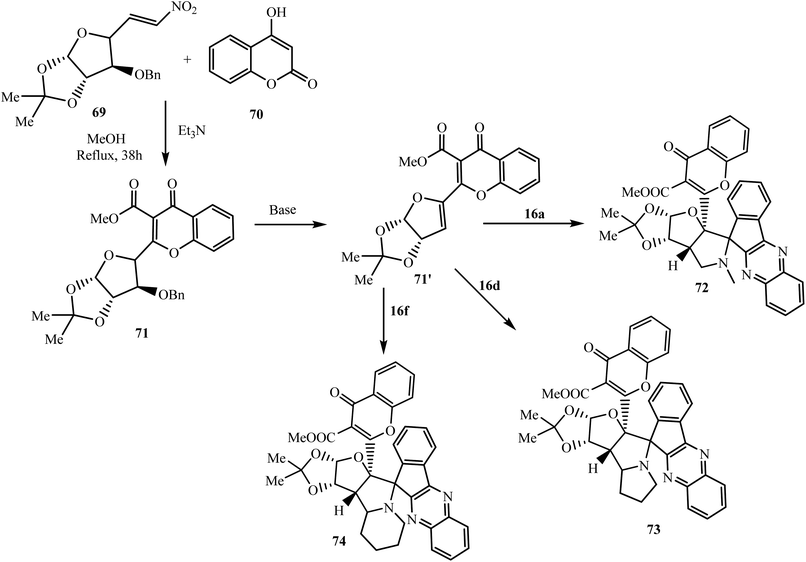
Raghunathan et al.75 have introduced a structurally unique sugar and chromene based dipolarophile hybrid, namely (4-oxo-2-glyco-4H-chromene-3-carboxylate) 71, and accomplished a 3 + 2 cycloaddition reaction with dipoles generated in situ by the reaction of indenoquinoxalinone 3 and α-amino acids such as sarcosine 16a, L-proline 16d, and pipecolinic acid 16f in the presence of base NaOMe to produce the corresponding glyco polycyclic spiroindenoquinoxalines 72–74, respectively. The dipolarophile was synthesized by the reaction of glyco-nitroalkene 69 with 4-hydroxycoumarin 70 in the presence of Et3N and methanol (Scheme 43).
The important feature of this reaction is that, in the absence of NaOMe, the [3 + 2] cycloaddition does not proceed due to steric hindrance around the double bond of the chromene moiety; but in the presence of base NaOMe, dehydrobenzyloxylation of dipolarophile 71′ occurs and 1,3-dipolar cycloaddition is completed across the sterically less hindered double bond of the diene with regio- and diastereoselectivity.
Raghunathan et al.76 also synthesized the glyco 3-nitrochromane hybrid spiroindenoquinoxaline-pyrrolidines/pyrrolizines 78/79 via a [3 + 2] cycloaddition reaction using glycol-3-nitrochromenes 77 as dipolarophiles, which were synthesized from glyco-β-nitroalkenes 75 with salicylaldehyde 76 in the presence of DABCO. In this reaction, the dipole attacks from the face opposite to the carbohydrate moiety in glyco-3-nitrochromenes 77 to avoid high steric crowding. This leads to the projection of a NO2 group and Ha proton towards the β-face. The regio- and stereochemical results of the cycloaddition reaction were confirmed by X-ray crystallographic analysis (Scheme 44).
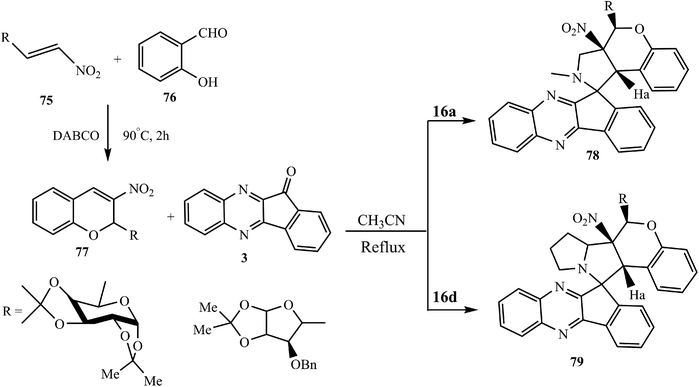 | ||
| Scheme 44 Synthesis of glyco-3-nitrochromane hybrid pyrrolidinyl spiro indenoquinoxalines 78 and 79. | ||
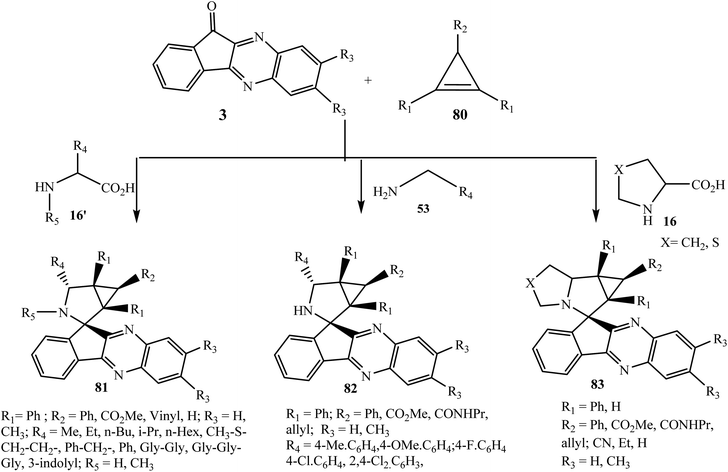
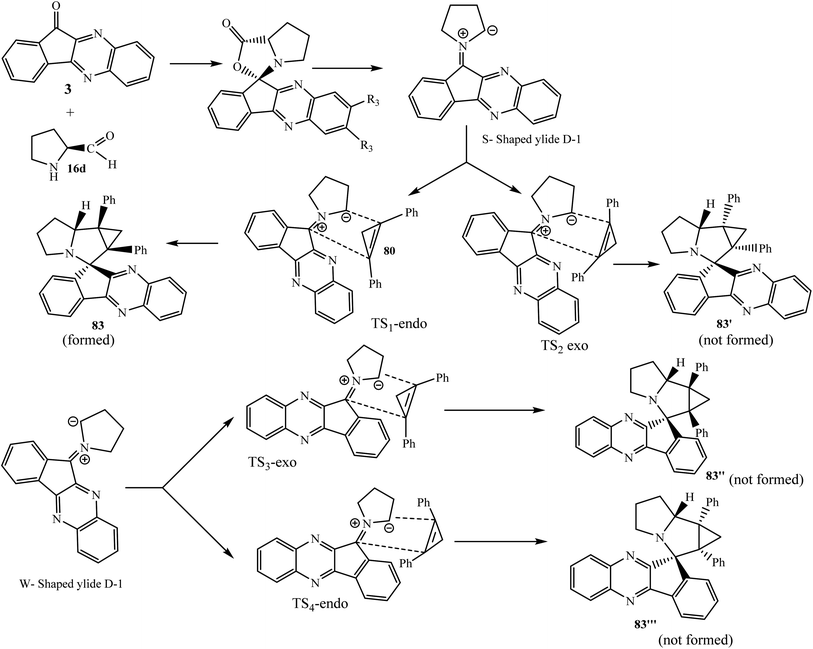
5.2.1.7. Using cyclopropenes as a dipolarophile. Boitsov et al.77 reported the efficient synthesis of complex spiro systems 81–83 by combining 11H-indeno[1,2-b]quinoxaline and cyclopropa[a]pyrrolizines or azabicyclo[3.1.0]hexane moieties via the one-pot three-component 1,3-dipolar cycloaddition reaction of azomethine ylides with substituted dipolarophile cyclopropenes 80 in MeOH (Scheme 45). The reaction is variously oriented and dipolarophile cyclopropane smoothly reacted with various azomethine ylides generated in situ from 11H-indeno[1,2-b]quinoxalin-11-ones 3 and amines, such as N-substituted and N-unsubstituted α-amino acids 16 and 16′, benzylamines 53, and also peptides (dipeptide Gly–Gly and tripeptide Gly–Gly–Gly) 16′. The desired cycloadducts were obtained in high yields with excellent diastereoselectivity. Some of the spiro compounds showed anticancer activity against the human leukemia K562 cell line via flow cytometry in vitro. The oxidation of their Ar–CH–NH bond with DDQ in toluene at 110 °C for 1 h.
The accepted mechanism of the reaction of 1,2-diphenylcyclo-propene 80 with the in situ generated azomethine ylide from 11H-indeno[1,2-b]quinoxalin-11-one 3 and L-proline 16d is presented in Scheme 46. The formation of only one diastereomer from four possible enantiomer pairs is explained on the basis of quantum chemistry investigations and calculated Gibbs free energies for the reagents, intermediates, transition states, and possible products. The formation of only single spiro compound 83 proceeds through energetically favorable S-shaped ylide D-1 not through the other possible W-shaped ylide D-2. Further, the interaction of S-shaped ylide D-1 and dipolarophile 1,2-diphenylcyclopropene 80 occurs via the less sterically hindered transition state TS1-endo, leading to product 83 selectively out of the other possible transition states TS-2 to TS-4.
5.2.2.1. Cycloaddition reaction at the exocyclic double bond of piperidinone-based dipolarophiles. The piperidinone heterocyclic moiety is an important class of pharmacophore as its derivatives possess interesting biological profiles, as potential antitumor78 and antimicrobial agents.79 Structurally diverse heterocyclic hybrids comprising spiropyrrolidine and piperidone units have been reported as anti-cancer,80 anti-mycobacterial,81 anti-Alzheimer82 and anti-microbial leads.83 These considerations prompted researchers to explore the synthesis of novel heterocyclic hybrids containing dispiropyrrolidine, piperidinone, and indeno[1,2-b]quinoxaline in a single frame to further enhance the biological profile. Perumal et al.84 synthesized a library of novel dispiro system 1-methyl-4-arylpyrrolo-(spiro[2.11′]-11H-indeno[1,2-b]quinoxaline)-spiro[3.3′′]-1′′-methyl/benzyl-5′′-(arylmethylidene)piperidin-4′′-ones 85 via the four-component 1,3-dipolar cycloaddition reaction of piperidinone-based dipolarophile 3,5-bis(4-chlorobenzylidene)-1-methylpiperidin-4-one 84, ninhydrin 1, o-phenylenediamine 2 and sarcosine 16a using [BMIm]Br ionic liquid as a reaction medium and reaction accelerator (Scheme 47).
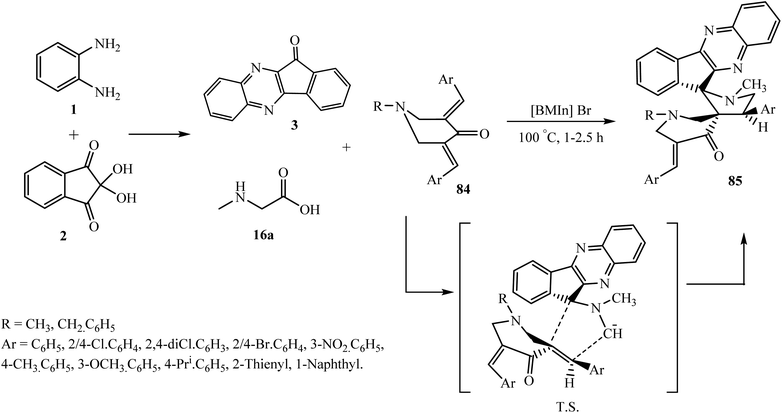 | ||
| Scheme 47 Synthesis of arylidene-substituted dispiropyrrolidinyl-indenoquinoxaline-piperidinones 85. | ||
This is an efficient and eco-compatible approach and spiro hybrid is formed by the simple attachment of the β-carbon of the dipolarophile to the less substituted side of the dipole through transition state A regio- and stereoselectively. The ionic liquid [BMIm]Br was also recyclable up to four times.
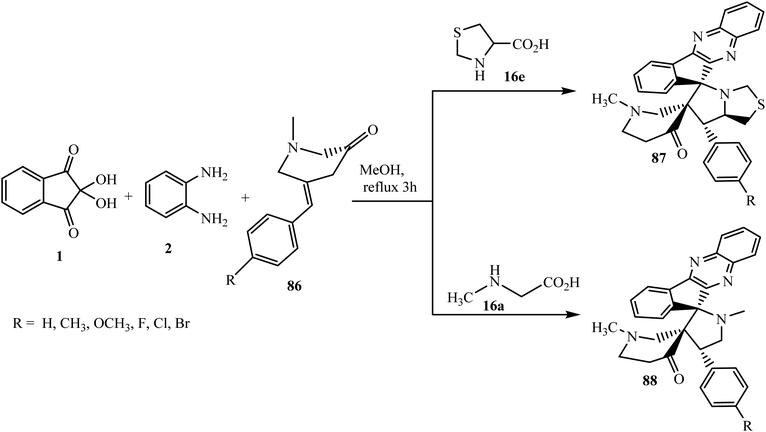
After that, Kumar et al.85 explored this work with dipolarophile mono-3-arylidene-1-methylpiperidin-4-ones 86 and reported the synthesis of novel polycyclic dispiro systems N-methyl-4-piperidinone-indenoquinoxaline-pyrrolothiazole 87/pyrrolidine hybrid heterocycles 88 using α-amino acid sarcosine 16a and L-thiaproline 16e, respectively. They also tried this reaction with amino acid L-proline 16d in place of 16a and 16e but the reaction failed to occur (Scheme 48).
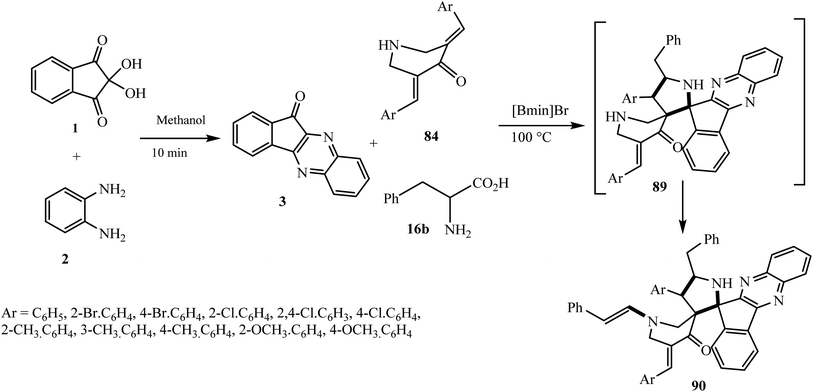
In 2019 Arumugam et al.86 reported the expedient synthesis of novel dispiropyrrolidinyl-N-styrylpiperidone-indeno[1,2-b]quinoxaline heterocyclic hybrid 90 via a domino multicomponent strategy employing N-unsubstituted 3,5-dibenzylidenepiperidin-4-ones 84 as a dipolarophile, L-phenylalanine 16b and indeno[1,2-b]quinoxalinone 3 in an ionic liquid, 1-butyl-3-methylimidazolium bromide (Scheme 49).
Mechanistically, this reaction is very interesting. The azomethine ylide A formed ‘in situ’ from the reaction of L-phenylalanine 16b and indeno[1,2-b]quinoxalinone 3 attacks regioselectively the β-carbon of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of the dipolarophile to form spiro compound 89.
C bond of the dipolarophile to form spiro compound 89.
Simultaneously, ylide A is attacked by a water molecule to furnish 2-phenylacetaldehyde 91 via intermediate B. Subsequently, the secondary amine of the piperidone ring of spirocycloadduct 89 reacts with 91 through an enamine reaction to afford spiro adduct 90 (Scheme 50).
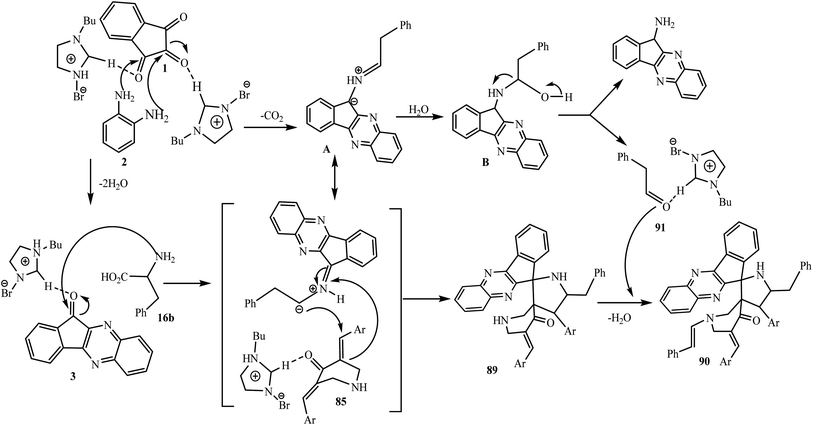 | ||
| Scheme 50 Mechanism for the synthesis of dispiropyrrolidinyl-N-styrylpiperidone-indenoquinoxaline 90. | ||
Synthesized compounds were tested for their antimicrobial activity against bacterial and fungal pathogens and compounds bearing chloro and methyl groups displayed significant activity against tested microbial pathogens. The synergistic effect revealed that the combination of a compound with a methyl group with streptomycin and vancomycin exhibited potent synergistic activity against E. coli ATCC 25922. In addition, molecular docking simulations were also studied for the most active compound.
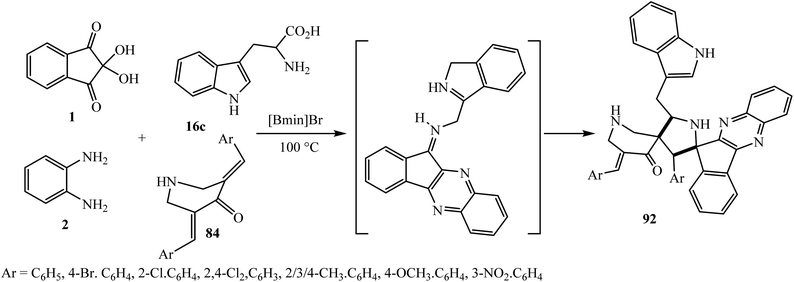
Simultaneously, Arumugam et al.87 accomplished the synthesis of a new class of dispiropyrrolidinyl-piperidone attached indeno[1,2-b]quinoxaline heterocyclic hybrids 92 by employing a new kind of azomethine ylide generated in situ from indenoquinoxalinone 3 and L-tryptophan 16c for the first time (Scheme 51).
The synthesized heterocyclic hybrids 92 were evaluated for their in vitro acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activities. Compounds bearing nitro and methyl substituents on the piperidinone ring displayed more potent AChE and BChE enzyme inhibition than the standard drug with IC50 values of 3.22, 2.01, 12.40 and 10.45 mM, respectively. Molecular docking studies have also been conducted for the most active compounds that showed interesting binding templates to the active site channel of the cholinesterase enzyme.
The mechanistic pathway is similar to those previously described. In all ionic liquid-mediated reactions the ionic liquids play a twin role as a solvent and catalyst, and accelerate the reaction by increasing the electrophilicity of the carbonyl carbons.
5.2.2.2. Cycloaddition reaction at the exocyclic double bond of indole-based dipolarophiles. The spiro-pyrrolidine and oxindole ring systems have acquired importance because of their specific structural motifs in many pharmacologically relevant alkaloids, as typified by rhyncophylline, coryn-oxeine, mitraphylline, horsifiline, and spirotryprostatins.88,89
Raghunathan et al.90 described the synthesis of novel spiro product 1-N-methyl-spiro[2.11′]indeno[1,2-b]quinoxaline-spiro[3.3′′]oxindole-4-benzoyl-pyrrolidines 94 by combining an indole and an indeno[1,2-b]quinoxaline moiety together in a single frame through the four-component 1,3-cycloaddition reaction of dipolarophile (E)-2-oxoindolino-3-ylidene acetophenones 93 with the azomethine ylide generated from ninhydrin 1, 1,2-phenylenediamine 2, and sarcosine 16a (Scheme 52).
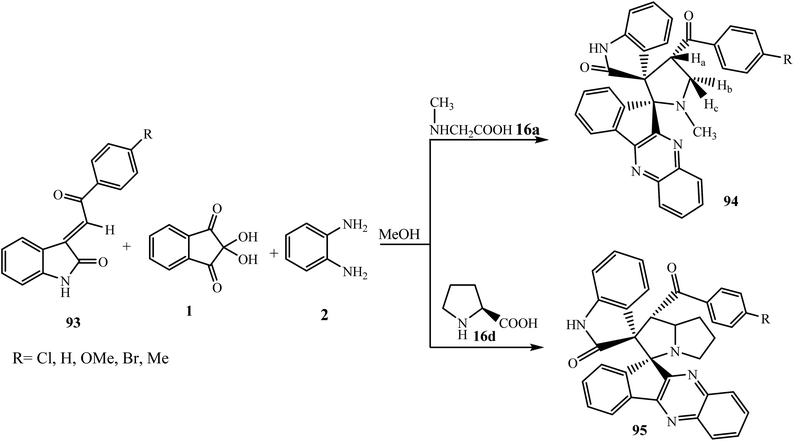 | ||
| Scheme 52 Synthesis of spiroindeno[1,2-b]quinoxaline-spiro[3.3′′]oxindole-4-benzoyl-pyrrolidines 94 and 95. | ||
Previously, Raghunathan et al.91 described an efficient heteropolyacid H4[Si(W3O10)3]–silica catalyzed synthesis of dispiroindenoquinoxaline-pyrrolizidine derivatives 95 via a four-component 1,3-dipolar cycloaddition reaction using dipolarophile 93 with an azomethine ylide generated from ninhydrin 1, 1,2-phenylenediamine 2, and L-proline 16d with high regioselectivity (Scheme 52).
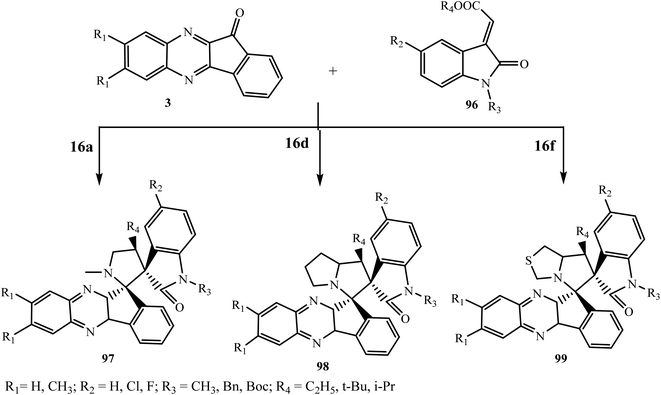
Recently Gu and coworkers92 designed and synthesized novel spirooxindole-indenoquinoxaline derivatives 97–99 through the 3 + 2 cycloaddition reaction of new indole-based dipolarophile 3-ylideneoxindole 96, α-amino acids (sarcocine 16a, L-proline 16d and L-thiaproline 16e) and in situ generated indeno[1,2-b]quinoxalinone 3 in acetonitrile (Scheme 53). These compounds were assayed by biochemical TrpRS inhibition, using in vitro experiments to test against various Gram-positive and Gram-negative strains, and using diffuse large B cell lymphoma cell lines. The results showed that these compounds can act as novel TrpRS inhibitors as potential lead compounds for antibiotics and as novel anticancer agents.
5.2.2.3. Cycloaddition reaction at the exocyclic double bond of chromanone-based dipolarophiles. Due to the remarkable catalytic properties of heteropolyacid H4[Si(W3O10)3]–silica in 1,3-dipolar cycloaddition reactions, Raghunathan et al.91 constructed chromanone-grafted novel dispiroindenoquinoxaline-pyrrolizidine derivatives 101 via a four-component reaction using dipolarophile (E)-3-arylidene-4-chromanones 100 with an azomethine ylide generated from ninhydrin 1, 1,2-phenylenediamine 3, and L-proline 16d selectively using heteropolyacid H4[Si(W3O10)3]–silica (Scheme 54).
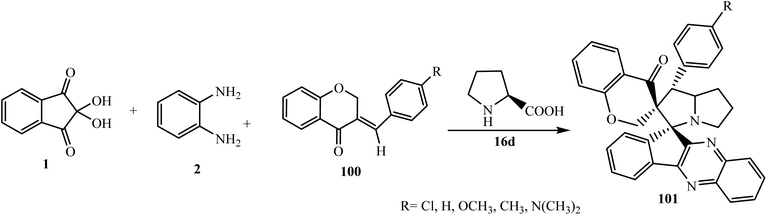 | ||
| Scheme 54 Synthesis of chromanone-grafted novel dispiroindenoquinoxaline-pyrrolizidine derivatives 101. | ||
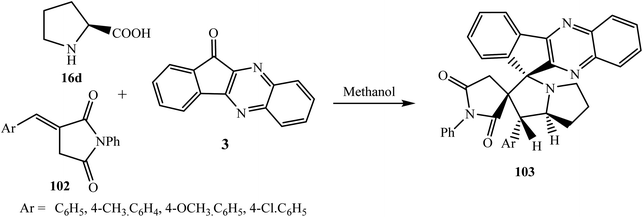
5.2.2.4. Cycloaddition reaction at the exocyclic double bond of pyrrolidine-based dipolarophiles. Askri et al.93 reported the synthesis of a series of spiro[2,11′′]indeno[1,2-b]quinoxaline-spiro[3,3′]-N-phenylsuccinimide-pyrrolizines 103 via the one-pot three-component [3 + 2] cycloaddition reaction of dipolarophiles (E)-3-arylidene-1-phenylpyrrolidine-2,5-diones 102, L-proline 16d and indenoquinoxalinone 3. The observed regio- and stereoselectivity was calculated using DFT at the B3LYP/6-31G(d,p) level and found to be under kinetic control (Scheme 55).
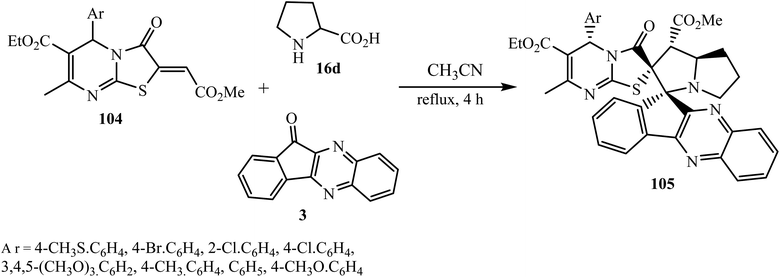
5.2.2.5. Cycloaddition reaction at the exocyclic double bond of sulfur-based dipolarophiles. Li et al.94 reported the synthesis of the novel dispiro system indeno[1,2-b]quinoxaline-11,3′-pyrrolizine-2′,2′′-[1,3]thiazolo[3,2a]pyrimidine-1′,6′′-dicarboxylates 105 via 3 + 2 cycloaddition reaction of a new dipolarophile, namely ethyl-5-aryl-2-(2-methoxy-2-oxoethylidene)-[1,3]thiazolo[3,2-a]pyrimidine-6-carboxylate 104, and azomethine ylide generated ‘in situ’ by the reaction of 11H-indeno[1,2-b]quinoxaline-11-one 3 and L-proline 16d (Scheme 56).
Kumar et al.95 reported a combinatorial four-component synthesis of novel dispiro-dihydrothiophenone-indenoquinoxaline-pyrrolidines 107 or pyrrolothiazole hybrids 108 heterocyclic systems utilizing bis(arylidine)dihydrothiophen-3(2H)-ones 106 as dipolarophile and azomethine ylide generated in situ from indenoquinoxalinone 3 and α-amino acids, i.e., sarcosine 16a/thioproline 16e, with high chemo-, regio- and stereoselectivity (Scheme 57).
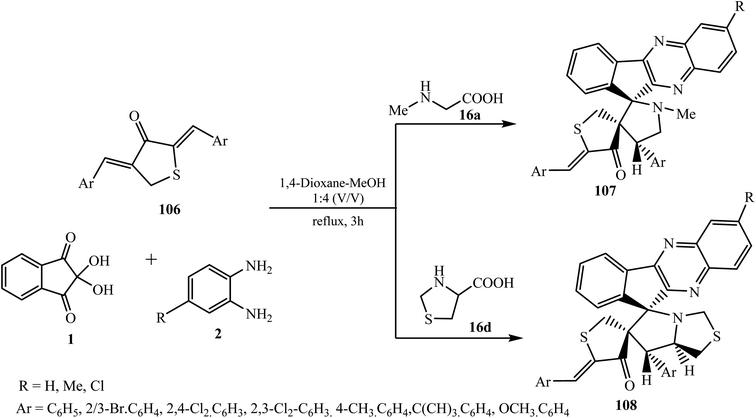 | ||
| Scheme 57 Synthesis of thiophene-grafted dispiroindenoquinoxaline-pyrrolidine/pyrrolothiazoles 107/108. | ||
5.2.2.6. Cycloaddition reaction at the exocyclic double bond of simple cycloalkane-based dipolarophiles. In 2014, Raghunathan et al.96 reported an efficient synthesis of highly substituted spiro-indenoquinoxaline-pyrrolidine heterocycles 110 via the [3 + 2] cycloaddition reaction of simple cycloalkane-based dipolarophiles 2,6-bis-arylmethylidenecyclohexanone and 2,5-bis-arylmethylidenecyclopentanones 109 regioselectively. The synthesized spiro adduct 110 further undergoes ring annulation using hydrazine hydrate to give a new pyrazolo-cyclohexane grafted spiro-indenoquinoxaline-pyrrolidine derivative 111 (Scheme 58). This facile, one-pot, sequential multicomponent reaction offers several advantages. It involves mild reaction conditions, straightforward easy workup, readily available and low-cost starting materials, excellent yield with high regioselectivity, without a catalyst and the use of green solvent and afforded a series of hitherto novel pyrazolo-cycloalkane grafted spiroindenoquinoxaline-pyrrolidine derivatives of biological significance.
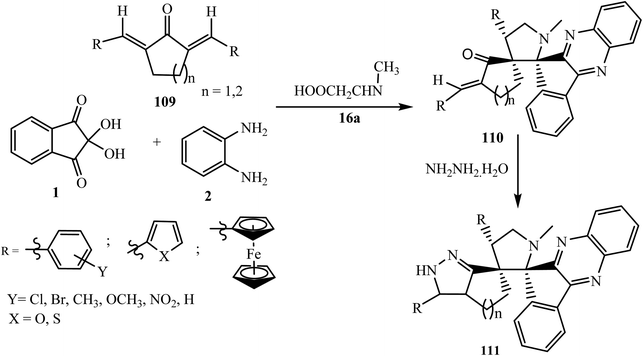 | ||
| Scheme 58 Synthesis of cycloalkane-grafted dispiroindenoquinoxaline-pyrrolidines 110 and their conversion to pyrazolo derivatives 111. | ||
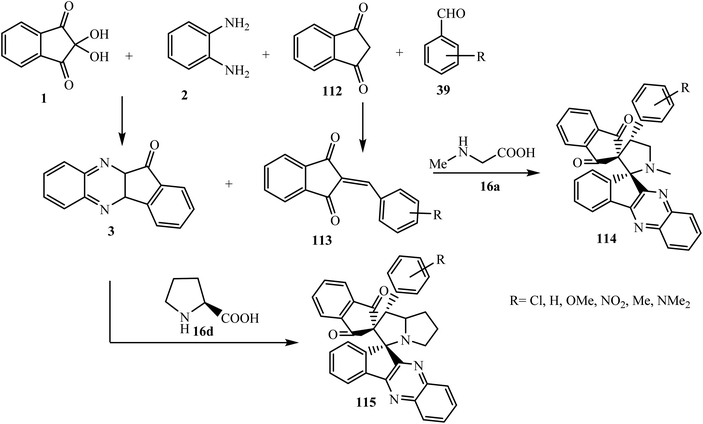
Liu et al.97 demonstrated an efficient and simple five-component cascade protocol to synthesize highly substituted dispiroindenoquinoxaline-indane-pyrrolidine derivatives 114 from ninhydrin 1, 1,2-phenylenediamine 2, sarcosine 16a, 1,3-indanedione 112, and aldehydes 39 via cascade reactions under mild conditions. In this five-component reaction the most electrophilic β-carbon of dipolarophile arylidene-1,3-indanedione 113 (formed in situ from 1,3-indanedione 112 and aldehyde 39) is attacked by the electron-rich carbon of the azomethine ylide in a thermodynamically more stable exo manner to construct a single regioisomer and diasteroisomer without any trace of another isomer. It is of significance to mention that the dispiroindenoquinoxaline derivatives 114 that have aromatic aldehydes with substituents at the ortho position lead to the formation of rotamers in ratios of around 0.76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.24 and 0.74
0.24 and 0.74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.26, as determined by 1H-NMR spectroscopy. Raghunathan et al. investigated the three-component 1,3-dipolar cycloaddition reaction of dipolarophile 113 with an azomethine ylide generated by the reaction of 3 and L-proline 16d in the presence of heteropolyacid H4[Si(W3O10)3]–silica to synthesize spiroindeno-pyrazolidines 115 (ref. 91) (Scheme 59).
0.26, as determined by 1H-NMR spectroscopy. Raghunathan et al. investigated the three-component 1,3-dipolar cycloaddition reaction of dipolarophile 113 with an azomethine ylide generated by the reaction of 3 and L-proline 16d in the presence of heteropolyacid H4[Si(W3O10)3]–silica to synthesize spiroindeno-pyrazolidines 115 (ref. 91) (Scheme 59).
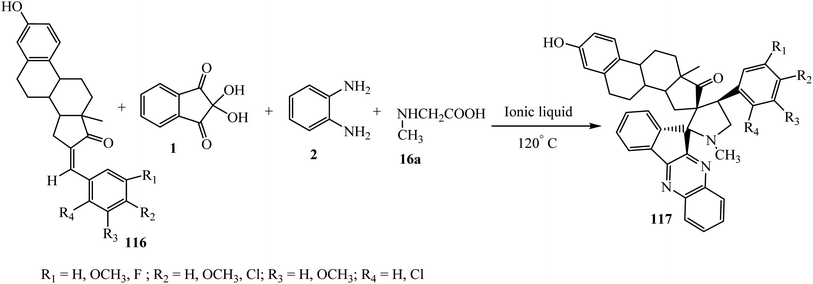
5.2.2.7. Cycloaddition reaction of a steroidal grafted exocyclic double bond. Raghunathan et al.98 investigated an expedient ionic liquid-assisted approach for the synthesis of a series of unknown novel steroidal grafted dispiroindenoquinoxaline-pyrrolidines 117 via a one-pot four-component [3 + 2] cycloaddition reaction. Concomitant cycloaddition of the azomethine ylide to various unusual estrone-derived dipolarophiles 116 affords a series of hitherto novel steroid grafted cyclo adducts 117 with excellent yield and high regioselective purity. In this sequential method, the ionic liquid N-(1-acroloyl)-N-(4-cyclopentyl)piperazinium phosphate plays a dual role, firstly as a recyclable green solvent in which the reactants have better solubility, especially the amino acids, and secondly because of its catalytic effect, which makes this approach more convenient and environmentally benign (Scheme 60).
5.3. Synthesis of spiro[indeno[1,2-b]quinoxaline[1,3,4]oxadiazole]
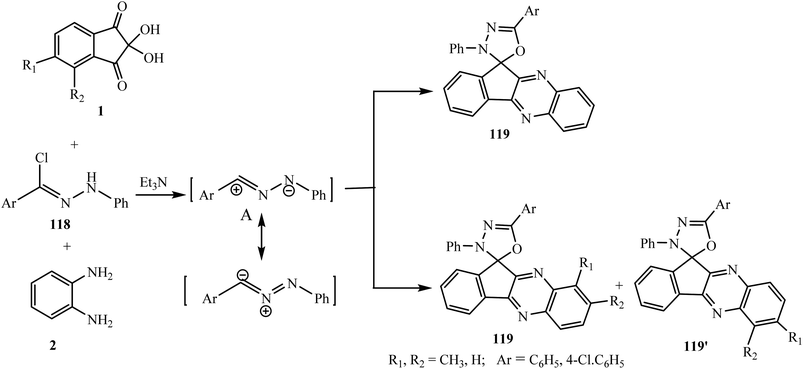
Alizadeh and Moafi99 accomplished the regio- and stereoselective synthesis of the novel spiro framework spiro[indeno[1,2-b]quinoxaline-11,2′-[1,3,4]oxadiazoles] 119 via the three-component reaction of ninhydrin 1, o-phenylenediamines 2 and hydrazonoyl chlorides 118 in the presence of Et3N in EtOH at room temperature.Mechanistically, quinoxalinone 3 formed by the reaction of ninhydrin 1 and o-phenylenediamine 2 reacted with intermediate nitrileimine A generated in situ from hydrazonoyl chloride 118 leading to the formation of the final product 119. This reaction is interesting as only one isomer of spiro adduct 119 is formed in the case of unsubstituted o-phenylenediamine 2; however, in the case of substituted o-phenylenediamines 2 two isomers 119 and 119′ were detected due to the presence of two nonequivalent amino groups (Scheme 61).
5.4. Synthesis of spiro[indeno[1,2-b]quinoxaline[11,2′]thiazolidine]-4′-ones
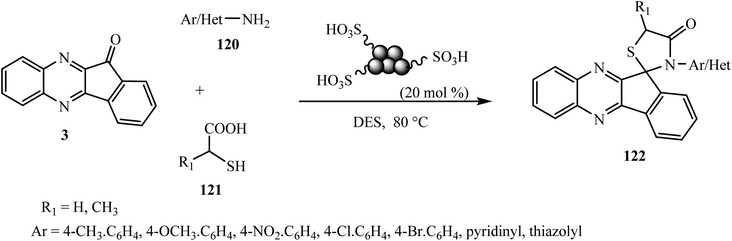
The 4-thiazolidinone moiety is also a very common and privileged heterocyclic target for building many pharmacologically important synthetic and naturally occurring compounds.100 Recently, our group101 developed a convenient and eco-compatible approach for the synthesis of medicinally important novel hybrid spiro[indeno[1,2-b]quinoxaline[11,2′]thiazolidine]-4′-ones 122 via the one-pot multicomponent reaction of indeno[1,2-b]quinoxalinone 3 with various types of amines 120 and α-mercaptocarboxylic acid 121 in the presence of carbon–SO3H as a solid acid catalyst using the green reaction medium urea–choline chloride as a deep eutectic solvent at 80 °C (Scheme 62).The proposed mechanistic pathway for the synthesis of spiro[indeno[1,2-b]quinoxaline[11,2′]thiazolidine]-4′-one 122 is shown in Scheme 63. Firstly, the reaction of indeno[1,2-b]quinoxalinone 3 with aromatic amines 120 affords the corresponding imine derivative A, and then in next step reacts with thioglycolic acid 121 to produce spirothiazolidinone 122.
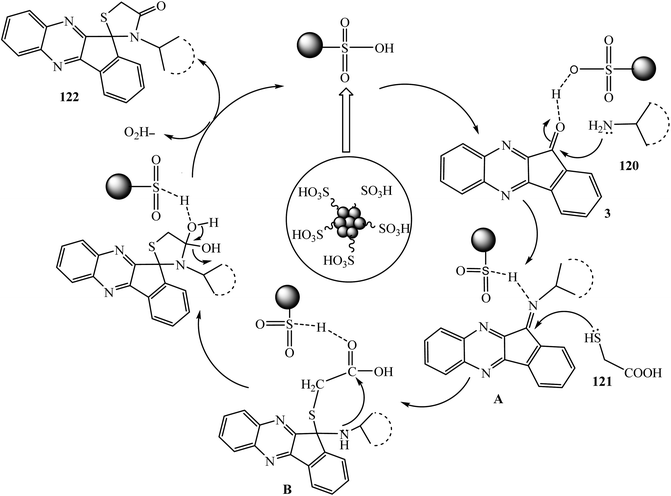 | ||
| Scheme 63 C–SO3H catalyzed synthesis of spiro[indeno[1,2-b]quinoxaline[11,2′]thiazolidine]-4′-ones 122. | ||
5.5. Synthesis of spiroindenoquinoxaline-pyran derivatives
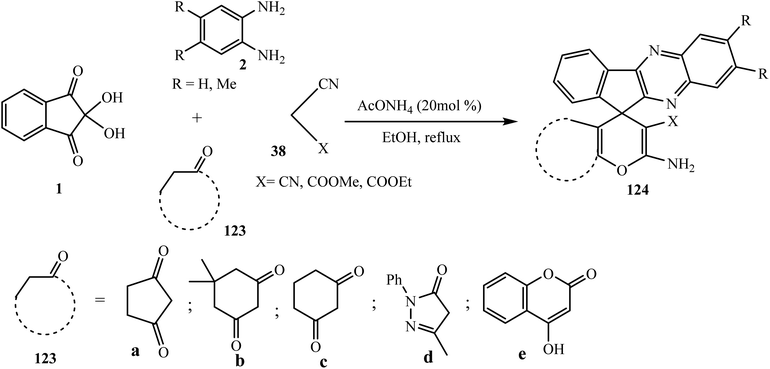
The spiropyrans have been reported for their good activities as hypertensive agents102 and attracted significant interest as potential novel analgesic agents.103 In this connection, Hasaninejad et al.104 in 2011 synthesized 2′-aminospiro[11H-indeno[1,2-b]quinoxaline-11,4′-[4H]pyran] derivatives 124 via the four-component reaction of ninhydrin 1, benzene-1,2-diamine 2, malononitrile and its derivatives 38, and α-methylene carbonyl compounds 123 in the presence of ammonium acetate as a neutral, inexpensive, and dual-activating catalyst. A variety of new spiro-indenoquinoxalines 124 were synthesized using various malononitrile derivatives 38 and α-methylene carbonyl compounds 123a–e (Scheme 64).The mechanism for the synthesis of spiro derivatives 124 involves firstly Knoevenagel condensation between indenoquinoxalinones 3 and malononitrile 38 in the presence of ammonium acetate to afford intermediate A. Thereafter the enol form of the α-methylene carbonyl compound undergoes Michael addition with intermediate A to give intermediate B. The carbonyl group of 123 further attacks the CN group followed by subsequent H-atom shift furnishing 2′-aminospiro[4H-indeno[1,2-b]quinoxaline-11,4′-[4H]pyran]-3′-carbonitriles 124 with 91% yield. In this transformation, AcONH4 plays a dual role as it activates the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of 3 via H-bond formation between one H-atom of NH4 and the O-atom of the C
O group of 3 via H-bond formation between one H-atom of NH4 and the O-atom of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and the malononitriles 38 are activated through deprotonation by the acetate ion derived from AcONH4 (Scheme 65).
O group and the malononitriles 38 are activated through deprotonation by the acetate ion derived from AcONH4 (Scheme 65).
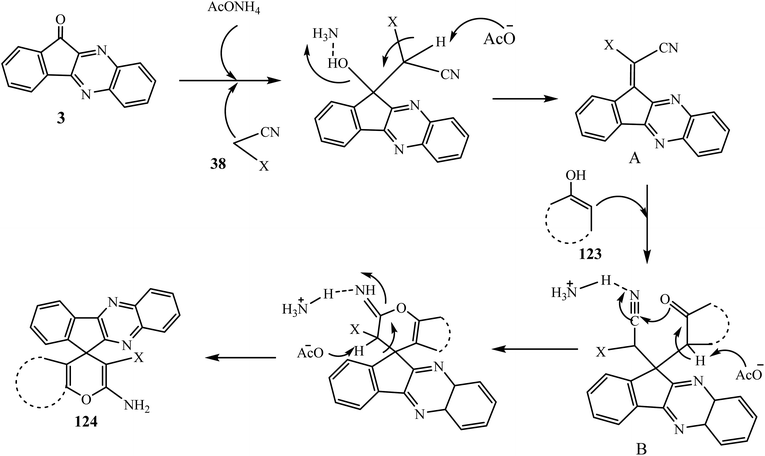 | ||
| Scheme 65 Synthesis of 2′-aminospiro[4H-indeno[1,2-b]quinoxaline-11,4′-[4H]pyran]-3′-carbonitriles 124 catalyzed by ammonium acetate. | ||
After that, the synthesis of substituted 2′-aminospiro[4H-indeno[1,2-b]quinoxaline-11,4′-[4H]pyran]-3′-carbonitrile derivatives 124 was investigated by various researchers via three- and four-component reactions under different reaction conditions (Scheme 66). Hasaninejad et al.105 described the four-component reaction in the presence of the inorganic catalyst indium(III) chloride in acetonitrile under reflux conditions. Previously, in 2007, Perumal et al. used silica gel impregnated with indium(III) chloride for the synthesis of 124 under microwave irradiation and solvent-free conditions via three-component reaction using indenoquinoxalinones 3 as a starting substrate.106
In an another report, Hasaninejad107 introduced a novel heterogeneous silica-supported ionic liquid catalyst, silica-bonded 5-n-propyloctahydropyrimido[1,2-a]azepinium chloride, (SB-DBU)Cl, for the synthesis of spiroindeno[1,2-b]quinoxaline-11′,4-pyrano derivatives 124 in EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent. The used catalyst was recycled and reused fifteen times with unchanged yield.
1) solvent. The used catalyst was recycled and reused fifteen times with unchanged yield.
Soleimani et al.108 reported the synthesis of spiro[indeno[1,2-b]quinoxaline-11′,4-pyrano[2,3-c]pyrazole] carbonitrile derivative 124 via the one-pot three-component reaction of 11H-indeno[1,2-b]quinoxalinone 3 with malononitrile and pyrazolone. In addition, various basic catalysts such as Na2CO3, K2CO3, Et3N, and NaOH were examined in this reaction with different organic solvents such as ethanol, methanol, ethyl acetate, acetonitrile, toluene, and dichloromethane. However, the best results were obtained when the reaction was carried out using Na2CO3 in ethanol.
In 2014, Chen et al.109 introduced a three-component one-pot reaction between indenoquinoxalinone 3, various α-methylene carbonyl compounds (β-diketones, pyrazolones) and malonitrile 38 to give spiroindenoquinoxaline derivative 124 using Et3N as a basic catalyst in EtOH.
In 2016, Zare et al.110 introduced a novel heterogeneous catalyst, acetic acid-functionalized poly(4-vinylpyridinium) bromide, for the synthesis of spiro[indeno[1,2-b]quinoxaline-11′,4-pyrano] derivatives 124 under solvent-free and mild reaction conditions. The prepared spiropyrans were subjected to antioxidant activity screening by DPPH free radical scavenging assay and antifungal activity screening against Fusarium oxysporum. The synthesized spiropyrans showed strong antioxidant activity and also good inhibition activity against Fusarium oxysporum.
Heravi et al. in 2017111 described the synthesis of spiro[indeno[1,2-b]quinoxaline-11′,4-pyrano] derivatives 124 under catalyst-free conditions from the condensation of malononitrile or ethyl 2-cyanoacetate 38, ninhydrin 1, 1,2-phenylenediamine 2, and 1,3-dicarbonyl compounds 123 using trifluoroethanol as an efficient and recyclable medium under ultrasound irradiation at room temperature.
In 2016 Ahmadi et al.112 amino-functionalized SBA-15 (SBA-Pr-NH2) with a pore size of 6 nm. The organocatalyst was used as reusable catalyst for the synthesis of spiro compounds 124 in high yields and in short reaction times.
Safari et al. in 2019113 demonstrated a four-component reaction using NiFe2O4/Ag3PO4 as a novel magnetically reusable nanocatalyst and reported the synthesis of spiro[indeno[1,2-b]quinoxaline-11,4-pyran]-2′-amines 124 in good yield in short reaction time with environmentally friendly conditions. Hojati and coworkers114 demonstrated the synthesis of spiro[indeno[1,2-b]quinoxaline-11′,4-pyrano] derivatives 124 via four-component reaction using magnetically separable heterogeneous nanocatalyst poly(Py-co-Ani)@GO–Fe3O4 in mild reaction conditions.
The mechanistic path for the synthesis of diverse spiroindenoquinoxalines-pyrans 124 involves an initial Knoevenagel condensation between indenoquinoxalinone 3 (derived from the reaction of ninhydrin 1 and benzene-1,2-diamine 2) with the active methylene substrate (malononitrile/ethylcyanoacetate) 38 and after that Michael addition with 1,3-dicarbonyl 123 and then cyclization.
5.6. Spiroindenoquinoxaline-pyridine derivatives
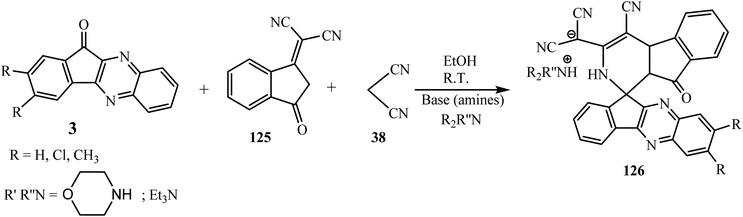
The indenopyridine framework is a privileged heterocyclic scaffold since it appears in the 4-azafluorenone group of alkaloids and is also well known due to biological applications since it has insecticidal, phosphodiesterase inhibiting, antifungal, anti-spermatogenic, antifertility, antagonistic, antidepressant and antiarrhythmic activities.115 As a result, the synthesis of new heterocycles containing both spiroindeno[1,2-b]quinoxalines and indenopyridine moieties may result in the discovery of new drug candidates.In 2016 Bazgir et al.116 designed and synthesized highly functionalized complex products dicyano(4′-cyano-9′-oxo-2′,9′-dihydrospiro[indeno[1,2-b]quinoxaline-11,1′-indeno[2,1-c]pyridine]-3′-yl)methanide salts 126 regioselectively in good yields via multicomponent reaction of 1,1-dicyanomethylene-3-indanone 125, indeno[1,2-b]quinoxalin-11-ones 3 and malononitrile 38 in the presence of amines as a base in ethanol (Scheme 67). The salt was successfully neutralized using dilute hydrochloric acid.
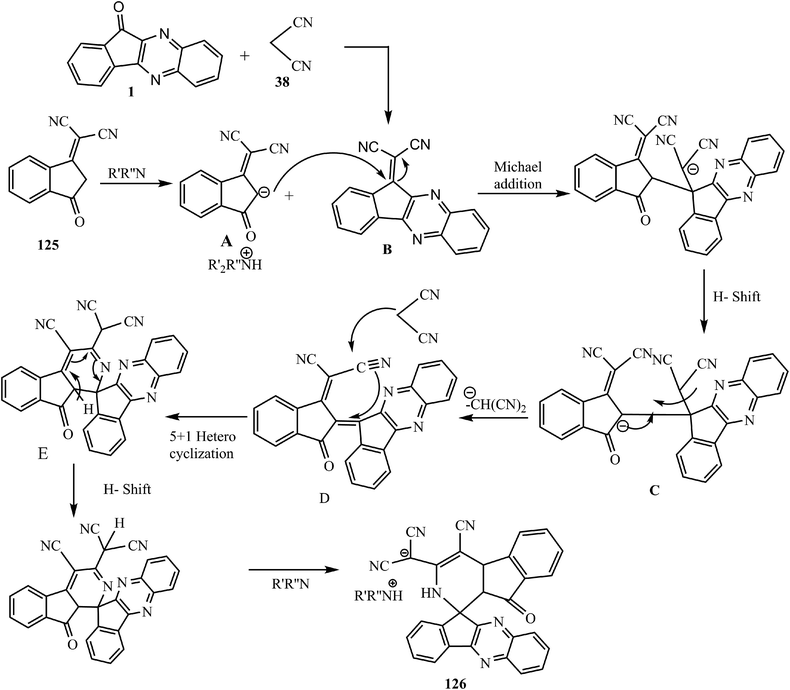
A feasible reaction mechanism for this one-pot process probably occurs via a domino reaction through a Knoevenagel/Michael/elimination/[5 + 1] cyclization series, as outlined in Scheme 68.
Initially, anion intermediate A is formed by the deprotonation of 125 in the presence of amine. Then, Michael-type addition reaction of A to intermediate B (formed in situ by the reaction of 3 and 38) followed by H-shift, produces a new intermediate C, which further converts to intermediate D after the loss of dicyanomethanide from C. Next, the [5 + 1] heterocyclization of this intermediate with malononitrile leads to E. Finally, the H-shift and deprotonation by the amine afford spiro product 126.
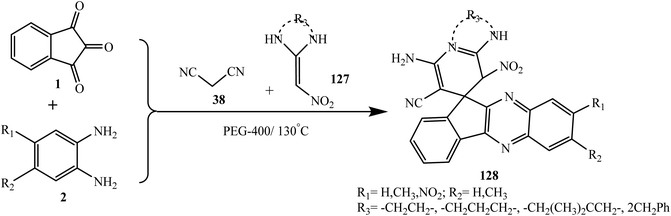
Hasaninejad et al.117 discovered a convenient synthesis of hitherto unknown spiroindenoquinoxaline-pyridine/pyridopyrimidine derivatives 128 via the one-pot multicomponent reaction of ninhydrin 1, substituted o-phenylenediamine 2, malononitrile 38 and N,N′-substituted 2-nitroethene-1,1-diamines 127 under catalyst-free green conditions using PEG-300 (Scheme 69). Synthesized spiro compounds were evaluated both in silico and in vitro for their ability to inhibit AChE and BChE and the results were similar to those of galantamine as a positive control standard.
A tentative mechanism for this transformation is proposed in Scheme 70. It is conceivable that the reaction involves the formation of Knoevenagel adduct 11-(propane-2-ylidene)-11H-indeno[1,2-b]quinoxaline A via the condensation reaction of malononitrile 38 with indenoquinoxalinone 3 (generated in situ from the condensation reaction of o-phenylenediamine 2 with ninhydrin 1) followed by the Michael addition of various 2-nitroethene-1,1-diamines 127. Subsequently, the cycloaddition of an amine group to the cyano moiety occurs with successive rearrangements to afford the desired corresponding spiroindenoquinoxaline derivatives 128 in good yields.
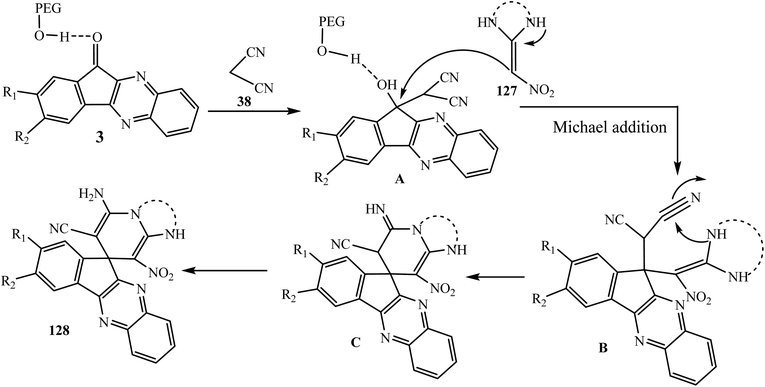 | ||
| Scheme 70 Plausible mechanism for the synthesis of spiroindenoquinoxaline-pyridine/pyridopyrimidines 128. | ||
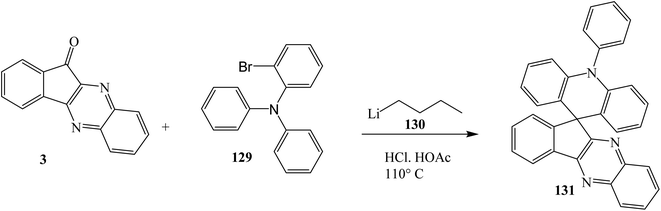
In 2018, Fan et al.118 reported the novel spirocyclic compound 10H-spiro[acridine-9,110-indeno[1,2-b]quinoxaline] (SAIQ) 131 containing an electron-donating acridine unit and an electron-withdrawing pyrazine segment by the reaction of indeno[1,2-b]quinoxalinone 3, 2-bromo-N,N-diphenylaniline 129 and n-butyl lithium 130 using THF at −78 °C (Scheme 71). In that report the basic physical and chemical properties of SAIQ were studied in detail. In addition, blue/green/red and green/red phosphorescent organic light-emitting diodes were fabricated with SAIQ as host materials and showed a relatively high device performance with maximum external quantum efficiencies.
5.7. Spiroindenoquinoxaline-perimidines
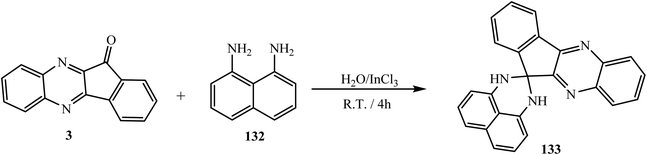
Bazgir et al.119 reported the synthesis of 1′,3′-dihydrospiro[indeno[2,1-b]quinoxaline-11,2′-perimidine] 133 via the cyclic condensation reaction of naphthalene-1,8-diamine 132 and indeno[2,1-b]quinoxalinone 3 using water as a “green” reaction medium at room temperature under mild reaction conditions (Scheme 72).6. Conclusions
This review summarizes the use of indenoquinoxalinone as a building block in the synthesis of the diverse spirocyclic frameworks of heterocyclic compounds to date. Indenoquinoxalinone has been utilized in the construction of spiro-β-lactams, spirofurans, mono- and dispiropyrrolidines/pyrrolizidines, spirothiazolidinones, and spiropyran skeletons grafted with other bioactive moieties. Most reactions described in this review involve multiple components and cycloaddition, and interesting examples of the regio- and stereoselective synthesis of biologically relevant compounds have also been presented. Many synthetic compounds also exhibit potential antimicrobial, anticancer and other interesting activities.We think that this review will draw the attention of researchers in the field of synthetic organic chemistry and biology for the design and development of new viable spirocyclic frameworks to deliver new drug candidates in the future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial assistance from the CSIR and DST (SERB), New Delhi, is gratefully acknowledged.References
- (a) N. O. Danylkova, S. R. Alcala, H. D. Pomeranz and L. K. McLoon, Exp. Eye Res., 2007, 84, 293–301 CrossRef CAS; (b) C. L. S. Sanz and E. M. Garcia-Sipido, Med. Clin., 2014, 142, 179–180 CrossRef; (c) R. C. Klesges, K. C. Johnson and G. Somes, JAMA, J. Am. Med. Assoc., 2006, 296, 94–95 CrossRef; (d) S. A. Kotharkar and D. B. Shinde, Bioorg. Med. Chem. Lett., 2006, 16, 6181–6184 CrossRef CAS.
- A. Kumbhar, S. Kamble, M. Barge, G. Rashinkar and R. Salunkhe, Tetrahedron Lett., 2012, 53, 2756–2760 CrossRef CAS.
- The Chemistry of Heterocyclic Compounds Quinoxalines: Supplements II, ed. J. D. Brown, C. E. Taylor and P. Wipf, John Wiley and Sons, New Jersey, 2004 Search PubMed.
- Z. Zhao, R. G. Robinson, S. F. Barnett, D. D. Jones, R. E. Jones, G. D. Hartman, H. E. Huber, M. E. Duggana and C. W. Lindsley, Bioorg. Med. Chem. Lett., 2008, 18, 49–53 CrossRef CAS.
- S. P. Tanis, J. W. Strohbach, T. T. Parker, M. W. Moon, S. Thaisrivongs, W. R. Perrault, T. A. Hopkins, M. L. Knechtel, N. L. Oien, J. L. Wieber, K. J. Stephanski and M. W. Wathen, Bioorg. Med. Chem. Lett., 2010, 20, 1994–2000 CrossRef CAS.
- P. Chen, D. Norris, E. J. Iwanowicz, S. H. Spergel, J. Lin, H. H. Gu, Z. Shen, J. Wityak, T.-A. Lin, S. Pang, H. F. D. Fex, S. Pitt, D. R. Shen, A. M. Doweyko, D. A. Bassolino, J. Y. Roberge, M. A. Poss, B.-C. Chen, G. L. Schievend and J. C. Barrisha, Bioorg. Med. Chem. Lett., 2002, 12, 1361–1364 CrossRef CAS.
- Z. Szekelyhidi, J. Pato, F. Waczek, P. Banhegyi, B. H. Barakonyi, D. Eros, G. Meszaros, F. Hollosy, D. Hafenbradl, S. Obert, B. Klebl, G. Keri and L. Orfi, Bioorg. Med. Chem. Lett., 2005, 15, 3241–3246 CrossRef CAS.
- S. Y. Hassan, S. N. Khattab, A. A. Bekhit and A. Amer, Bioorg. Med. Chem. Lett., 2006, 16, 1753–1756 CrossRef CAS.
- C. H. Tseng, Y. R. Chen, C. C. Tzeng, W. Liu, C. K. Chou, C. C. Chiu and Y. L. Chen, Eur. J. Med. Chem., 2016, 27, 258–273 CrossRef.
- I. A. Schepetkin, A. I. Khlebnikov, A. S. Potapov, A. R. Kovrizhina, V. V. Matveevskaya, M. L. Belyanin, D. N. Atochin, S. O. Zanoza, N. M. Gaidarzhy, S. A. Lyakhov, L. N. Kirpotina and M. T. Quinn, Eur. J. Med. Chem., 2019, 1, 179–191 CrossRef.
- R. A. Rajasekaran, Iran. J. Pharm. Res., 2007, 3, 251–262 Search PubMed.
- C.-H. Tseng, C.-C. Tzeng, C.-L. Yang, P.-J. Lu, H.-L. Chen, H.-Y. Li, Y.-C. Chuang, C.-N. Yang and Y.-L. Chen, J. Med. Chem., 2010, 53, 6164–6179 CrossRef CAS.
- B. Obot and N. O. Obi-Egbedi, Mater. Chem. Phys., 2010, 122, 325–328 CrossRef.
- Y. Zheng, C. M. Tice and S. B. Singh, Bioorg. Med. Chem. Lett., 2014, 24, 3673–3682 CrossRef CAS.
- E. Chupakhin, O. Babich, A. Prosekov, L. Asyakina and M. Krasavin, Molecules, 2019, 24, 4165–4204 CrossRef CAS.
- H. A. Etman, H. M. Metwally, M. M. Elkasaby, A. M. Khalil and M. A. Metwally, Am. J. Org. Chem., 2011, 1, 10–13 CrossRef.
- D. L. Boger, T. V. Hughes and M. P. Hedrick, J. Org. Chem., 2001, 66, 2207–2216 CrossRef CAS.
- S. Yoshida, T. C. Rosen, O. G. J. Meyer, M. J. Sloan, S. Ye, G. Haufe and K. L. Kirk, Bioorg. Med. Chem., 2004, 12, 2645–2652 CrossRef CAS.
- J. Salaün, Topics in Current Chemistry, Springer Berlin, Heidelberg, 2000, vol. 207, pp. 1–67 Search PubMed.
- R. Faust, Angew. Chem., Int. Ed., 2001, 40, 2251–2253 CrossRef CAS.
- M. Shaabanzadeh and F. Khabari, ARKIVOC, 2009, 11, 307–315 Search PubMed.
- H. G. Lindwall and J. S. Maclennan, J. Am. Chem. Soc., 1932, 54, 4739–4744 CrossRef CAS.
- M. Shaabanzadeh, H. Hashemimoghaddam, M. B. Torbati and T. S. Ahoee, J. Theor. Comput. Chem., 2012, 11, 1227–1236 CrossRef CAS.
- J. Azizian, M. Shaabanzadeh, F. Hatamjafari and M. R. Mohammadizadeh, ARKIVOC, 2006, 11, 47–58 Search PubMed.
- R. Southgate, C. Branch, S. Coulton and E. Hunt, Recent Progress in Chemical Synthesis of Antibiotics and Related Microbial Products, ed. G. Lukacs, Springer Press, Berlin, 1993, vol. 2, p. 621 Search PubMed.
- J. A. Rad, A. Jarrahpour, C. C. Ersanlı, Z. A. glu, M. Akkrut and E. Turos, Tetrahedron, 2017, 73, 1135–1142 CrossRef.
- H. Nakajima, T. Hamasaki, S. Maeta, Y. Kimura and Y. Takeuchi, Phytochemistry, 1990, 29, 1739–1743 CrossRef CAS.
- J. Azizian, A. R. Karimi, A. A. Mohammadi and M. R. Mohammadizadeh, Heterocycles, 2004, 63, 2225–2229 CrossRef CAS.
- N. Sabouri, G. H. Mahdavinia and B. Notash, Chin. Chem. Lett., 2016, 27, 1040–1043 CrossRef CAS.
- M. Zarei-Haji-Abadi, R. Mohebat and M. H. Mosslemin, Lett. Org. Chem., 2017, 14, 43–48 CrossRef CAS.
- M. T. Maghsoodlou, S. M. Habibi-Khorassani, A. Moradi, N. Hazeri, A. Davodi and S. S. Sajadikhah, Tetrahedron, 2011, 67, 8492–8495 CrossRef CAS.
- A. Yazdani-Elah-Abadia, M.-T. Maghsoodlou, R. Mohebat and R. Heydaria, J. Chem. Sci., 2017, 129, 691–698 CrossRef.
- P. Dauban and G. Malik, Angew. Chem., Int. Ed., 2009, 48, 9026–9029 CrossRef CAS.
- S. Kobayashi, K. A. Jorgensen, K. V. Gothelf and K. A. Jorgensen, Chem. Rev., 1998, 98, 863–909 CrossRef.
- M. S. Singh, S. Chowdhury and S. Koley, Tetrahedron, 2016, 72, 1603–1644 CrossRef CAS.
- N. Lashgari and M. Ziarani, ARKIVOC, 2012, 1, 277–320 Search PubMed.
- M. Narayanarao, L. Koodlur, V. G. Revanasiddappa, S. Gopal and S. Kamila, Beilstein J. Org. Chem., 2016, 12, 2893–2897 Search PubMed.
- L. Maiuolo, V. Algieri, F. Olivito and A. De Nino, Catalysts, 2020, 10, 65–92 Search PubMed.
- M. R. Mohammadizadeh and N. Firoozi, Bull. Korean Chem. Soc., 2009, 30, 1877–1880 CrossRef CAS.
- M. Moemeni, H. Arvinnezhad, S. Samadi, M. Tajbakhsh, K. Jadidi and H. R. Khavasi, J. Heterocycl. Chem., 2012, 49, 190–194 CrossRef CAS.
- M. Moemeni, H. Arvinnezhad, S. Samadi, F. Salahi, K. Jadidi and B. Notash, J. Heterocycl. Chem., 2014, 6, 34–45 Search PubMed.
- A. Yu. Barkov, N. S. Zimnitskiy, V. Yu. Korotaev, I. B. Kutyashev, V. S. Moshkin and V. Y. Sosnovskikh, Chem. Heterocycl. Compd., 2017, 53, 1315 CrossRef CAS.
- A. A. Karsalarya, M. R. Mohammadizadeh, A. R. Hasaninejad, A. A. Mohammadic and A. R. Karimi, J. Iran. Chem. Soc., 2010, 7, 45–50 CrossRef.
- (a) A. Pejovic, I. Damljanovic, D. Stevanovic, M. Vukicevic, S. B. Novakovic, G. A. Bogdanovic, N. Rudulovic and R. D. Vukicevic, Polyhedron, 2012, 31, 789–795 CrossRef CAS; (b) S. L. Shen, J. H. Shao, J. Z. Luo, J. T. Liu, J. Y. Miao and B. X. Zhao, Eur. J. Med. Chem., 2013, 63, 256–268 CrossRef CAS.
- A. R. Suresh Babu, D. Gavaskar and R. Raghunathan, Tetrahedron Lett., 2012, 53, 6676–6681 CrossRef CAS.
- A. R. Suresh Babu, D. Gavaskar and R. Raghunathan, J. Organomet. Chem., 2013, 745–746, 409–416 CrossRef CAS.
- B. V. Kumar, D. Gavaskar, T. Srinivasan, R. Raghunathan and D. Velmurugan, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2012, 68, 1382–1383 Search PubMed.
- B. V. Kumar, D. Gavaskar, T. Srinivasan, R. Raghunathan and D. Velmurugan, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2012, 68, 1576–1577 Search PubMed.
- N. Arumugam, A. I. Almansour, R. S. Kumar and N. Dege, J. King Saud Univ., Sci., 2020, 32, 2500–2504 CrossRef.
- D. Gavaskar, A. R. Suresh Babu, R. Raghunathan, M. Dharani and S. Balasubramanian, J. Organomet. Chem., 2014, 768, 128–135 CrossRef CAS.
- A. M. Akondi, S. Mekala, M. L. Kantam, R. Trivedi, L. R. Chowhane and A. Das, New J. Chem., 2017, 41, 873–878 RSC.
- E. Ramesh, M. Kathiresan and R. Ragunathan, Tetrahedron Lett., 2007, 48, 1835–1839 CrossRef CAS.
- A. Yu. Barkov, N. S. Zimnitskiy, V. Yu. Korotaev, I. B. Kutyashev, V. S. Moshkin and V. Y. Sosnovskikh, Chem. Heterocycl. Compd., 2017, 53, 451–459 CrossRef CAS.
- J. Nie, H. C. Guo, D. Cahard and J.-A. Ma, Chem. Rev., 2011, 111, 455–529 CrossRef CAS.
- A. Yu. Barkov, N. S. Zimnitskiy, I. B. Kutyashev, V. Yu. Korotaev, V. S. Moshkin and V. Y. Sosnovskikh, J. Fluorine Chem., 2017, 204, 37–44 CrossRef CAS.
- N. Arumugam, A. I. Almansour, R. S. Kumar, S. I. Alaqeel, V. S. Krishna and D. Sriram, Bioorg. Chem., 2020, 99, 103799–103806 CrossRef CAS.
- N. Arumugam, A. I. Almansour, R. S. Kumar, A. J. Mohammad Ali Al-Aizari, S. I. Alaqeel, S. Kansız, V. S. Krishna, D. Sriram and N. Dege, RSC Adv., 2020, 10, 23522–23531 RSC.
- M. Li, F.-M. Gong, L.-R. Wen and Z.-R. Li, Eur. J. Org. Chem., 2011, 3482–3490 CrossRef CAS.
- A. V. Velikorodov, N. N. Stepkina, E. A. Shustova and V. A. Ionova, Russ. J. Org. Chem., 2015, 51, 674–679 CrossRef CAS.
- A. Yu. Barkov, N. S. Zimnitskiy, I. B. Kutyashev, V. Yu. Korotaev and V. Y. Sosnovskikh, Chem. Heterocycl. Compd., 2018, 54, 43–50 CrossRef CAS.
- R. Wen, L. Cen, Y. Ma, J. Wang and S. Zhu, Tetrahedron Lett., 2018, 59, 1686–1690 CrossRef CAS.
- N. Shahrestani, F. Salahi, N. Tavakoli, K. Jadidi, M. Hamzehloueian and B. Notasha, Tetrahedron: Asymmetry, 2015, 26, 1117–1129 CrossRef CAS.
- M. S. Reddy, L. R. Chowhan, N. S. Kumar, P. Ramesh and S. B. Mukkamala, Tetrahedron Lett., 2018, 59, 1366–1371 CrossRef CAS.
- M. R. Reddy, N. S. Kumar and L. R. Chowhan, RSC Adv., 2018, 8, 35587–35593 RSC.
- S. Gupta and J. M. Khurana, ChemistrySelect, 2019, 4, 7200–7203 CrossRef CAS.
- J. Azizian, A. R. Karimi, R. Dastkhan, A. A. Mohammadi and M. R. Mohammadizadeh, J. Chem. Res., 2004, 347–349 CrossRef CAS.
- P. Pattanaik, S. Nayak, D. R. Mishra, P. Panda, B. P. Raiguru, N. P. Mishra, S. Mohapatra, A. Mallampuri and C. S. Purohit, Tetrahedron Lett., 2018, 59, 2688–2694 CrossRef CAS.
- S. Nayak, P. Pattanaik, S. Mohapatra, D. R. Mishra, P. Panda, B. P. Raiguru, N. P. Mishra, S. Jena and H. S. Biswal, Synth. Commun., 2019, 49, 1823–1835 CrossRef CAS.
- K. S. Mani, W. Kaminsky and S. P. Rajendran, New J. Chem., 2018, 42, 301–310 RSC.
- K. S. Mani, B. Murugesapandian, W. Kaminsky and S. P. Rajendran, Tetrahedron Lett., 2018, 59, 2921–2929 CrossRef.
- D. A. Calarese, C. N. Scanlan, M. B. Zwick, S. Deechongkit, Y. Mimura, R. Kunert, P. Zhu, M. R. Wormald, R. L. Stanfield, K. H. Roux, J. W. Kelly, P. M. Rudd, R. A. Dwek, H. Katinger, D. R. Burton and I. A. Wilson, Science, 2003, 300, 2065–2071 CrossRef CAS.
- V. R. Doddi, H. P. Kokatla, A. P. J. Pal, R. K. Basak and Y. D. Vankar, Eur. J. Org. Chem., 2008, 5731–5739 CrossRef CAS.
- L. Dammak, M. Kammoun, N. Allouche, H. Ammar and S. Abid, Org. Commun., 2017, 10, 32–39 CrossRef CAS.
- R. Mallikarjuna, M. Yedukondalu, P. S. S. D. Varma, D. M. Vandana and D. Manidhar, Pharma Chem., 2015, 7, 117–129 CAS.
- J. N. S. Rao and R. Raghunathan, Tetrahedron Lett., 2015, 56, 1539–1544 CrossRef CAS.
- J. N. S. Rao and R. Raghunathan, Tetrahedron Lett., 2015, 56, 2276–2279 CrossRef.
- A. S. Filatov, N. A. Knyazev, M. N. Ryazantsev, V. V. Suslonov, A. G. Larina, A. P. Molchanov, R. R. Kostikov, V. M. Boitsov and A. V. Stepakov, Org. Chem. Front., 2018, 5, 595–605 RSC.
- Y. Santiago-Vazquez, S. Das, U. Das, E. Robles-Escajeda, N. M. Ortega, C. Lema, A. Varela-Ramírez, R. J. Aguilera, J. Balzarini and E. D. Clercq, Eur. J. Med. Chem., 2014, 77, 315–322 CrossRef CAS.
- S. T. Harini, H. V. Kumar, J. Rangaswamy and N. Naik, Bioorg. Med. Chem. Lett., 2012, 22, 7588–7592 CrossRef CAS.
- R. S. Kumar, A. I. Almansour, N. Arumugam, F. Mohammad, W. S. Alshahrani, D. Kotresha, M. Altaf, M. Azam and J. C. Menendez, RSC Adv., 2018, 8, 41226–41236 RSC.
- R. S. Kumar, S. M. Rajesh, D. Banerjee, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2010, 45, 411–422 CrossRef CAS.
- N. Arumugam, R. S. Kumar, A. I. Almansour, M. Altaf, R. Padmanaban, P. S. babu, G. Angamuthu, D. Kotresha, T. S. Manohar and S. Venketesh, Bioorg. Chem., 2018, 79, 64–71 CrossRef CAS.
- N. Arumugam, G. Periyasami, R. Raghunathan, S. Kamalraj and J. Muthumary, Eur. J. Med. Chem., 2011, 46, 600–607 CrossRef CAS.
- S. M. Rajesh, B. D. Bala and S. Perumal, Tetrahedron Lett., 2012, 53, 5367–5371 CrossRef.
- K. Malathi, S. Kanchithalaivan, R. R. Kumar, A. I. Almansour, R. S. Kumar and N. Arumugam, Tetrahedron Lett., 2015, 56, 6132–6135 CrossRef CAS.
- A. I. Almansour, N. Arumugam, R. S. Kumar, D. M. Al-thamili, G. Periyasami, K. Ponmurugan, N. A. Al-Dhabi, K. Perumal and D. Premnath, Molecules, 2019, 24, 1962 CrossRef.
- N. Arumugam, A. I. Almansour, R. S. Kumar, D. Kotresha, R. Saiswaroop and S. Venketesh, Bioorg. Med. Chem., 2019, 27, 2621–2628 CrossRef CAS.
- (a) S. Fujimori, Jap. Pat. Appl. 882912 Chem. Abstr. 1990, 112, 98409 Search PubMed; (b) R. T. Brown, in Indoles, Part 4, The Monoterpenoid Indole Alkaloids, ed. J. E. Saxton, John Wiley, New York, 1983, pp. 147–199 Search PubMed.
- J. B. Hendrickson and R. A. Silva, J. Am. Chem. Soc., 1962, 34, 643 CrossRef.
- A. R. S. Babu and R. Raghunathan, Synth. Commun., 2008, 38, 1433–1438 CrossRef CAS.
- A. R. S. Babu and R. Raghunathan, Tetrahedron Lett., 2006, 47, 9221–9225 CrossRef CAS.
- W. Ren, Q. Zhao, M. Yu, L. Guo, H. Chang, X. Jiang, Y. Luo, W. Huang and G. He, Mol. Diversity, 2020, 24, 1043–1063 CrossRef CAS.
- S. Haddad, S. Boudriga, F. Porzio, A. Soldera, M. Askri, M. Knorr, Y. Rousselin, M. M. Kubicki, C. Golz and C. Strohmann, J. Org. Chem., 2015, 80, 9064–9075 CrossRef CAS.
- D. Ren, X. Hu, Y. Huang and X. Fang Li, J. Chem. Res., 2018, 42, 453–455 CrossRef CAS.
- M. A. Rani, S. V. Kumar, K. Malathi, M. Muthu, A. I. Almansour, R. S. Kumar and R. R. Kumar, ACS Comb. Sci., 2017, 19, 308–314 CrossRef.
- D. Gavaskar, A. R. S. Babu, R. Raghunathan, M. Dharani and S. Balasubramanian, Steroids, 2016, 109, 1–6 CrossRef CAS.
- F.-H. Liu, Y.-B. Song, L.-J. Zhai and M. Li, J. Heterocycl. Chem., 2015, 52, 322–329 CrossRef CAS.
- D. Gavaskar, R. Raghunathan and A. R. S. Babu, Tetrahedron Lett., 2014, 55, 2217–2220 CrossRef CAS.
- A. Alizadeh and L. Moafi, Heterocycl. Commun., 2017, 23, 375–378 CAS.
- R. C. Gadwood, B. V. Kamdar, L. A. C. Dubray, M. L. Wolfe, M. P. Smith, W. Watt, S. A. Mizsak and V. E. Groppi, J. Med. Chem., 1993, 36, 1480–1487 CrossRef CAS.
- R. Singh, S. A. Ganaie, A. Singh and A. Chaudhary, Synth. Commun., 2019, 49, 80–93 CrossRef CAS.
- B. L. Bourdonnec, R. T. Windh, L. K. Leister, Q. J. Zhou, C. W. Ajello, M. Gu, G.-H. Chu, P. A. Tuthill, W. M. Barker, M. Koblish, D. D. Wiant, T. M. Graczyk, S. Belanger, J. A. Cassel, M. S. Feschenko, B. L. Brogdon, S. A. Smith, M. J. Derelanko, S. Kutz, P. J. Little, R. N. DeHaven, D. L. DeHaven-Hudkins and R. E. Dolle, J. Med. Chem., 2009, 52, 5685–5702 CrossRef.
- F. Cardano, E. D. Canto and S. Giordani, Dalton Trans., 2019, 48, 15537–15544 RSC.
- A. Hasaninejad, N. Golzara, M. Shekouhya and A. Zare, Helv. Chim. Acta, 2011, 94, 2289–2294 CrossRef CAS.
- A. Hasaninejad, N. Golzar and A. Zare, J. Heterocycl. Chem., 2013, 50, 608–614 CrossRef CAS.
- G. Shanthi, G. Subbulakshmi and P. T. Perumal, Tetrahedron, 2007, 63, 2057–2063 CrossRef CAS.
- A. Hasaninejad, N. Golzar, M. Beyrati, A. Zare and M. M. Doroodmand, J. Mol. Catal. A: Chem., 2013, 372, 137–150 CrossRef CAS.
- E. Soleimani, M. Hariri and P. Saei, C. R. Chim., 2013, 16, 773–777 CrossRef CAS.
- F. Chen, J. Zheng, M. Huang and Y. Li, Res. Chem. Intermed., 2015, 41, 5545–5554 CrossRef CAS.
- A. R. Moosavi-Zare, M. A. Zolfigol, E. Noroozizadeh, M. Zarei, R. Karamian and M. Asadbegy, J. Mol. Catal. A: Chem., 2016, 425, 217–228 CrossRef CAS.
- M. R. P. Heravi and F. Norouzy, Res. Chem. Intermed., 2017, 43, 4265–4282 CrossRef.
- T. Ahmadi, G. M. Ziarani, S. Bahar and A. Badiei, J. Iran. Chem. Soc., 2018, 15, 1153–1161 CrossRef CAS.
- N. H. Nasab and J. Safari, Polyhedron, 2019, 164, 74–79 CrossRef.
- S. F. Hojati, A. Amiri and E. Fardi, Appl. Organomet. Chem., 2020, 34, e5604 CrossRef CAS.
- (a) S. D. Crawford, E. G. Rowley, J. R. Eldridge, F. Schuler, D. M. Roush, J. W. Lyga, B. Frank and S. Sehgel, WO 2006089038A2, 2006Chem. Abstr., 2006, 145, 243208; (b) C. E. Cook, C. D. Sloan, B. F. Thomas and H. A. Navarro, US Pat. 2004147539A1, 2004transChem. Abstr., 2004, 141, 157039 Search PubMed; (c) S. A. Hild, M. L. Meistrich, R. P. Blye and J. R. Reel, Biol. Reprod., 2001, 65, 165 CrossRef CAS; (d) C. Upton, R. H. Osborne and M. Jaffar, Bioorg. Med. Chem. Lett., 2000, 10, 1277–1279 CrossRef CAS; (e) C. Safak, R. Simsek, Y. Altas, S. Boydag and K. Erol, Boll. Chim. Farm., 1997, 136, 665–669 CAS; (f) C. E. Cook, Y. W. Lee, M. C. Wani, P. A. Fail and J. M. Jump, US Pat. 5319084, 1993Chem. Abstr.,1995, 122, 265250a Search PubMed; (g) M. D. Meyer, J. F. De Bernardis and A. A. Hancock, J. Med. Chem., 1994, 37, 105–112 CrossRef CAS; (h) R. Kunstmann and G. Fischer, J. Med. Chem., 1984, 27, 1312–1316 CrossRef CAS.
- G. I. Shakibaei and A. Bazgir, RSC Adv., 2016, 6, 22306–22311 RSC.
- A. Maryamabadi, A. Hasaninejad, N. Nowrouzi and G. Mohebbi, Bioorg. Med. Chem., 2017, 25, 2057–2064 CrossRef CAS.
- X.-Y. Liu, Yi-J. Zhang, X. Fei, Q. Ran, M.-K. Fung and J. Fan, J. Mater. Chem. C, 2019, 7, 1370–1378 RSC.
- Z. Yasaei, P. Mirzaei and A. Bazgir, C. R. Chim., 2010, 13, 1308–1312 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |