Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A regioselective C7 bromination and C7 palladium-catalyzed Suzuki–Miyaura cross-coupling arylation of 4-substituted NH-free indazoles†
Khalid Boujdiabc,
Nabil El Brahmia,
Jérôme Graton b,
Didier Dubreuilb,
Sylvain Colletb,
Monique Mathé-Allainmat*b,
Mohamed Akssirac,
Jacques Lebretonb and
Saïd El Kazzouli
b,
Didier Dubreuilb,
Sylvain Colletb,
Monique Mathé-Allainmat*b,
Mohamed Akssirac,
Jacques Lebretonb and
Saïd El Kazzouli *a
*a
aEuromed Research Center, School of Engineering in Biomedical and Biotechnology, Euromed University of Fes (UEMF), Route de Meknes, 30000 Fez, Morocco. E-mail: s.elkazzouli@ueuromed.org
bLaboratoire CEISAM–UMR 6230, Université de Nantes, CNRS, Faculté des Sciences et des Techniques, 2 rue de la Houssinière, BP 92208, 44322 Nantes Cedex 3, France
cFaculty of Sciences and Technologies Mohammedia, University Hassan 2, URAC 22 FSTM University Hassan II – Casablanca, BP 146, 28800 Mohammedia, Morocco
First published on 10th February 2021
Abstract
A direct and efficient regioselective C7-bromination of 4-substituted 1H-indazole has been achieved. Subsequently, a successful palladium-mediated Suzuki–Miyaura reaction of C7-bromo-4-substituted-1H-indazoles with boronic acids has been performed under optimized reaction conditions. A series of new C7 arylated 4-substituted 1H-indazoles was obtained in moderate to good yields.
Introduction
The aryl-heteroaryl compounds are an important class of organic entities used as promising building blocks of many biologically active molecules and drugs1–6 and the ability to combine aryl and heteroaryl fragments by the formation of new C–C bonds7–11 is an important and challenging field in organic chemistry. The palladium-catalyzed Suzuki–Miyaura coupling process12–16 is probably one of the most efficient methods nowadays to create C(sp2)–C(sp2) bonds because of its mild reaction conditions, broad substrate scope, broad functional group tolerance, and the high air and water stability of the boronic acids.12,17–20Indazoles are often the key fragments in several important compounds, with a broad range of biological activities for anticancer,21–23 HIV-protease inhibition,24 antimicrobial25 and anti-inflammatory26 purposes. For this reason, various procedures have been developed for their synthesis27–30 and functionalization.31–45
In particular, indazoles and bioisosteres containing sulfonamide moieties on either the C7 or C4 position have shown interesting anticancer activities.46–48 For these reasons we decided to introduce sulfonamides at position C4 of the indazole ring prior to the functionalization at the C7 position in order to give access to a diversity of new potentially bioactive compounds.
Recently, our group and others were actively involved in the functionalization of NH-free or protected 1H and 2H-indazoles. Despite the recent advances made in this field, namely the direct C3 and C7-arylations32,49–51 and the Suzuki–Miyaura coupling at C3, C4, C5 and C6 positions,31,52–54 to date, no example of selective arylation of NH-free or protected indazoles using Suzuki–Miyaura process, has been described at the C7 position. It is important to note that we previously reported only two examples of C7 direct arylation of indazoles containing a C4 nitro group in which C3 position was already substituted with a phenyl group. Moreover, during these investigations with nitro substrates, we were unable to introduce heteroaryl substituents.55 At this point, it was clear to us that the higher reactivity of the C3 position over C7 unsubstituted indazole derivatives could be a limitation to efficiently prepare new C7 substituted and C3 free scaffolds.
To bypass this limitation, we examine herein the influence of the electronic properties of some C4 sulfonamido- or amido-substituents at the 1H-indazole nucleus, on the course of a regioselective C7-halogenation, followed by Suzuki–Miyaura coupling reactions, aiming at developing novel series of 7-aryl-4-sulfonamido or 7-aryl-4-amido-1H-indazole compounds.
Results and discussion
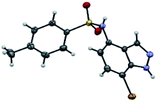
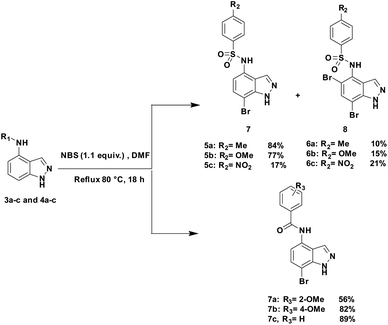
The first step in our investigation pathway was to prepare the 4-substituted 1H-indazoles 3a–c, 4a–c, used as starting materials in this study. They were synthesized following known procedures starting from 4-nitroindazole according to the Scheme 1.47,56 The sulfonylation of free 4-amino-indazole 2 with one equivalent of the selected sulfonyl chlorides gave the expected sulfonamides 3a–c in 75–83% yields. The 4-amino acylation of indazole 2 with a carboxylic acid in the presence of coupling reagents afforded amide 4a with a low yield.56 Satisfyingly, in dichloromethane as the solvent and with acyl chloride as the acylating agent, the yield could be optimized, due to the precipitation of the expected amide 4b and 4c during the reaction, probably avoiding the formation of N-1 acyl side-product. These compounds were isolated by simple filtration with high yields of 82% and 87%, respectively.The regioselective C7 bromination of N-(1H-indazol-4-yl)-4-methylbenzenesulfonamide 3a, used as model substrate, was attempted with N-bromosuccinimide (NBS)57,58 as depicted in Table 1. Compared to room temperature conditions (Table 1, entry 1), we were pleased to find that the treatment of N-(1H-indazol-4-yl)-4-methylbenzenesulfonamide 3a with 1.1 equivalents of NBS in DMF at 80 °C provided the desired C7 halogenated product 5a in 84% yield along with 10% of the 5,7-dibrominated compound 6a, (Table 1, entry 2). Testing microwave activation conditions afforded degradation (Table 1, entry 3). Moreover, when 2 equivalents of NBS were used, the 5,7-dibrominated compound 6a was obtained with 88% yield, without identification of any 3-brominated derivative (Table 1, entry 4). Based on previous results obtained for indazole halogenation in basic conditions,46 we observed in this case a rapid formation of the dibrominated compound 6a, in the presence of 2 equivalents of NaOH or KOH (Table 1, entries 5 and 6). The structure of the compound 5a as its 7-bromo-1H-indazole form was proved by X-ray diffraction analysis (Fig. 1) (see the ESI†).
| Entry | T (°C) | NBS (equiv.) | t (h) | Base | Yieldsa 5a/6a |
|---|---|---|---|---|---|
a Yields after column chromatography purification.b Reaction conditions optimized for 5a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a (1 mmol), NBS (1.1 mmol), DMF (5 mL), 80 °C for 18 h.c MW = microwaves.d 2 equivalents of base.e tr = traces. 3a (1 mmol), NBS (1.1 mmol), DMF (5 mL), 80 °C for 18 h.c MW = microwaves.d 2 equivalents of base.e tr = traces. |
|||||
| 1 | rt | 1.1 | 18 | None | 26/4 |
| 2 | 80 | 1.1 | 18 | None | 84/10b |
| 3 | 120c | 1.1 | 0.5 | None | Degradation |
| 4 | Reflux | 2.0 | 18 | None | tre/88 |
| 5 | 80 | 1.1 | 18 | NaOHd | 45/28 |
| 6 | 80 | 1.1 | 18 | KOHc | 18/45 |
With this sequence in hand, we set out to extend the halogenation to the series of indazole derivatives containing sulfonamide or amide groups at C4 position (Scheme 2). These experiments highlighted the regioselectivity of the halogenation reaction with NBS but also the influence of the nature and the electronic effect of the C4 substituent group. Sulfonamides with electron-donating groups such as methyl or methoxy at para-position of the phenylsulfonyl group gave the desired C7 monohalogenated products 5 in high yields along with small amounts of 5,7-dihalogenated compounds 6 (5a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6a, 84%/10% and 5b
6a, 84%/10% and 5b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6b, 77%/15%). Strong electron-withdrawing substituents such as NO2, also gave a mixture of both mono- and dihalogenated compounds but with a drastic decrease of the C7-mono brominated expected product 5c (5c, 17%; 6c, 21%) (Scheme 2). Satisfyingly, the bromination reaction with indazoles 4a–c containing benzamide groups at C4 position and bearing electron-donating group at either the ortho- or para-position of the aryl ring, gave only the C7 halogenated products 7a–c, and no traces of dihalogenated products were observed. In the case of the bromination reaction with 4a, the reaction was not total and starting material 4a was recovered.
6b, 77%/15%). Strong electron-withdrawing substituents such as NO2, also gave a mixture of both mono- and dihalogenated compounds but with a drastic decrease of the C7-mono brominated expected product 5c (5c, 17%; 6c, 21%) (Scheme 2). Satisfyingly, the bromination reaction with indazoles 4a–c containing benzamide groups at C4 position and bearing electron-donating group at either the ortho- or para-position of the aryl ring, gave only the C7 halogenated products 7a–c, and no traces of dihalogenated products were observed. In the case of the bromination reaction with 4a, the reaction was not total and starting material 4a was recovered.
DFT calculations have been performed to identify the main electrophilic, nucleophilic or radical reaction sites of compound 3a and 3c, with the aim to rationalize the preferred site of bromination experimentally observed. For compound 3a, the projection of the fk+ Fukui function on the isodensity surface suggests that this compound will preferentially undergo a nucleophilic attack from a Br− anion on its C5 and C7 carbon atoms, as illustrated by the regions in blue in Fig. 2A. Likewise, compound 3c is predicted to be the subject of a nucleophilic attack on the same sites (Fig. 2B). If experimentally, the C7 atom is the preferred site of bromination, it can be due to the greater steric hindrance at C5 on the second face of the aromatic moiety. Indeed, only one electrophilic site on C7 can be detected on the back side of the indazole moieties of 3a and 3c, as shown by the open surfaces in Fig. 2. Considering an electrophilic attack from a Br+ cation, the representation of the fk− Fukui function is less convincing, with a main site of interaction that would be between C5 and C6 for compound 3a, and a secondary site on C7 (Fig. 3A). Furthermore, none of the indazole carbon atoms of 3c seem to be prone to such an electrophilic attack (Fig. 3B). Similar trends are found with the assumption of a radical attack (Fig. S1, see ESI†), without any favorable carbon sites in 3c and, for 3a, two active sites on the C7 atom for the former and between the C5 and C6 atoms for the latter. The comparison of these different theoretical models mainly suggests that C3 bromination is never predicted, as experimentally observed. Additionally, following our bromination conditions at high temperature with NBS involving an electrophilic Br+, we could observe a higher reactivity of compound 3a over compound 3c.
A direct and efficient regioselective C7-bromination of 4-substituted 1H-indazole has been achieved. Subsequently, a successful palladium-mediated Suzuki–Miyaura reaction of C7-bromo-4-substituted-1H-indazoles with boronic acids has been performed under optimized reaction conditions. A series of new C7 arylated 4-substituted 1H-indazoles was obtained in moderate to good yields.
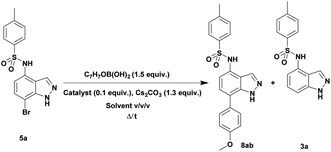
Once the halogenation has been carried out on 3a, the obtained N-(7-bromo-1H-indazol-4-yl)-4-methylbenzenesulfonamide 5a was employed to optimize the Suzuki–Miyaura reaction with (4-methoxyphenyl)boronic acid as coupling partner under various reaction conditions exploring the effects of different bases, catalysts, solvents, and reaction times (Table 2). We began this study using 10 mol% of PdCl2(PPh3)2 as catalyst and K2CO3 or Cs2CO3 as base in DMF at reflux for 48 h. These conditions failed to give the desired product 8ab (entries 1 and 2). Using Pd(PPh3)4 as the catalyst instead of PdCl2(PPh3)2, only traces of the desired product 8ab were detected along with dehalogenated product 3a (entries 3 and 4). Unfortunately, only a modest yield of 11% of coupled product 8ab was obtained carrying out the reaction under microwaves irradiation for 2 h (entry 5). Then various parameters such as solvents (pure or as a mixture), temperature, pressure (sealed tube) or microwave activation conditions were changed (entries 6–10). We found that protocols realized in a mixture of dioxane/EtOH/H2O (3/1.5/0.5) as solvents in a sealed tube either under conventional heating or microwave irradiation provided the coupled product 8ab in a good yield (70%) together with traces of both starting material 5a and dehalogenated product 3a (entries 9 and 10).
| Entry | Catalyst 10 mol% | T (°C)/t (h) | Solvent (v/v) | Yields% 3a/5a/8ab |
|---|---|---|---|---|
| a Optimized conditions: (5a) (1 mmol), Pd(PPh3)4 (10 mol%), Cs2CO3 (1.3 mmol), dioxane/EtOH/H2O (3/1.5/0.5 mL), 140 °C for 4 h. Yields of products after column chromatography purification.b K2CO3 was used as base.c tr = traces. | ||||
| 1b | PdCl2(PPh3)2 | Reflux/48 | DMF | 0/100/0 |
| 2 | PdCl2(PPh3)2 | Reflux/48 | DMF | 0/100/0 |
| 3b | Pd(PPh3)4 | Reflux/48 | DMF | 18/75/trc |
| 4 | Pd(PPh3)4 | Reflux/48 | DMF | tr/80/tr |
| 5 | Pd(PPh3)4 | 140 MW/2 | DMF | 68/tr/11 |
| 6 | Pd(PPh3)4 | Reflux/2 | Dioxane | 0/100/0 |
| 7 | Pd(PPh3)4 | Reflux/48 | Dioxane/EtOH 3/1 | 14/69/tr |
| 8 | Pd(PPh3)4 | Reflux/48 | Dioxane/EtOH/H2O 3/1.5/0.5 | 14/32/46 |
| 9 | Pd(PPh3)4 | 140 MW/2 | Dioxane/EtOH/H2O 3/1.5/0.5 | tr/tr/70 |
| 10 | Pd(PPh3)4 | 140 sealed tube/2 | Dioxane/EtOH/H2O 3/1.5/0.5 | tr/tr/70 |
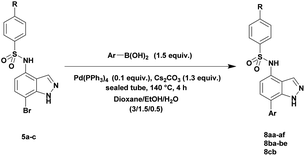
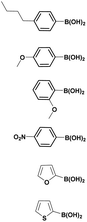
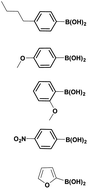
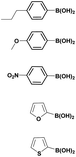
The optimized reaction conditions (1 equiv. of 5a–c, 10 mol% of Pd(PPh3)4, 1.3 equiv. of Cs2CO3, dioxane/EtOH/H2O (3/1.5/0.5 mL), 140 °C (2 h under MW or 4 h in a sealed tube)) were used to explore the substrate scope and limitations. A variety of aryl and heteroaryl boronic acids were successfully coupled with the 4-substituted-7-bromo-1H-indazoles 5a–c (Table 3). The reactions of 5a and 5b bearing electron donating groups with phenylboronic acids bearing also electron donating groups resulted in the formation of the desired products 8aa–8ac and 8ba–8bc in good yields (Table 3, entries 1–3 and 7–9). The aryl boronic acid bearing electron-withdrawing group NO2 at the C4 position, was also efficiently coupled with 7a and 5b, giving 8ad and 8bd in 78% and 75% yield, respectively (Table 3, entries 4 and 10). Additionally, the reaction of indazole 5c containing NO2 on the sulfonamide moiety reacted with 4-methoxyphenyl boronic acid, to give the coupled product 8cb in 71% yield (entry 12). These optimized conditions were also successfully applied to couple heteroaryl boronic acids such as thienyl and furyl-boronic acid. Thus, these derivatives were coupled with 5a and 5b to lead to 8ae and 8be in 75% and 72% yield, respectively (Table 3, entries 5 and 11). Comparatively, 2-thienyl boronic acid was coupled to 5a to give 8af with an excellent yield (80%, entry 6). It is noticed that except for the boronic acids bearing alkyl groups which gave the desired products 8aa and 8ba in moderate yields (entries 1 and 7, Table 3), the Suzuki–Miyaura cross-coupling reaction was not influenced by electronic or steric hindrance of the substituents on the boronic acid partners.
Finally, using the optimized conditions, the scope of this protocol was also extended to 4-amido-7-bromo-1H-indazole 7a–c, in order to examine the effect of the nature of the functional group at C4 on the Suzuki–Miyaura reaction. With an amido substituent at C4 position, the starting material 7 efficiently reacted with various boronic acids to provide the corresponding C7 arylated products 9 in good yields either with aryl (Table 4, entries 1–3, 6–8) or heteroaryl reagents (Table 4, entries 4 and 5). In this case, the reaction yields were not influenced by the electronic or steric hindrance of the substituents on the boronic acids. It is noticed so that in the case of unprotected 7-bromo-1H-indazoles bearing benzamide groups at C4 position, the heteroaryl boronic acids as coupling partners gave excellent yields.
Conclusions
We have prepared a novel series of C7 substituted unprotected NH indazoles in a two steps manner from 4-sulfonamido-1H-indazoles. A simple, fast and regioselective bromination reaction at the C7 position of the 4-sulfonamido-1H-indazoles was observed with N-bromosuccinimide and a computational study was performed to estimate the reactivity of the 4-sulfonamido NH-indazole ring. From the bromo NH-indazole precursors, a Suzuki–Miyaura cross-coupling reaction with a set of aryl boronic acids afforded the expected C7 (hetero)arylated NH-indazole derivatives, in moderate to excellent yields, regardless of any electronic influence or steric hindrance by the substituent on the boronic partner. Then, we have shown also that the bromination of unprotected NH indazoles bearing benzamide groups at C4 position took place in regioselective manner at C7 position. Again, the Suzuki–Miyaura cross-coupling reaction with various (hetero)aryl boronic acids led to the desired C7 (hetero)arylated NH-indazole derivatives in good yields.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Euromed University of Fes, the CNRST of Rabat and the University of Nantes (CEISAM laboratory-Symbiose group). Authors thank Prof Gérald Guillaumet for his help during the preparation of the revised version.Notes and references
- C. K. Chung, P. G. Bulger, B. Kosjek, K. M. Belyk, N. Rivera, M. E. Scott, G. R. Humphrey, J. Limanto, D. C. Bachert and K. M. Emerson, Process Development of C–N Cross-Coupling and Enantioselective Biocatalytic Reactions for the Asymmetric Synthesis of Niraparib, Org. Process Res. Dev., 2014, 18, 215–227 CrossRef CAS.
- Y. Jia, J. Zhang, J. Feng, F. Xu, H. Pan and W. Xu, Design, Synthesis and Biological Evaluation of Pazopanib Derivatives as Antitumor Agents, Chem. Biol. Drug Des., 2014, 83, 306–316 CrossRef CAS.
- H. Shen, S. Gou, J. Shen, Y. Zhu, Y. Zhang and X. Chen, Synthesis and Biological Evaluations of Novel Bendazac Lysine Analogues as Potent Anticataract Agents, Bioorg. Med. Chem. Lett., 2010, 20, 2115–2118 CrossRef CAS.
- A. Veerareddy, G. Surendrareddy and P. K. Dubey, Total Syntheses of AF-2785 and Gamendazole—Experimental Male Oral Contraceptives, Synth. Commun., 2013, 43, 2236–2241 CrossRef CAS.
- S. B. Yan, V. L. Peek, R. Ajamie, S. G. Buchanan, J. R. Graff, S. A. Heidler, Y. H. Hui, K. L. Huss, B. W. Konicek, J. R. Manro, C. Shih, J. A. Stewart, T. R. Stewart, S. L. Stout, M. T. Uhlik, S. L. Um, Y. Wang, W. Wu, L. Yan, W. J. Yang, B. Zhong, A. Richard and R. A. Walgren, LY2801653 is an Orally Bioavailable Multi-kinase Inhibitor with Potent Activity Against MET, MST1R, and Other Oncoproteins, and Displays Anti-tumor Activities in Mouse Xenograft Models, Invest. New Drugs, 2013, 31, 833–844 CrossRef CAS.
- T. Li, M. A. Pobanz, C. Shih, Z. Wu, W. J. Yang and B. Zhong, Amidophenoxyindazoles useful as inhibitors of c-Met – Google Patents, US Pat. US8030302B2, https://patents.google.com/patent/US8030302B2/en (accessed May 9, 2020) Search PubMed.
- A. F. P. Biajoli, C. S. Schwalm, J. Limberger, T. S. Claudino and A. L. Monteiro, Recent Progress in the Use of Pd-Catalyzed C-C Cross-Coupling Reactions in the Synthesis of Pharmaceutical Compounds, J. Braz. Chem. Soc., 2014, 25, 2186–2214 CAS.
- L. Yin and J. Liebscher, Carbon-carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts, Chem. Rev., 2007, 107, 133–173 CrossRef CAS.
- X. Chen, K. M. Engle, D. H. Wang and J. Q. Yu, Palladium(II)-catalyzed C-H Activation/C-C Cross-coupling Reactions: Versatility and Practicality, Angew. Chem., Int. Ed., 2009, 48, 5094–5115 CrossRef CAS.
- D. Alberico, M. E. Scott and M. Lautens, Aryl−Aryl Bond Formation by Transition-Metal-Catalyzed Direct Arylation, Chem. Rev., 2007, 107, 174–238 CrossRef CAS.
- S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Recent Advances in the Transition Metal-catalyzed Twofold Oxidative C–H Bond Activation Strategy for C–C and C–N Bond Formation, Chem. Soc. Rev., 2011, 40, 5068–5083 RSC.
- N. Miyaura and A. Suzuki, Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS.
- N. Miyaura Organoboron Compounds; Springer, Berlin, Heidelberg, 2002; pp. 11–59. DOI:10.1007/3-540-45313-X_2.
- G. A. Molander and B. Canturk, Organotrifluoroborates and Monocoordinated Palladium Complexes as Catalysts—A Perfect Combination for Suzuki–Miyaura Coupling, Angew. Chem., Int. Ed., 2009, 48, 9240–9261 CrossRef CAS.
- C. Torborg and M. Beller, Recent Applications of Palladium-Catalyzed Coupling Reactions in the Pharmaceutical, Agrochemical, and Fine Chemical Industries, Adv. Synth. Catal., 2009, 351, 3027–3043 CrossRef CAS.
- M. A. Düfert, K. L. Billingsley and S. L. Buchwald, Suzuki-Miyaura Cross-Coupling of Unprotected, Nitrogen-Rich Heterocycles: Substrate Scope and Mechanistic Investigation, J. Am. Chem. Soc., 2013, 135, 12877–12885 CrossRef.
- A. C. Suzuki, Cross-Coupling Reactions Of Organoboranes: An Easy Way To Construct C-C Bonds (Nobel Lecture), Angew. Chem., Int. Ed., 2011, 50, 6722–6737 CrossRef CAS.
- R. Martin and S. L. Buchwald, Palladium-catalyzed Suzuki-Miyaura Cross-coupling Reactions Employing Dialkylbiaryl Phosphine Ligands, Acc. Chem. Res., 2008, 41, 1461–1473 CrossRef CAS.
- G. C. Fu, The Development of Versatile Methods for Palladium-Catalyzed Coupling Reactions of Aryl Electrophiles through the Use of P(t-Bu)3 and PCy3 as Ligands, Acc. Chem. Res., 2008, 41, 1555–1564 CrossRef CAS.
- C. Valente, S. Çalimsiz, K. H. Hoi, D. Mallik, M. Sayah and M. G. Organ, The Development of Bulky Palladium NHC Complexes for the Most-Challenging Cross-coupling Reactions, Angew. Chem., Int. Ed., 2012, 51, 3314–3332 CrossRef CAS.
- S. Qian, J. Cao, Y. Yan, M. Sun, H. Zhu, Y. Hu, Q. He and B. Yang, SMT-A07, a 3-(Indol-2-yl) Indazole Derivative, Induces Apoptosis of Leukemia Cells in Vitro, Mol. Cell. Biochem., 2010, 345, 13–21 CrossRef CAS.
- M. De Lena, V. Lorusso, A. Latorre, G. Fanizza, G. Gargano, L. Caporusso, M. Guida, A. Catino, E. Crucitta, D. Sambiasi and A. Mazzei, Paclitaxel, Cisplatin and Lonidamine in Advanced Ovarian Cancer. A Phase II Study, Eur. J. Cancer, 2001, 37, 364–368 CrossRef CAS.
- J. B. Cross, J. Zhang, Q. Yang, M. F. Mesleh, J. A. C. Romero, B. Wang, D. Bevan, K. M. Poutsiaka, F. Epie, T. Moy, A. Daniel, J. Shotwell, B. Chamberlain, N. Carter, O. Andersen, J. Barker, M. D. Ryan, A. Chester, C. A. Metcalf, J. Silverman, K. Nguyen, B. Lippa, E. Roland and R. E. Dolle, Discovery of Pyrazolopyridones as a Novel Class of Gyrase B Inhibitors Using Structure Guided Design, ACS Med. Chem. Lett., 2016, 7, 374–378 CrossRef CAS.
- W. Han, J. C. Pelletier and C. N. Hodge, Tricyclic Ureas: A New Class of HIV-1 Protease Inhibitors, Bioorg. Med. Chem. Lett., 1998, 8, 3615–3620 CrossRef CAS.
- X. Li, S. Chu, V. A. Feher, M. Khalili, Z. Nie, S. Margosiak, V. Nikulin, J. Levin, K. G. Sprankle, M. E. Tedder, R. Almassy, K. Appelt and K. M. Yager, Structure-based Design, Synthesis, and Antimicrobial Activity of Indazole-derived SAH/MTA Nucleosidase Inhibitors, J. Med. Chem., 2003, 46, 5663–5673 CrossRef CAS.
- C. Runti and L. Baiocchi, The Chemistry of Benzydamine, Int. J. Tissue React., 1985, 7, 175–186 CAS.
- N. Halland, M. Nazaré, O. R’kyek, J. Alonso, M. Urmann and A. Lindenschmidt, A General and Mild Palladium-Catalyzed Domino Reaction for the Synthesis of 2H-Indazoles, Angew. Chem., Int. Ed., 2009, 48, 6879–6882 CrossRef CAS.
- A. H. Shinde, S. Vidyacharan and D. S. Sharada, BF3·OEt2 Mediated Metal-free One-pot Sequential Multiple Annulation Cascade (SMAC) Synthesis of Complex and Diverse TetrahydroisoquinolineFused Hybrid Molecules, Org. Biomol. Chem., 2016, 14, 3207–3211 RSC.
- H. Bel Abed, N. Weißing, J. Schoene, J. Paulus, N. Sewald and M. Nazaré, Novel Strategy for the Preparation of 3-perfluoroalkylated-2 H-indazole Derivatives, Tetrahedron Lett., 2018, 59, 1813–1815 CrossRef CAS.
- M. R. Kumar, A. Park, N. Park and S. Lee, Consecutive Condensation, C–N and N–N Bond Formations: A Copper- Catalyzed One-Pot Three-Component Synthesis of 2H-Indazole, Org. Lett., 2011, 13, 3542–3545 CrossRef CAS.
- M. Naas, S. El Kazzouli, E. M. Essassi, M. Bousmina and G. Guillaumet, Palladium-Catalyzed Oxidative Direct C3- and C7-Alkenylations of Indazoles: Application to the Synthesis of Gamendazole, Org. Lett., 2015, 17, 4320–4323 CrossRef CAS.
- K. Basu, M. Poirier and R. T. Ruck, Solution to the C3–Arylation of Indazoles: Development of a Scalable Method, Org. Lett., 2016, 18, 3218–3221 CrossRef CAS.
- G. Bogonda, H. Y. Kim and K. Oh, Direct Acyl Radical Addition to 2H-Indazoles Using Ag-Catalyzed Decarboxylative Cross-Coupling of α-Keto Acids, Org. Lett., 2018, 20, 2711–2715 CrossRef CAS.
- T. Y. H. Wu, P. G. Schultz and S. Ding, One-Pot Two-Step Microwave-Assisted Reaction in Constructing 4,5-Disubstituted Pyrazolopyrimidines, Org. Lett., 2003, 5, 3587–3590 CrossRef CAS.
- N. Kudo, M. Perseghini and G. C. Fu, A Versatile Method for Suzuki Cross-Coupling Reactions of Nitrogen Heterocycles, Angew. Chem., Int. Ed., 2006, 118, 1304–1306 CrossRef.
- K. Billingsley and S. L. Buchwald, Highly Efficient Monophosphine-Based Catalyst for the Palladium-Catalyzed Suzuki−Miyaura Reaction of Heteroaryl Halides and Heteroaryl Boronic Acids and Esters, J. Am. Chem. Soc., 2007, 129, 3358–3366 CrossRef CAS.
- M. Prieto, E. Zurita, E. Rosa, L. Muñoz, P. Lloyd-Williams and E. Giralt, Arylboronic Acids and Arylpinacolboronate Esters in Suzuki Coupling Reactions Involving Indoles. Partner Role Swapping and Heterocycle Protection, J. Org. Chem., 2004, 69, 6812–6820 CrossRef CAS.
- J. E. Grob, J. Nunez, M. A. Dechantsreiter and L. G. Hamann, One-Pot Reductive Amination and Suzuki–Miyaura Cross-Coupling of Formyl Aryl and Heteroaryl MIDA Boronates in Array Format, J. Org. Chem., 2011, 76, 4930–4940 CrossRef CAS.
- P. Čapek, M. Vrábel, Z. Hasník, R. Pohl and M. Hocek, Aqueous-Phase Suzuki-Miyaura Cross-Coupling Reactions of Free Halopurine Bases, Synthesis, 2006, 20, 3515–3526 Search PubMed.
- F. Y. Kwong, K. S. Chan, C. H. Yeung and A. S. C. Chan, An active ferrocenyl triarylphosphine for palladium-catalyzed Suzuki–Miyaura cross-coupling of aryl halides, Chem. Commun., 2004, 20, 2336–2337 RSC.
- C. M. So, W. K. Chow, P. Y. Choy, C. P. Lau and F. Y. Kwong, Remarkably Effective Phosphanes Simply with a PPh2 Moiety: Application to Pd-Catalysed Cross-Coupling Reactions for Tetra-ortho-substituted Biaryl Syntheses, Chem. – Eur. J., 2010, 16, 7996–8001 CrossRef CAS.
- Y. Kitamura, S. Sako, T. Udzu, A. Tsutsui, T. Maegawa, Y. Monguchi and H. Sajiki, Ligand-free Pd/C-catalyzed Suzuki–Miyaura Coupling Reaction for the Synthesis of Heterobiaryl Derivatives, Chem. Commun., 2007, 47, 5069–5071 RSC.
- O. Navarro, N. Marion, J. Mei and S. P. Nolan, Rapid Room Temperature Buchwald–Hartwig and Suzuki–Miyaura Couplings of Heteroaromatic Compounds Employing Low Catalyst Loadings, Chem. – Eur. J., 2006, 12, 5142–5148 CrossRef CAS.
- D. W. Robbins and J. F. Hartwig, A C–H Borylation Approach to Suzuki–Miyaura Coupling of Typically Unstable 2–Heteroaryl and Polyfluorophenyl Boronates, Org. Lett., 2012, 14, 4266–4269 CrossRef CAS.
- K. I. Kusakabe, N. Ide, Y. Daigo, Y. Tachibana, T. Itoh, T. Yamamoto, H. Hashizume, Y. Hato, K. Higashino, Y. Okano, Y. Sato, M. Inoue, M. Iguchi, T. Kanazawa, Y. Ishioka, K. Dohi, Y. Kido, S. Sakamoto, K. Yasuo, M. Maeda, M. Higaki, K. Ueda, H. Yoshizawa, Y. Baba, T. Shiota, H. Murai and Y. Nakamura, Indazole-Based Potent and Cell-Active Mps1 Kinase Inhibitors: Rational Design from Pan-Kinase Inhibitor Anthrapyrazolone (SP600125), J. Med. Chem., 2013, 56, 4343–4356 CrossRef CAS.
- L. Bouissane, S. El Kazzouli, S. Léonce, B. Pfeiffer, E. M. Rakib, M. Khouili and G. S. Guillaumet, Synthesis and Biological Evaluation of N-(7-indazolyl)benzenesulfonamide Derivatives as Potent Cell Cycle Inhibitors, Bioorg. Med. Chem., 2006, 14, 1078–1088 CrossRef CAS.
- N. Abbassi, H. Chicha, E. M. Rakib, A. Hannioui, M. Alaoui, A. Hajjaji, D. Geffken, C. Aiello, R. Gangemi, C. Rosano and M. Viale, Synthesis, Antiproliferative and Apoptotic Activities of N-(6(4)-Indazolyl)-benzenesulfonamide Derivatives as Potential Anticancer Agents, Eur. J. Med. Chem., 2012, 57, 240–249 CrossRef CAS.
- Y. Oda, T. Owa, T. Sato, B. Boucher, S. Daniels, H. Yamanaka, Y. Shinohara, A. Yokoi, J. Kuromitsu and T. Nagasu, Quantitative Chemical Proteomics for Identifying Candidate Drug Targets, Anal. Chem., 2003, 75, 2159–2165 CrossRef CAS.
- S. El Kazzouli and G. Guillaumet, Functionalization of Indazoles by Means of Transition Metal-catalyzed Cross-coupling Reactions, Tetrahedron, 2016, 72, 6711–6727 CrossRef CAS.
- S. Faarasse, S. El Kazzouli, M. Naas, J. Jouha, F. Suzenet and G. Guillaumet, “On Water” Direct C-3 Arylation of 2H-Pyrazolo[3,4-b]pyridines, J. Org. Chem., 2017, 82, 12300–12306 CrossRef CAS.
- S. Faarasse, S. El Kazzouli, F. Suzenet and G. Guillaumet, Palladium-Catalyzed C3-Arylations of 1H- and 2H-Pyrazolo[4,3-b]pyridines on Water, J. Org. Chem., 2018, 83, 12847–12854 CrossRef CAS.
- J. Elie, J. Vercouillie, N. Arlicot, L. Lemaire, R. Bidault, S. Bodard, C. Hosselet, J. B. Deloye, S. Chalon, P. Emond, D. Guilloteau, F. Buron and S. Routier, Design of Selective COX-2 Inhibitors in the (aza)indazole Series. Chemistry, in Vitro Studies, Radiochemistry and Evaluations in Rats of a [18F] PET Tracer, J. Enzyme Inhib. Med. Chem., 2019, 34, 1–7 CrossRef CAS.
- A. Ben-Yahia, M. Naas, N. El Brahmi, S. El Kazzouli, J.-P. Majoral, E. M. Essassi and G. Guillaumet, Microwave-assisted Suzuki-Miyaura Cross-Coupling of Free (NH) 3-Bromoindazoles, Curr. Org. Chem., 2013, 17, 304–309 CrossRef CAS.
- S. El Kazzouli, L. Bouissane, M. Khouili and G. Guillaumet, Synthesis of 4-substituted and 3,4-disubstituted Indazole Derivatives by Palladium-mediated Cross-coupling Reactions, Tetrahedron Lett., 2005, 46, 6163–6167 CrossRef CAS.
- M. Naas, S. El Kazzouli, E. M. Essassi, M. Bousmina and G. Guillaumet, Palladium-Catalyzed Direct C7-Arylation of Substituted Indazoles, J. Org. Chem., 2014, 79, 7286–7293 CrossRef CAS.
- K. Down, A. Amour, I. R. Baldwin, A. W. J. Cooper, A. M. Deakin, L. M. Felton, S. B. Guntrip, C. Hardy, Z. A. Harrison, K. L. Jones, P. Jones, S. E. Keeling, J. Le, S. Livia, F. Lucas, C. J. Lunniss, J. Nigel, N. J. Parr, E. Robinson, P. Rowland, S. Smith, D. A. Thomas, G. Vitulli, Y. Washio and J. N. Hamblin, Optimization of Novel Indazoles as Highly Potent and Selective Inhibitors of Phosphoinositide 3-Kinase δ for the Treatment of Respiratory Disease, J. Med. Chem., 2015, 58, 7381–7399 CrossRef CAS.
- J. A. May, N. A. Sharif, M. A. McLaughlin, H. H. Chen, B. S. Severns, C. R. Kelly, W. F. Holt, R. Young, R. A. Glennon, M. R. Hellberg and T. R. Dean, Ocular Hypotensive Response in Nonhuman Primates of (8R)-1-[(2S)-2-Aminopropyl]-8,9-dihydro-7H-pyrano[2,3-g]indazol-8-ol a Selective 5-HT2 Receptor Agonist, J. Med. Chem., 2015, 58, 8818–8833 CrossRef CAS.
- E. Lohou, J. Sopkova-De Oliveira Santos, P. Schumann-Bard, M. Boulouard, S. Stiebing, S. Rault and V. Collot, New Hypotheses for the Binding Mode of 4- and 7-substituted Indazoles in the Active Site of Neuronal Nitric Oxide Synthase, Bioorg. Med. Chem., 2012, 20, 5296–5304 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2020826. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra08598g |
| This journal is © The Royal Society of Chemistry 2021 |