Open Access Article
Open Access ArticleQuantitative evaluation of the non-thermal effect in microwave induced polymer curing†
Kun Lia,
Tuo Pingb,
Haobo Zhanga,
Junying Zhang a,
Jue Cheng
a,
Jue Cheng *a and
Feng Gao
*a and
Feng Gao *a
*a
aKey Laboratory of Carbon Fiber and Functional Polymers, Ministry of Education, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: gaofeng@mail.buct.edu.cn; chengjue@mail.buct.edu.cn; Tel: +86-10-64425439
bBeijing Spacecrafts, China Academy of Space Technology, Beijing 100194, China
First published on 19th January 2021
Abstract
Microwave irradiation is one of the most effective strategies to accelerate the curing of resin. However, the mechanism is still unclear. The debates mainly focus on how to quantitatively evaluate the ‘non-thermal’ effect of the microwave. In this work, the non-thermal effect on DGEBA with amine (D230) and anhydride (MHHPA) hardeners respectively was evaluated via an isothermal microwave curing reactor. The ‘thermal effect’ caused by the microwave was peeled off accurately. Iso-conversional kinetic analysis was performed based on the reaction extent from the real time FTIR spectrum, and the apparent activation energy (Ea) was calculated for the quantitative evaluation of the microwave non-thermal effect. The influence caused by the polarized functional groups, reaction temperature and the transition state were explored, and the microwave non-thermal effect on the mechanical performance of the cured thermosets was evaluated. Results indicated that the microwave non-thermal effect was able to reduce the Ea and accelerate the curing speed of epoxy resin. At the same time, the final curing extent was increased when the non-thermal effect of microwave was induced leading to enhanced mechanical and thermal performance.
1. Introduction
Microwave irradiation is one of the most applicable strategies to accelerate the thermoset matrix curing in the fabrication of composite materials.1–7 The rapid direction variation of the electromagnetic field motivated the polarized groups on the polymer chains and made it vibrational leading to the raise of the mean kinetic energy and bulk temperature.8–11 This effect can accelerate the curing rate and enhance the curing extent of the resin, and it is regarded as the ‘thermal effect’ of the microwave irradiations. The microwave can transfer energy instantly and uniformly to the affected molecules and avoid the generation of the temperature gradient in the matrix, which leading to better mechanical performance compared with the conduction and convention methods.6,12–17 This effect becomes more obvious with larger thickness of the thermoset.7,17 Thus, microwave is widely used in the mass industrial production of composite materials. Moreover, microwave irradiation can work under vacuum, and it is an effective way to enhance the curing extent and rate of the coating during the on-orbit maintenance in the aerospace industry.18–20 The cost of microwave initiator is much lower than the other high energy radiation strategies, such as ultraviolet, X-ray and electron beam. Besides, the frequency of the microwave ranges from 109 to 1012 Hz, which is similar with the rotational4,8 and vibration frequency1,11 of reactive functional group,12,16 thus, the microwave can change the chain conformation and active the reactive groups selectively in the resin matrix.21 Microwave hardly damages the structure of the molecule, and has the better penetrability.1,3,10 However, the mechanism of the microwave ‘non-thermal effect’ is still not clear, and the effective quantitative evaluation strategy is required. The thermal effect, which is related to the fast increase of temperature when polarized groups were irradiated by microwave, can be estimated by the temperature measurements easily,8 and the non-thermal effect was classified as the direct interaction of the electric field with specific reaction molecules and be evaluated from the changes in activation energy (entropy) in the Arrhenius equation.13,19,21 The non-thermal effect must arise from interactions between the microwave field and material, and the microwave heating interferes with possible non-thermal effects, which can't be easily separated in real experimental studies.14,23The non-thermal effect of microwave includes the targeted stimulation of reactive groups, the altering of the dipole moment,1 the polarity,9 and the matrix regularity5 when the bulk reaction temperature is constant or totally under control.1,11 It is necessary to find a way quantitatively evaluating the non-thermal effect towards the matrix curing dynamic and make the influence factors (e.g.: functional groups, backbone polymer, bulk temperature, polarity, and transition state) comparable between different polymer formulations, which keeps being one of the bottleneck questions in the field of microwave induced polymer curing.4,6 The major challenge investigating the non-thermal effect is peeling off the ‘thermal effect’ from the microwave irradiation which inevitably increase the bulk temperature of the resin effecting the reaction kinetic.1,3 As the results, the only way to clarify the mechanism of microwave non-thermal effect during the thermosets curing was to perform the appropriate experiments comparing the thermosets curing dynamic with/without the microwave irradiation under the isothermal environment.21,24
The research work about the microwave non-thermal focused on two aspects: (1) the microwave induced isothermal reactors were tried to be fabricated in the past decades: Hosseini et al.2 designed a CEM-Discover monomode microwave oven under an inert argon atmosphere minimizing the microwave thermal effect, and the results indicated that the reaction time was significantly reduced (61% reduction). However, the infrared temperature sensors used in monomode microwave oven hardly reflected the bulk temperature of the resin, and the temperature fluctuation was more than 10 °C. Obermayer et al.6 used silicon carbide reactor to prevent the microwave penetrating the vessel and interacting with the reaction mixture, the selective heating by microwave irradiation of specific materials was experimentally confirmed using fully microwave transparent solvents and reaction vessels. However, part of the electric field was still present inside the silicon carbide vessel, which in turn did not completely block the microwave effects leading to temperature fluctuations (8–16 K) in the experiment. It remains a challenge constructing the microwave induced isothermal reactor. (2) The kinetic analysis and mechanical performance deduced by microwave non-thermal effect: Boey et al.23,24 explored the reaction kinetic of bisphenol A diglycidyl ether (DGEBA) and m-phenylenediamine (m-PDA) with the same temperature changing rates under heat and microwave irradiation. The results indicated that the curing rate under microwave irradiation was faster than that under heat conduction (43% reduction in curing time), and the mechanical performance under microwave was greater than that under heat curing. The kinetic analysis showed that the activation energy for the system irradiated by microwave was 26.2% lower than that by heating according to the 2nd order autocatalytic model. P. Navabpour et al.25 used differential scanning calorimeter (DSC) combining with microwave calorimeter to explore the reaction kinetics of epoxy/anhydride system and the results that the ‘non-thermal effect’ of microwave irradiation was reduce the curing time (27.5% reduction), and the reaction activation energy (21.2% reduction) according to the autocatalytic kinetic model. However, the matrix temperature in this work varied during the reaction process due to the thermal effect of microwave, and the influence of the microwave non-thermal effect can't be evaluated quantitatively.25–31
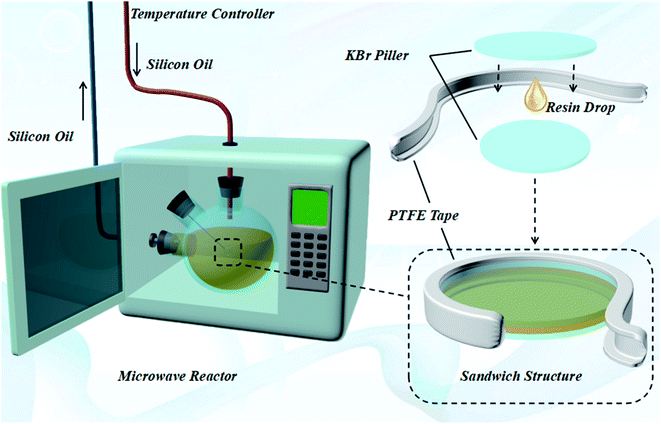
In this work, an isothermal reactor (Fig. 1) was constructed to explore the non-thermal effect of microwave towards epoxy resin system. Silicone oil was chosen as the cooling fluid to stabilize the temperature of the resin due to its low dielectric constant. Thus, the microwave hardly affected the temperature of the cooling fluid. The bulk temperature was controlled by the microwave induced isothermal resin reactor. The curing temperature was defined according to the analysis of non-isothermal kinetic of resin based on the Málek method for better accuracy. Samples were sealed between two pieces of KBr pills by Teflon tape, and immersed in the silicone oil. The reaction extent was tracked by an IR detector in the real time. The Ea was calculated by the kinetic analysis of the reactions to evaluate quantitatively the non-thermal effect of the microwave, and the mechanical performance of the cured resin was examined.
DGEBA was researched in this work, and it is widely used as the matrix resin in composite material. Polyether amine (D230) and methyl hexahydrophthalic anhydride (MHHPA) were chosen as hardener. The influence of curing agent polarity and ratio to microwave non-thermal effect was evaluated. DMA, TGA and tensile tests were conducted on the thermoset sets to explore microwave non-thermal effect on material mechanical and thermal performances. The tensile sections after yield were investigated by SEM.
Two hypotheses were raised in this work: (1) the non-thermal effect of microwave does enhance the curing rate and mechanical performance of epoxy matrix in isothermal environment, and this ‘non-thermal’ effect can be quantitatively evaluated by the change of Ea and pre-exponential factors (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα); (2) the non-thermal effect of microwave becomes more obvious with more polarized functional groups and lower reaction temperature.
Aα); (2) the non-thermal effect of microwave becomes more obvious with more polarized functional groups and lower reaction temperature.
2. Experimental section
2.1 Material
DGEBA (epoxy equivalent: 248 g mol−1) was supplied by Yueyang Baling Huaxing Petrochemical Co., Ltd. (China). The curing agent D230 was obtained from Beijing Yinuokai Technology Co., Ltd. (China); MHHPA was obtained from Beijing Minerida Technology Co., Ltd. (China). The accelerator tris-2,4,6-dimethyl aminomethyl phenol (DMP-30) was obtained from Beijing Minerida Technology Co., Ltd. (China). The thermal silicone oil Mantherm SF-15 was obtained from Jiangsu Manto Technology Co., Ltd. (China). All other chemicals and reagents were purchased from Beijing Chemical Works (China) (Fig. S1†).2.2 The construction of microwave induced isothermal resin reactor with in situ IR characterization system
The microwave induced isothermal resin reactor (Fig. 1) was designed and assembled, which could eliminate the influence of thermal effect of microwave on epoxy curing reaction and non-thermal effect of microwave was investigated individually. The temperature of entire device was controlled by silicone oil circulation in Table S1† and ‘sandwich’ structure was immersed in silicone oil. The parameters of reactor were presented by Table S2.†2.3 Sample preparation
DGEBA and curing agent (anhydride, amine) were mixed according to the chemical equivalent ratio of reactive groups to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 0.5 wt% DMP-30 was added as accelerator. The mixture was stirred uniformly and removed the bubbles under vacuum. A drop of the mixture (≈1 mg) was applied between two KBr sheets and sealed by PTFE tape. The KBr sheet thickness was 0.7 mm, diameter was 13 mm, and reactant thickness was 0.8 mm. The ‘sandwich’ structure was placed in microwave induced isothermal resin reactor, and reaction extent was tracked by Fourier transform infrared spectroscopy in real time.
1, and 0.5 wt% DMP-30 was added as accelerator. The mixture was stirred uniformly and removed the bubbles under vacuum. A drop of the mixture (≈1 mg) was applied between two KBr sheets and sealed by PTFE tape. The KBr sheet thickness was 0.7 mm, diameter was 13 mm, and reactant thickness was 0.8 mm. The ‘sandwich’ structure was placed in microwave induced isothermal resin reactor, and reaction extent was tracked by Fourier transform infrared spectroscopy in real time.
2.4 Measurements
The curing process of raw materials was monitored with DSC (Differential Scanning Calorimeter, and calibrated) in 50 mL min−1 nitrogen flow and sample weight ranged from 5 mg to 8 mg. The temperature was set from 40 to 240 °C in order to determine the total reaction enthalpy and heating rates were determined as 5, 10, 15 and 20 °C min−1. FTIR spectrum was recorded by an Alpha-T spectrometer (Bruker, Germany) with a resolution of 4 cm−1 in the range of 4000–400 cm−1. The thermal property of the sample sets was investigated with dynamic mechanical analysis (DMA, Q800, TA instrument). The original films were cut into small pieces with a size of 20 mm × 3 mm × 0.8 mm. The specimens were heated from −50 to 200 °C under a heating rate of 5 °C min in a forced convection oven with the nitrogen environment and were measured using tensile mode. The cured samples were deformed in a sinusoidal manner with controlled strain amplitude of 125% at a fixed frequency of 1 Hz. All samples were tested for each formulation. Thermal stability of thermal and microwave curing epoxy resin was evaluated with a Thermos geometrical analyzer (TGA, NETZSCH TG209C) at a constant heating rate (10 °C min−1) from 40 to 600 °C in the nitrogen stream (10 mL min−1). The samples were cured in the isothermal microwave reactor between two KBr slices (0.7 mm) in silicone oil bath, and the thickness of the resin was controlled as 0.8 mm with the help of spacers ensuring the formulation of the sample was consistent with those in the thermal analysis. The sample slides were cut into dumbbell spline with the length 21.2 mm and width 1.6 mm. Tensile test was performed at room temperature with 20 kN loading cell and 5 mm min−1 speed. Tensile test was performed five times for each sample sets. The fractured morphology was characterized by scanning electron microscopy (SEM, JEOL, JSM-6700M) at an accelerating voltage of 5 kV, and all the surfaces of the samples were sputtered (Denton Desk III) with gold to improve the conductivity and prevent charging.2.5 Calculation of isothermal curing reaction kinetics
The characteristic peak of aromatic groups is around 1508 cm−1 and the area of this peak is used as the reference in the calculation. The epoxy conversion α is expressed by eqn (1)
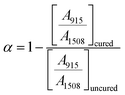 | (1) |
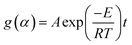 | (2) |
Eqn (3) was obtained after a series rearrangement:
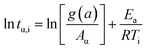 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tα,i vs. 1/Ti, 1000 obtained from conversional curve is linear fitted where the slope is Ea,α.32,36
tα,i vs. 1/Ti, 1000 obtained from conversional curve is linear fitted where the slope is Ea,α.32,36
By analytical comparing of the experimental normalized conversion curves with theoretical models of the normalized conversion curves, it was established that the epoxy kinetics with heat curing could be described by eqn (4):
| g(α) = [1 − (1 − α)1/3] | (4) |
And the kinetics under microwave irradiation could be described by eqn (5):
| g(α) = −ln(1 − α) | (5) |
The value of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα was intercept subtracted ln[1 − (1 − α)1/3] under heat curing, and the value of ln
Aα was intercept subtracted ln[1 − (1 − α)1/3] under heat curing, and the value of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα was the intercept subtracted ln[−ln(1 − α)] under microwave irradiation.32,37
Aα was the intercept subtracted ln[−ln(1 − α)] under microwave irradiation.32,37
3. Results and discussion
3.1 Reaction temperature correction
The silicone oil was used to stabilize the temperature of ‘sandwich’ structure in this iso-thermal microwave reactor. The dielectric constant of the epoxy resin was higher than silicone oil, and it caused the temperature of resin slightly higher than that of silicone oil (eqn S1†) when the system was steady operating. It was measured that the temperature difference ranged from 1.1 to 1.3 °C under different reaction temperature with different types of the matrix resin (Table S3†), however, they were non-negligible. So, reaction temperature of resin without microwave irradiation was corrected and reaction temperature in oven has been raised to ensure that matrix has the same curing temperature, and study scientifically the non-thermal effect of accelerated reaction mechanism of microwave curing. According to the hypothesis mentioned in introduction, the effect of reaction temperature to the microwave non-thermal effect would be explored, and a temperature gradient should be defined for a series of isothermal reaction.The model free advanced iso-conversion kinetic analysis was used to investigate the reactions. The data of non-isothermal DSC curing reaction of EP-D230 and EP-MHHPA systems was presented by Fig. S2–S6 and Table S4 in ESI file.†
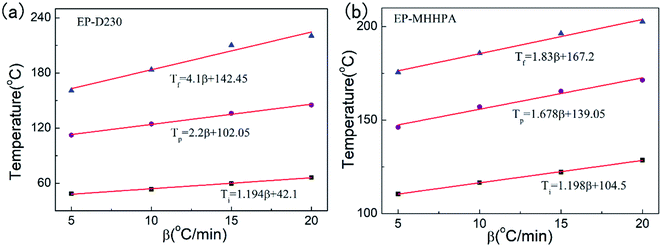
The initial curing temperature of EP-D230 was found to be 48.6 °C, 53.4 °C, 59.7 °C and 66.4 °C, the peak temperature was 112.4 °C, 124.5 °C, 136.1 °C and 145.2 °C, the final temperature was 160.8 °C, 183.7 °C, 210.2 °C and 220.4 °C. The initial curing temperature of EP-MHHPA was found to be 110.5 °C, 116.5 °C, 122.2 °C and 128.6 °C, the peak temperature was 146.2 °C, 157.1 °C, 165.4 °C and 171.4 °C, the final temperature was 175.6 °C, 185.8 °C, 196.3 °C and 220.6 °C (Fig. 2).
Hence,the static curing temperature was obtained by plotting Ti, Tp, and Tf versus different heating rates and extrapolating to β = 0, where the intercepts of T–β fitting curves were equal to the temperature parameters of the isothermal curing,38,39 and the temperature of isothermal curing under heat and microwave curing were determined that EP-D230 at 50, 55, 60 and 65 °C; EP-D230 at 70, 75, 80 and 85 °C; EP-MHHPA at 110, 115, 120 and 125 °C.38,40
3.2 Kinetic analysis of microwave irradiated epoxy resin
The real-time FTIR spectra of epoxy matrix under different reaction conditions were plotted in Fig. S7,† and the characteristic peak of epoxy group was around 915 cm−1. The temperature of ‘sandwich’ structure reactor was constant approximately with or without the microwave irradiation. Thus, all the difference presented by the samples when microwave induced was caused by the non-thermal effect. The conversion rates for the fully cured samples were significantly higher compared with the samples without microwave irradiation. The non-thermal effect of the microwave did exist, and it was able to increase the curing speed and the final conversion rate of epoxy resin with polyether amine and anhydride as the hardener.Microwave field excites polarizable electrons in molecules and creates rotational motion from dielectric loss. This rotational energy provides increase collision frequencies and more favourable alignment of the reactive species which is different from conventional heating of molecular translational energy. Microwave excited rotations occur not only on the side chains but also along the backbones of polymers, greatly enhancing the mobility of the entire polymer network.41,42 The frequency of the microwave adopted was 2450 MHz, which belong to the nonionizing radiation and the microwave was not able to break down the chemical bonds of the matrix. This was consistent with the conclusion of Chaowasakoo et al. that the microwave was not able to change the chemical structure of the resin.43
The implementation of model-fitting methods is aimed at extracting the value of the activation energy for an overall process which does not reflect changes in the reaction mechanism and kinetics with the temperature and the extent of conversion.35 The drawbacks can be avoided using the iso-conversional methods. Firstly, these methods determined the activation energy as a function of the conversion extent or temperature. Secondly, this dependence is determined without any assumptions about the reaction model.32,35
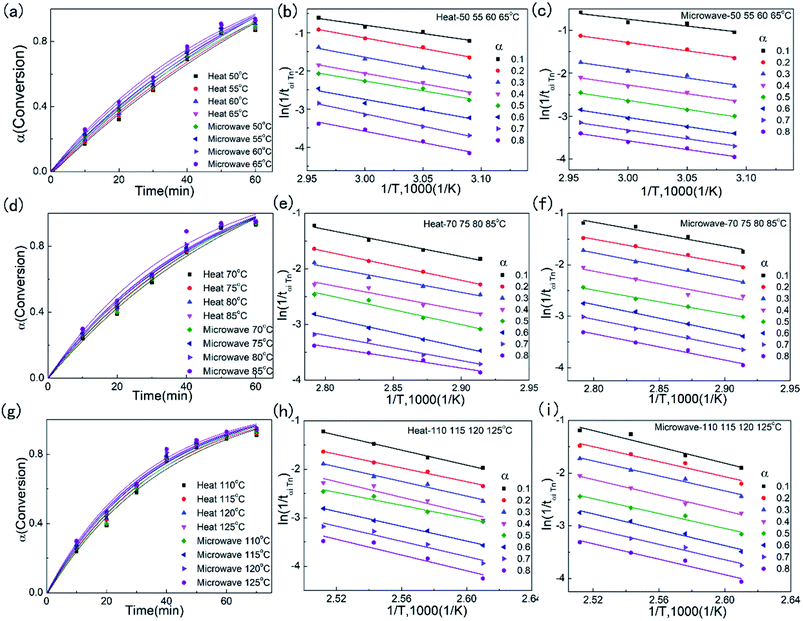
The conversion extent of epoxy and the dependences of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tα,i on inverse temperature (1/Ti, 1000) for different conversion of three samples under conventional heating and iso-thermal microwave irradiation at different reaction temperature were presented in Fig. 3, and the entire data was represented in Tables S5–S7.† The data was linear fitted, and the slopes and intercepts of these fitted lines represented the apparent activation energy (Ea) and pre-exponential factors (ln
tα,i on inverse temperature (1/Ti, 1000) for different conversion of three samples under conventional heating and iso-thermal microwave irradiation at different reaction temperature were presented in Fig. 3, and the entire data was represented in Tables S5–S7.† The data was linear fitted, and the slopes and intercepts of these fitted lines represented the apparent activation energy (Ea) and pre-exponential factors (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα) respectively.32,37 The plots of Ea,α and ln
Aα) respectively.32,37 The plots of Ea,α and ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα versus α were shown in Fig. 4 and Tables 1–3.
Aα versus α were shown in Fig. 4 and Tables 1–3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα values for the EP-D230 at 50–65 °C under heat and iso-thermal microwave irradiation
Aα values for the EP-D230 at 50–65 °C under heat and iso-thermal microwave irradiation
| α (%) | Heat 50–65 °C (Ea kJ mol−1) | Microwave 50–65 °C (Ea kJ mol−1) | Heat 50–65 °C (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα) Aα) |
Microwave 50–65 °C (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα) Aα) |
|---|---|---|---|---|
| 10 | 46.8 | 43.1 | 17.1 | 14.4 |
| 20 | 47.1 | 43.5 | 16.1 | 13.9 |
| 30 | 47.6 | 43.7 | 16.0 | 13.2 |
| 40 | 47.5 | 43.5 | 15.4 | 13.2 |
| 50 | 48.1 | 44.2 | 15.5 | 12.8 |
| 60 | 48.3 | 44.1 | 15.0 | 12.6 |
| 70 | 48.9 | 44.5 | 15.0 | 12.7 |
| 80 | 49.4 | 46.3 | 15.2 | 12.7 |
| Average | 48.0 | 44.1 | 15.7 | 13.2 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα values for the EP-D230 at 70–85 °C under heat and iso-thermal microwave irradiation
Aα values for the EP-D230 at 70–85 °C under heat and iso-thermal microwave irradiation
| α (%) | Heat 70–85 °C (Ea kJ mol−1) | Microwave 70–85 °C (Ea kJ mol−1) | Heat 70–85 °C (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα) Aα) |
Microwave 70–85 °C (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα) Aα) |
|---|---|---|---|---|
| 10 | 43.5 | 41.2 | 15.6 | 12.8 |
| 20 | 44.2 | 42.3 | 14.8 | 12.3 |
| 30 | 44.5 | 42.1 | 14.7 | 12.1 |
| 40 | 44.8 | 42.2 | 14.4 | 12.0 |
| 50 | 44.5 | 42.5 | 14.1 | 11.9 |
| 60 | 44.7 | 42.8 | 14.0 | 11.2 |
| 70 | 45.1 | 43.1 | 14.0 | 11.2 |
| 80 | 45.5 | 43.4 | 14.2 | 11.4 |
| Average | 44.6 | 42.4 | 14.5 | 11.9 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα values for the EP-MHHPA at 110–125 °C under heat and iso-thermal microwave irradiation
Aα values for the EP-MHHPA at 110–125 °C under heat and iso-thermal microwave irradiation
| α (%) | Heat 110–125 °C (Ea kJ mol−1) | Microwave 110–125 °C (Ea kJ mol−1) | Heat 110–125 °C (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα) Aα) |
Microwave 110–125 °C (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα) Aα) |
|---|---|---|---|---|
| 10 | 63.4 | 57.4 | 21.2 | 17.5 |
| 20 | 64.7 | 57.8 | 20.7 | 17.3 |
| 30 | 65.9 | 58.1 | 20.6 | 16.6 |
| 40 | 66.3 | 58.8 | 20.3 | 16.1 |
| 50 | 65.8 | 58.7 | 20.3 | 16.1 |
| 60 | 66.7 | 59.2 | 19.5 | 15.9 |
| 70 | 66.9 | 59.8 | 19.5 | 15.6 |
| 80 | 67.8 | 60.9 | 20.0 | 15.6 |
| Average | 65.9 | 58.8 | 20.3 | 16.3 |
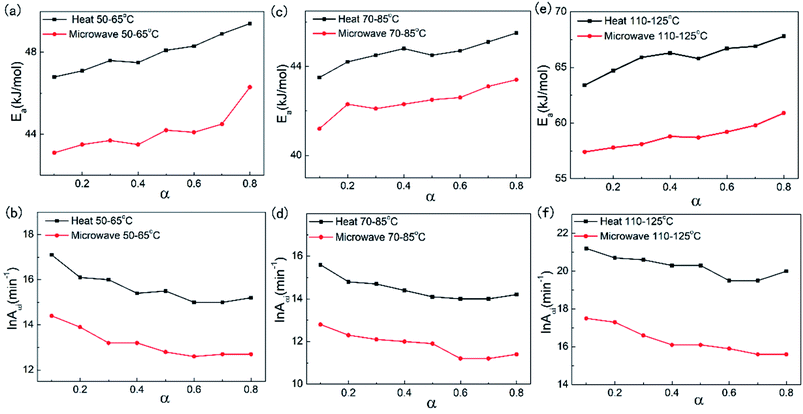
The Ea and ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα values for EP-D230 and EP-MHHPA with different reaction temperature decreased when the microwave irradiation was induced in the isothermal environment. All the resin slices were cooled rapidly by silicone oil in the isothermal environment and the mean molecular kinetic energy of the polymer bulk was not significantly changed, indicating the extra energy for becoming the activated molecules was reduced. The energy of the microwave was insufficient to break down the chemical bonds during the reaction, however, it matched the energy level of rotation of the polarized functional groups,4 and could help to activate them.11 Besides, the non-thermal effect changed the distribution of electron cloud of the functional groups and further increased the degree of polarization, which led to the increase of the reactivity. Moreover, the microwave field motivated the polarizable electrons of polymer chains and created rotational motion from the dielectric loss,1,8 which increased the collision frequency of the polymer matrix.6,41,42 The ln
Aα values for EP-D230 and EP-MHHPA with different reaction temperature decreased when the microwave irradiation was induced in the isothermal environment. All the resin slices were cooled rapidly by silicone oil in the isothermal environment and the mean molecular kinetic energy of the polymer bulk was not significantly changed, indicating the extra energy for becoming the activated molecules was reduced. The energy of the microwave was insufficient to break down the chemical bonds during the reaction, however, it matched the energy level of rotation of the polarized functional groups,4 and could help to activate them.11 Besides, the non-thermal effect changed the distribution of electron cloud of the functional groups and further increased the degree of polarization, which led to the increase of the reactivity. Moreover, the microwave field motivated the polarizable electrons of polymer chains and created rotational motion from the dielectric loss,1,8 which increased the collision frequency of the polymer matrix.6,41,42 The ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα value was proportional to the concentration of the active centers. The microwave irradiation of the reaction led to the rapid energy transferred and absorption causing the non-equilibrium distribution which reduced the account of the activation centers.1,15,18 As the result, the ln
Aα value was proportional to the concentration of the active centers. The microwave irradiation of the reaction led to the rapid energy transferred and absorption causing the non-equilibrium distribution which reduced the account of the activation centers.1,15,18 As the result, the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα value decreased by the microwave non-thermal effect.
Aα value decreased by the microwave non-thermal effect.
The value of Eα increased and ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα decreased when the α value raised up. With the polymer matrix curing, the chain mobility was restricted by increasing molecular weight, which led to the climb up of the activation energy. The curing rate in the glassy state decreased dramatically which relied on the diffusion of unreacted groups remined in the matrix. Other research works also indicated that the vitrification caused the considerable decrease in the molecular mobility leading to the increase of the effective activation energy with the increasing reaction extent.32,35,37 The account of the activation centers reduced with the climb up of α, as the result, the ln
Aα decreased when the α value raised up. With the polymer matrix curing, the chain mobility was restricted by increasing molecular weight, which led to the climb up of the activation energy. The curing rate in the glassy state decreased dramatically which relied on the diffusion of unreacted groups remined in the matrix. Other research works also indicated that the vitrification caused the considerable decrease in the molecular mobility leading to the increase of the effective activation energy with the increasing reaction extent.32,35,37 The account of the activation centers reduced with the climb up of α, as the result, the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα decreased.
Aα decreased.
3.3 Affection caused by the reaction temperature towards the microwave non-thermal effect
There were two sets of reaction temperature for EP-D230 samples which were 50–65 °C and 70–85 °C respectively. As from Tables 1 and 2, the Ea under iso-thermal microwave irradiation was 42.4 kJ mol−1 for the 70–85 °C sample set, which was 4.82% lower than that without microwave irradiation. This reduction became distinct for the 50–65 °C sample sets. In this case, the Ea value decreased 8.02% from 48.0 kJ mol−1 to 44.1 kJ mol−1.The decrease extent of Ea is less than that reported in other literatures, for example, P. Navabpour et al. reported that comparison of the curing kinetics of a DGEBA/acid anhydride using differential scanning calorimetry and a microwave-heated calorimeter, and the values obtained were 78.6 ± 0.2 and 91.2 ± 0.2 kJ mol−1 for the conventional and microwave curing, respectively.25 This is the result peeling the ‘thermal effect’ from microwave in isothermal reactor, and it leads to the weakening of microwave enhancement.
The microwave non-thermal effect to the activation energy was more distinct with lower reaction temperature, that the reduction portion of the activation energy should be less with higher reaction temperature. Robert L. H. et al. have pointed that the microwave field was able to affect the reaction entropy.11 The polar molecules were forced to move according to the direction of the applied electromagnetic field, and the molecule chaotic motion was suppressed leading to the reduction of the collision chance between epoxy and hardeners.29,43
The pre-exponential factor value was decreased by the microwave non-thermal effect. The thermal energy initiated a high number of activation centers without microwave irradiation and this value of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα was proportional to the concentration of activation centers. The microwave irradiation of the reaction led to the rapid energy transferred and absorption which reduced the account of the activation centers.1,15,18 As the result, the ln
Aα was proportional to the concentration of activation centers. The microwave irradiation of the reaction led to the rapid energy transferred and absorption which reduced the account of the activation centers.1,15,18 As the result, the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα value was decreased by the microwave non-thermal effect. The microwave field affects molecules by the Lorentz force, and mean kinetic energy of molecules was increased which should lead to the climb up of ln
Aα value was decreased by the microwave non-thermal effect. The microwave field affects molecules by the Lorentz force, and mean kinetic energy of molecules was increased which should lead to the climb up of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα value.27,28 However, all the molecules were forced to move according to the direction of the applied electromagnetic field, and the molecule chaotic motion was suppressed leading to the reduction of ln
Aα value.27,28 However, all the molecules were forced to move according to the direction of the applied electromagnetic field, and the molecule chaotic motion was suppressed leading to the reduction of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα value. This suppression affection became more obvious compared with the increase caused by Lorentz force under higher reaction temperature.
Aα value. This suppression affection became more obvious compared with the increase caused by Lorentz force under higher reaction temperature.
3.4 Affection caused by the polarity of the hardener towards the microwave non-thermal effect
The reaction temperature of EP-MHHPA sample set ranged from 110 to 125 °C which was higher than that of EP-D230. As from Table 3, the Ea value reduced by 10.77% from 65.9 kJ mol−1 to 58.8 kJ mol−1 EP-MHHPA when microwave was induced. This reduction proportion was significantly larger compared with that of EP-D230.The amine equivalent is lower than the anhydride equivalent, which results in higher polar functional group content in the EP-MHHPA than EP-D230. The microwave non-thermal effect was amplified by the polarized transition state caused by the formation of oxygen anion in EP-MHHPA samples.16 The energy of activation could be reduced by the microwave field stabilizing the polar transition state.41,44 As a result, the microwave non-thermal effect on the EP-MHHPA was stronger than that of the EP-D230.
3.5 Microwave non-thermal effect towards the thermoset mechanical and thermal performance
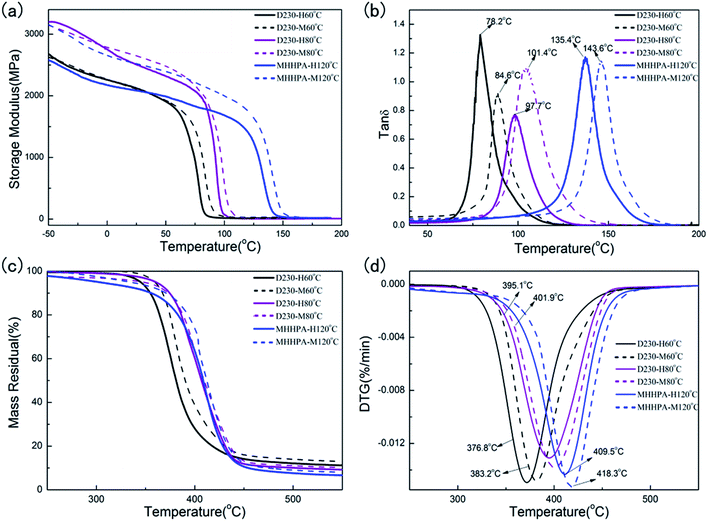
The DMA data for these samples was plotted in Fig. 5(a) and (b). The solid lines represented the storage moduli (G′) of the thermoset without microwave, and the dash lines were those when microwave induced. Results showed the G′ and Tg increased in all the sample sets when microwave induced. The microwave non-thermal effects arose upon directly interaction of electric field with target molecules, under the microwave field.45,46 A preferential orientation of epoxy and hardener in the microwave field (as opposed to random orientation in the thermal field) shortened the curing time, made the molecular packing more compact and increased the density of the epoxy resin. The frequency of the microwave was close to the revolving vibration frequency of chemical radicals, and the conformation of the molecules can be changed. The preferential orientation of epoxy and hardener in the microwave field led to few inter-twinements in the process of formation of a molecular net.47 In the thermal field a randomly cross-linked network would start to form the onset of reactions while the microwave field would favor the initial formation of a linear polymer with cross-linking occurring at later stages of cure, which was favorable for improving the modulus and Tg of epoxy resin. It should be noticed that the microwave irradiation could increase the Tg of epoxy resin more significantly when the microwave thermal effect was not peeled off, for an example, the Tg of R2512/H2409 epoxy/hardener system increased 19.8 °C (88.9 °C to 108.7 °C) after the microwave was induced,43 however, the Tg of EP-MHHPA in this presented work increased only 8.2 °C (135.4 °C to 143.6 °C).Interestingly, the G′ increase of EP-MHHPA was much more significant than that of EP-D230 demonstrated by Fig. 5(a). At the same time, the Tg increase of EP-MHHPA was 8.2 °C (135.4 °C to 143.6 °C) which was higher than that of EP-D230 (78.2 °C for 84.6 °C, and 97.7 °C for 101.4 °C). Results indicated the microwave non-thermal effect was more obvious on the epoxy-anhydride system than the epoxy-amine system. The microwaves field stabilizing the polar transition state of the epoxy-anhydride, which lead to the decrease of Ea and improved cross-linking density.44 The speculated explanation was that the microwave non-thermal effect was amplified by the polarized transition state caused by the formation oxygen anion in EP-MHHPA matrix. The effect of accelerator (DMP-30) on the epoxy resin with and without microwave irradiation in isothermal environment was evaluated in order to verify the speculation. The curing of EP-D230 systems were accelerated by DMP-30 promoting the formation of hydroxyl groups at the initial curing stage with the polarity undisturbed. However, the generation of carboxylate anion in the EP-MHHPA samples was accelerated leading to the increase of polarized oxygen anion in the transition state. Calculation results from the FTIR spectrum (Fig. S8†) showed that the conversion rate of EP-MHHPA with 0.5% DMP-30 increased 7.5% (0.86 to 0.93, 120 °C) when microwave induced. This value was much higher than that of EP-D230 with 0.5% DMP-30, which was 4.6% (0.87 to 0.91, 60 °C).
Similar trend was reflected by the TGA analysis of these matrix. Fig. 5(c) and (d) presented the TGA and DTG plots of these samples, and the initial decomposition temperature (T5%), the maximum decomposition temperature (Tmax) and the carbon yield (Mresidue) were listed in Table 4. All these values were increased under the iso-thermal microwave irradiation. The results reflected the increase of the crosslinking degree of these thermosets, as more energy was required for the decomposition. The introduction of the microwave increased the kinetic energy of polar molecules selectively and instantly. The polar functional groups were vibrating under the applied electromagnetic field together with the main chain. It increased the migration rate and helped to generate the ‘unrestricted network structure’ in the matrix resin under isothermal environment.48,49 The microwave nonthermal effect increased both chemical and regional selectivity of the polar functional groups during the reaction, and enhanced the cross-linking leading to homogeneous structure of the cured thermoset.
| Samples | T5% (°C) | Tmax (°C) | Mresidue (%) |
|---|---|---|---|
| D230 heat-60 °C | 345 | 376 | 7.7 |
| D230 microwave-60 °C | 356 | 383 | 10.1 |
| D230 heat-80 °C | 351 | 396 | 9.8 |
| D230 microwave-80 °C | 361 | 401 | 11.4 |
| MHHPA heat-120 °C | 311 | 409 | 12.1 |
| MHHPA microwave-120 °C | 319 | 418 | 15.3 |
3.6 Effect on the resin tensile property and the fractured morphology
The tensile properties of the thermosets were measured and listed in Table 5. The tensile moduli of EP-D230 increased from 65 ± 5.0 MPa to 68 ± 4.5 MPa after the iso-thermal microwave irradiation under 60 °C. Similar trend was presented by the EP-MHHPA which increased from 60 ± 5.5 MPa to 63 ± 6.5 MPa by the iso-thermal microwave irradiation under 120 °C. The elongation of EP-D230 and EP-MHHPA after the iso-thermal microwave irradiation were higher than those after thermal curing. Microwave irradiation rises the bulk energy of the resin uniformly and avoiding the generation of energy gradient in the material, reducing the internal residual stress.| Samples | Tensile moduli (MPa) | Elongation (%) |
|---|---|---|
| D230 heat-60 °C | 65 ± 5.0 | 12 ± 0.8 |
| D230 microwave-60 °C | 68 ± 4.5 | 13 ± 0.7 |
| D230 heat-80 °C | 73 ± 6.0 | 12 ± 0.6 |
| D230 microwave-80 °C | 75 ± 5.5 | 13 ± 0.4 |
| MHHPA heat-120 °C | 60 ± 5.5 | 8 ± 0.4 |
| MHHPA microwave-120 °C | 63 ± 6.5 | 9 ± 0.6 |
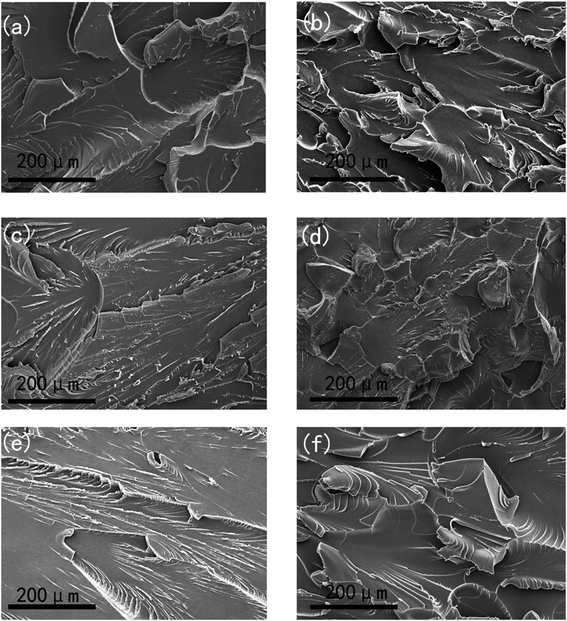
The tensile fractured sections of these thermosets with and without iso-thermal microwave irradiation were presented in Fig. 6. According to the microscopic observations of the fractured surfaces, the mechanisms of rupture were identical for microwave and thermal curing.29 The crack distributions in both EP-D230 and EP-MHHPA became more uniform when cured under iso-thermal microwave irradiation. This was consistent with the results reported by S. L. Bai et al.22 Randomly crosslinked net-work was generated in the thermal environment. However, linear crosslinking structure was the dominating product when the iso-thermal microwave irradiation was induced due to the preferential orientation of epoxy and hardener. As the result, the nonthermal effect of microwave was able to generate well distributed network structure enlarging the stress concentration region and improving the mechanical performance of the thermoset.50
4. Conclusion
An iso-thermal reactor was constructed, and matrix were sealed in ‘sandwich structure’ to explore the non-thermal effect of microwave on the epoxy curing. Results indicate the non-thermal effect of microwave did enhanced the polymer curing. The non-thermal effect of microwave was evaluated quantitatively by calculation of the apparent activation energy (Ea) and pre-exponential factors (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aα), the decrease of Ea for EP-D230 (70–85 °C) is 4.82% and EP-MHHPA (110–125 °C) is 10.77%. The non-thermal effect became more obvious with lower reaction temperature as the decrease of Ea for EP-D230 (50–65 °C) is 4.82%. Besides, the microwave non-thermal effect was more obvious on the EP-MHPPA than EP-D230 due to the higher concentration of polar functional groups. The iso-thermal microwave irradiation did not change the chemical structure of the matrix resin, and that activated the polar functional groups selectively and generated the un-restricted network structure instantly, which was led to the higher crosslink degree, and it was reflected by the DMA, TGA and tensile test. Besides, the micrographs of the fractured section showed that the iso-thermal microwave irradiation was enlarged the stress concentration region in all the matrix. This presented work clearly explored the non-thermal effect of microwave irradiation on the EP-D230 and EP-MHHPA matrix, and it could help to better understand the mechanism of microwave induced resin curing.
Aα), the decrease of Ea for EP-D230 (70–85 °C) is 4.82% and EP-MHHPA (110–125 °C) is 10.77%. The non-thermal effect became more obvious with lower reaction temperature as the decrease of Ea for EP-D230 (50–65 °C) is 4.82%. Besides, the microwave non-thermal effect was more obvious on the EP-MHPPA than EP-D230 due to the higher concentration of polar functional groups. The iso-thermal microwave irradiation did not change the chemical structure of the matrix resin, and that activated the polar functional groups selectively and generated the un-restricted network structure instantly, which was led to the higher crosslink degree, and it was reflected by the DMA, TGA and tensile test. Besides, the micrographs of the fractured section showed that the iso-thermal microwave irradiation was enlarged the stress concentration region in all the matrix. This presented work clearly explored the non-thermal effect of microwave irradiation on the EP-D230 and EP-MHHPA matrix, and it could help to better understand the mechanism of microwave induced resin curing.
Conflict of interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.Acknowledgements
This work is supported by Chinese Defense Advance Research Program of Science and Technology, China, 61409230605.References
- C. O. Mgbemena, D. Li, M. F. Lin, P. D. Liddel and K. B. Katnam, Composites, Part A, 2018, 115, 88–103 CrossRef CAS.
- M. Hosseini, N. Stiasni, V. Barbieri and C. O. Kappe, J. Org. Chem., 2007, 72, 1417–1424 CrossRef CAS.
- M. Kwak, P. Robinson, A. Bismarck and R. Wise, Composites, Part A, 2015, 75, 18–27 CrossRef CAS.
- O. V. Kharissova, B. I. Kharisov and J. J. R. Valdés, Ind. Eng. Chem. Res., 2010, 49, 1457–1466 CrossRef CAS.
- T. K. S. Bindu, N. B. Ajalesh, A. T. Beena and T. T. Eby, Polymer, 2014, 55, 3614–3627 CrossRef.
- J. Robinson, S. Kingman and D. Obermayer, Phys. Chem. Chem. Phys., 2010, 12, 10793–10800 RSC.
- U. Riaz and S. M. Ashraf, RSC Adv., 2014, 4, 47153–47162 RSC.
- X. Y. Liu, Y. N. He, D. C. Qiu and Z. Q. Yu, Compos. Struct., 2019, 230, 111529 CrossRef.
- S. Li, G. S. Zhang, H. S. Zheng, N. N. Wang, Y. G. Zheng and P. Wang, RSC Adv., 2016, 6, 82439–82446 RSC.
- J. F. Chang, G. Z. Liang, A. J. Gu, S. D. Cai and L. Yuan, Carbon, 2012, 50, 689–698 CrossRef CAS.
- R. L. Hubbard, S. M. Strain, C. Willemsen and D. R. Tyler, J. Appl. Polym. Sci., 2016, 133, 44222 CrossRef.
- X. C. Wu, Y. G. Li, N. Y. Li, J. Zhou and X. Z. Hao, High Perform. Polym., 2017, 29, 1165–1174 CrossRef CAS.
- R. Pal, A. K. Jha, M. J. Akhtar, K. K. Kar, R. Kumar and D. Nayak, Adv. Powder Technol., 2017, 28, 1281–1290 CrossRef CAS.
- F. Colangelo, P. Russo, F. Cimino, R. Cioffi, I. Farina, F. Fraternali and L. Feo, Composites, Part B, 2017, 126, 100–107 CrossRef CAS.
- R. Pal, M. J. Akhtar and K. K. Kar, Polym. Test., 2018, 70, 8–17 CrossRef CAS.
- X. Liu, J. T. Luo, J. F. Fan, S. Lin, L. Y. Jia, X. L. Jia, Q. Cai and X. P. Yang, Composites, Part B, 2019, 174, 106909 CrossRef CAS.
- G. Jaroslaw and B. Dariusz, Polymer, 2003, 44, 7795–7800 CrossRef.
- Z. Liming, K. C. Leo and H. C. Martin, Polymer, 2005, 46, 2638–2645 CrossRef.
- M. G. B. Odom, C. B. Sweeney, D. Parviz, L. P. Sill, M. A. Saed and M. J. Green, Carbon, 2017, 120, 447–453 CrossRef CAS.
- J. Zhou, Y. G. Li, N. Y. Li, X. Z. Hao and C. Q. Liu, Compos. Sci. Technol., 2017, 133, 173–183 CrossRef.
- L. Outifa, H. Jullien, C. Moré and M. Delmotte, Ind. Eng. Chem. Res., 1995, 34, 688–698 CrossRef CAS.
- S. L. Bai, V. Djafari, M. Andreani and D. Francois, Eur. Polym. J., 1995, 31, 875–884 CrossRef CAS.
- F. Y. C. Boey and B. H. Yap, Polym. Test., 2001, 20, 837–845 CrossRef CAS.
- F. Y. C. Boey, B. H. Yap and L. Chia, Polym. Test., 1999, 18, 93–109 CrossRef CAS.
- P. Navabpour, A. Nesbitt, T. Mann and R. J. Day, J. Appl. Polym. Sci., 2007, 104, 2054–2063 CrossRef CAS.
- V. Tanrattanakul and K. S. Tiaw, J. Appl. Polym. Sci., 2005, 97, 1442–1461 CrossRef CAS.
- X. Zhao, X. L. Wang, F. Tian, W. L. An, S. M. Xu and Y. Z. Wang, Green Chem., 2019, 21, 2487–2493 RSC.
- J. H. Wei, M. C. Hawley and J. D. Delong, Polym. Eng. Sci., 1993, 33, 1132–1140 CrossRef CAS.
- J. Mijović and J. Wijaya, Macromolecules, 1990, 23, 3671–3674 CrossRef.
- J. Mijovic, A. Fishbain and J. Wijaya, Macromolecules, 1992, 25, 986–989 CrossRef CAS.
- K. Kempe, C. R. Becer and U. S. Schubert, Macromolecules, 2011, 44, 5825–5842 CrossRef CAS.
- S. Vyazovkin, A. K. Burnham, J. M. Criado and C. Popescu, Thermochim. Acta, 2011, 520, 1–19 CrossRef CAS.
- S. P. Dubey, H. A. Abhyankar, V. Marchante, J. L. Brighton, B. Bergmann, G. Trinh and C. David, RSC Adv., 2017, 7, 18529–18538 RSC.
- H. W. Cui, K. Suganuma and H. Uchida, RSC Adv., 2015, 5, 2677–2683 RSC.
- S. Vyazovkin and C. A. Wight, Thermochim. Acta, 1999, 340–341, 53–68 CrossRef CAS.
- U. L. Vergarra, M. Sarrionandia, K. Gondra and J. Aurrekoetxea, Thermochim. Acta, 2014, 581, 92–99 CrossRef.
- P. Spasojević, J. Jovanović and B. Adnadjevic, Mater. Chem. Phys., 2013, 141, 882–890 CrossRef.
- P. B. Zhang, S. A. A. Shah, F. Gao, H. Sun, Z. C. Cui, J. Cheng and J. Y. Zhang, Thermochim. Acta, 2019, 676, 130–138 CrossRef CAS.
- V. L. Zvetkov, E. S. Ivanova and V. Calado, Thermochim. Acta, 2014, 596, 42–48 CrossRef CAS.
- V. L. Zvetkov, S. Djoumaliisky and E. S. Ivanova, Thermochim. Acta, 2013, 553, 16–22 CrossRef CAS.
- C. Y. Zhang, H. H. Zhang, R. Li and Y. J. Xing, RSC Adv., 2017, 7, 48189–48198 RSC.
- P. B. Zetterlund and S. Perrier, Macromolecules, 2011, 44, 1340–1346 CrossRef CAS.
- T. Chaowasakoo and N. Sombatsompop, Compos. Sci. Technol., 2007, 67, 2282–2291 CrossRef CAS.
- J. Y. Ma, J. Phys. Chem. A, 2016, 120, 7989–7997 CrossRef CAS.
- A. Anshuman, S. S. Yarahmadi and B. Vaidhyanathan, RSC Adv., 2018, 8, 7709–7715 RSC.
- M. Hosseini, N. Stiasni, V. Barbieri and C. O. Kappe, J. Org. Chem., 2007, 72, 1417–1424 CrossRef CAS.
- J. Zhou, C. Shi, B. C. Mei, R. Z. Yuan and Z. Y. Fu, J. Mater. Process. Technol., 2003, 137, 156–158 CrossRef CAS.
- K. M. Huang, X. Q. Yang, W. Hua, G. Z. Jia and L. J. Yang, New J. Chem., 2009, 33, 1486–1489 RSC.
- E. J. Lee, J. Bae, K. M. Choi and N. C. Jeong, ACS Appl. Mater. Interfaces, 2019, 11, 35155–35161 CrossRef CAS.
- J. Jacob and F. Y. C. Boey, J. Mater. Sci., 1995, 30, 5321–5327 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08427a |
| This journal is © The Royal Society of Chemistry 2021 |