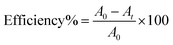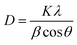Open Access Article
Open Access ArticleFacile synthesis of superparamagnetic Fe3O4@noble metal core–shell nanoparticles by thermal decomposition and hydrothermal methods: comparative study and catalytic applications†
Eman A.
Bakr
*,
Marwa N.
El-Nahass
 *,
Wafaa M.
Hamada
and
Tarek A.
Fayed
*,
Wafaa M.
Hamada
and
Tarek A.
Fayed
Department of Chemistry, Faculty of Science, Tanta University, 31527, Tanta, Egypt. E-mail: marwa.elnahas@science.tanta.edu.eg; eman.bakr@science.tanta.edu.eg; Fax: +20-403350804; Tel: +20-403344352
First published on 24th December 2020
Abstract
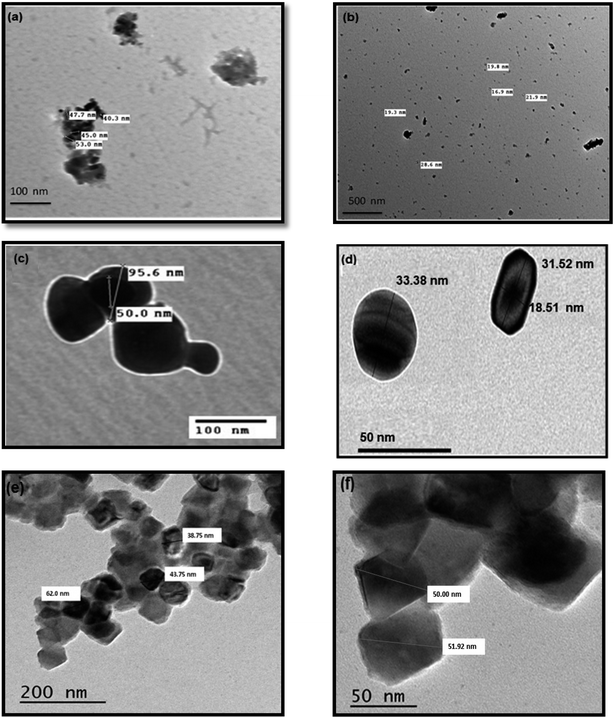
Herein, we report on developing a facile synthetic route for reusable nanocatalysts based on a combination of the supermagnetic properties of magnetite with the unique optical and catalytic properties of noble metal hybrid nanomaterials. We compare two different synthetic methods, to find out which is best from synthetic and application points of view, for the synthesis of Fe3O4 and Fe3O4@M (M = Ag or Au) core–shell hybrid nanostructures. The two different single-step synthetic methods are: thermal decomposition and hydrothermal. The structural, morphological and magnetic properties of the obtained Fe3O4 and Fe3O4@M nanoparticles were characterized by various techniques. XRD of the Fe3O4 nanoparticles exhibited sharp and strong diffraction peaks, confirming the highly crystalline structure of the Fe3O4 particles synthesized by the hydrothermal method, while broad and weak peaks were observed on using the thermal decomposition method. Both Fe3O4@Ag and Fe3O4@Au core–shells obtained by the hydrothermal method showed the reflection planes of Fe3O4 and additional planes of Ag or Au. But on the formation of Fe3O4@Ag/Au by the thermal decomposition method the peak for Fe3O4 disappeared and only the diffraction peaks of Ag or Au appeared. According to TEM analysis there was a broad particle-size distribution, random near-spherical shapes and slight particle agglomeration for Fe3O4 synthesized by the thermal decomposition method. However, there was a moderate size distribution, spherical shapes and well-dispersed particles without large aggregations for the hydrothermal method. TEM images of the synthesized nanoparticles by the two methods used showed a pronounced difference in both size and morphological shape. The catalytic performance of the synthesized nanoparticles was examined for the reduction of Congo red dye in the presence of NaBH4. The Fe3O4 nanocatalyst maintained its catalytic activity for only one cycle. In the cases of Fe3O4@Au and Fe3O4@Ag, the catalytic activity was conserved for four and ten successive cycles, respectively. Based on the obtained results, it was concluded that the hydrothermal synthesis of Fe3O4, Fe3O4@Ag and Fe3O4@Au nanostructures is highly recommended due to their selectivity and merits.
1. Introduction
Core–shell hybrid nanoparticles formed from the combination of two or more types of nanoparticles are highly regarded by the scientific community. They have the potential to merge the unique physicochemical properties of two or more nanomaterials into one entity, leading to enormous advances by these structures towards various applications in the areas of catalysis, electronics, photonics, and biotechnology and environmental applications.1–7 Superparamagnetic Fe3O4 has found applications in medicine for drug transport as a local magnetic therapy, to diagnose and treat cancer using magnetic hyperthermia (ablation), for magnetic marking of cells (immunological separation) or for contrast strengthening in cell magnetic resonance imaging.8,9 Among these applications catalysis technologies are chosen to contribute to alleviation of the water pollution problem. It is a global issue and the world community is facing the worst effects of polluted water. One of the major sources of water pollution is the discharge of organic dyes as industrial waste. There is an urgent need to find an ideal remover that not only reduces these organic dyes but also achieves their selective separation and recovery to achieve a green chemistry concept. So, it is of utmost importance to develop new materials with tailored properties that can meet strong connections between eco-efficiency and performance, processing and manufacturing, recyclability, and costs. Actually, through nanocomposites with core–shell nanostructures, the catalytic properties can be enhanced to a great extent, which may not be achievable by the use of monometallic catalysts. Magnetic iron oxide nanoparticles (MIONPs) are ideal materials for industrial and biomedical applications because of their very low toxicity and biodegradability.10–15 Superparamagnetic iron oxide nanoparticles, such as magnetite nanoparticles (Fe3O4), are extremely attractive as a component of core–shell hybrid nanoparticles where the saturation magnetization value for the bulk material is 92 emu g−1, so they connect with external fields, providing them with the capability to be easily recovered and subsequently reused or else pulled by a magnetic bar.16,17 However, Fe3O4 nanoparticles have large specific surface areas; they are prone to oxidation and aggregation leading to low magnetism and poor dispersion. Recently, attempts have been made to apply certain facial modifications to Fe3O4 through the development of a wide range of hybrid nanoparticles.18 Distinct consideration is being paid now to the effect of noble metals, such as Ag and Au NPs, on the properties of Fe3O4, due to their specific characteristics like: (i) intense absorption in the optical region associated with localized surface plasmon resonance (LSPR),19 and (ii) their stability at high temperatures under conditions where base metals are rapidly oxidized.20 This was confirmed by an improvement in the stability of and an enhancement in the catalytic and antibacterial activities of the Fe3O4@noble metal hybrid nanoparticles, suggesting their importance for potential applications in water treatment and biomedicine.21–24 A wide variety of types of chemical processing can be used to synthesize hybrid nanoparticles, such as co-precipitation, thermal decomposition, electrodeposition, sol–gel, sonochemical and hydrothermal methods. Each technique used to produce hybrid nanoparticles has different characteristics and displays some advantages and disadvantages. The selection of a specific technique will be based on several factors, such as the kind of desired structure, the necessity for size control, time available to produce the particles, and the required interaction between both components. Thermal decomposition is a common method used in industry to synthesize narrow magnetic iron oxide nanoparticles (MIONPs). In this method, the iron precursor and reducing agent are injected into a solvent containing surfactant solution at a high temperature, which in turn decomposes the iron precursor in the presence of the surfactant that will coat and stabilize the formation of MIONPs. The thermal decomposition method is characterized by some advantages, in comparison with other methods: the process is non-toxic, inexpensive and achieves high crystallinity with a uniform size distribution, allowing a good amount of nanocrystals to be produced in a single reaction without a further size-sorting process.25 Hydrothermal synthesis of MIONPs in aqueous media is carried out in autoclaves/reactors at high temperature and pressure (above 200 °C and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 kPa).26–31 Rapid nucleation and faster growth occur at high temperature and lead to the formation of MIONPs. The crystals formed by hydrothermal treatment are generally narrow with high purity and density. They do not aggregate due to the presence of stabilizing agents.
790 kPa).26–31 Rapid nucleation and faster growth occur at high temperature and lead to the formation of MIONPs. The crystals formed by hydrothermal treatment are generally narrow with high purity and density. They do not aggregate due to the presence of stabilizing agents.
The main goal of the present study is to optimize a synthetic method for Fe3O4@M (M = Ag or Au) core–shell NPs. Herein, we applied two different single-step synthetic methods; (i) thermal decomposition and (ii) hydrothermal, to compare them to find which is better in terms of both synthetic and catalytic approaches. Different complementary analytical tools were used to characterize the synthesized nano-core–shells such as FTIR, XRD, TEM, EDX, UV-Vis, and magnetization. Congo red was selected as a target pollutant, due to its solubility in water and extensive use in the textile, paper, rubber and plastic industries. Congo red is a carcinogenic benzidine-based anionic diazo dye used for the coloration of paper products. The catalytic activities of Fe3O4@M core–shell NPs were utilized for the degradation of Congo red dye in aqueous solution using NaBH4 as a reducing agent. The effects of NaBH4, initial dye concentration and catalyst amount on the degradation process were also studied in detail.
2. Experimental
2.1 Materials and reagents
All chemicals were commercial grade and used without additional purification. They include ferric nitrate nonahydrate Fe(NO3)3·9H2O, silver nitrate AgNO3, tetrachloroauric acid HAuCl4·3H2O, tri-disodium citrate dihydrate C6H5O7Na3·2H2O, urea (CON2H4), polyacrylamide (PAM), sodium borohydride NaBH4 (99.0%) and Congo red. Deionized water was used for all synthesis and measurement procedures.2.2 Methods for synthesis of Fe3O4, Fe3O4@Ag, and Fe3O4@Au NPs
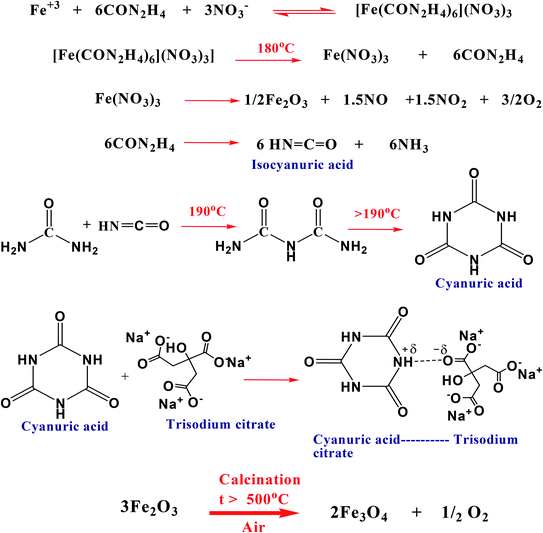
2.2.1.1 Synthesis of magnetite nanoparticles (Fe3O4). Magnetite nanoparticles were synthesized using an iron–urea complex, iron(III) hexaureatrinitrate [Fe(CON2H4)6](NO3)3, in the presence of sodium citrate as a stabilizing agent. This route was relatively environmentally-friendly because ferric nitrate and urea were the precursors for the iron complex. In a typical synthesis; 1 mmol Fe(NO3)3·9H2O, 6.2 mmol urea (CON2H4) and 3 mmol sodium citrate (C6H5O7Na3·2H2O) in 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.2
6.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
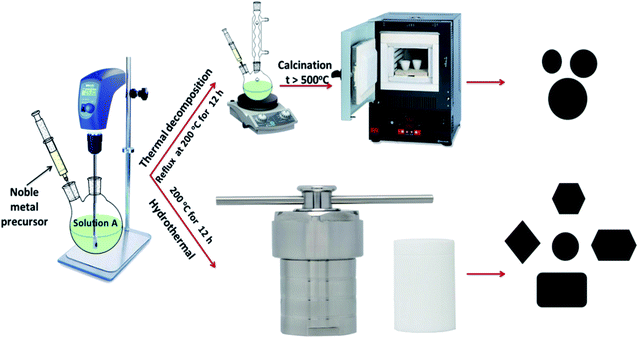
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio were dissolved in about 15 mL of distilled water. The obtained light green colored precipitate was vigorously stirred for 1 h. Then 0.15 g of polyacrylamide (PAM) as a capping agent was added under continuous stirring until complete dissolution. The mixture was then taken in a round-bottom flask and refluxed with stirring in the air at 200 °C for about 12 h. After cooling down to room temperature, the precipitate was collected by magnetic separation, washed several times with ethanol, then with distilled water, and kept in an oven at 40 °C overnight for drying. The prepared sample was calcined at 600 °C in a muffle oven (heating rate = 2 °C min−1) under air atmosphere.
3 molar ratio were dissolved in about 15 mL of distilled water. The obtained light green colored precipitate was vigorously stirred for 1 h. Then 0.15 g of polyacrylamide (PAM) as a capping agent was added under continuous stirring until complete dissolution. The mixture was then taken in a round-bottom flask and refluxed with stirring in the air at 200 °C for about 12 h. After cooling down to room temperature, the precipitate was collected by magnetic separation, washed several times with ethanol, then with distilled water, and kept in an oven at 40 °C overnight for drying. The prepared sample was calcined at 600 °C in a muffle oven (heating rate = 2 °C min−1) under air atmosphere.
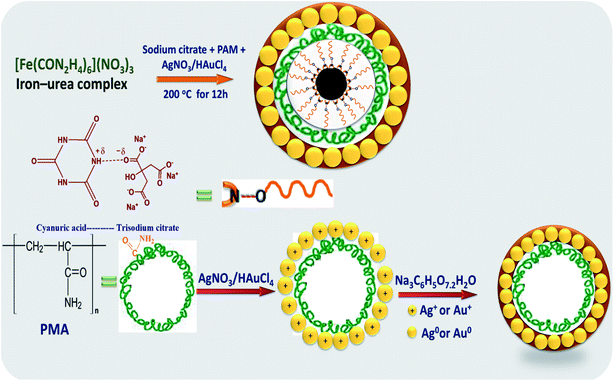
2.2.1.2 Synthesis of core–shell hybrid nanoparticles of Fe3O4@M (M = Ag or Au). The preparation of Fe3O4@M core–shell nanoparticles was carried out following the same procedures as in Section 2.2.1.1, with only one additional step, where 0.5 mmol AgNO3 was used for the synthesis Fe3O4@Ag, and 0.5 mmol HAuCl4·3H2O for the synthesis Fe3O4@Au, where each of these additives was dissolved in 5 mL of distilled water, then dropped slowly into the former solution until it dissolved completely. The molar ratio of the noble metal precursor to that of iron was 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The remaining steps were followed exactly until the required product was obtained.
2. The remaining steps were followed exactly until the required product was obtained.
2.2.2.1 Synthesis of magnetite nanoparticles (Fe3O4). Fe(NO3)3·9H2O (1 mmol), urea (CON2H4) (6.2 mmol), and sodium citrate (C6H5O7Na3·2H2O) (3 mmol) were dissolved in about 15 mL of distilled water followed by intensive stirring for 1 h until the reactants were completely converted to a light green precipitate. Then, 0.15 g of polyacrylamide (PAM) was slowly added under vigorous stirring until complete dissolution of all the materials. The solution was subsequently transferred to a 50 mL Teflon-lined autoclave. The autoclave was then sealed and subsequently put into an oven at 200 °C for 12 h. After the autoclave had cooled down to room temperature, the black precipitate was collected by an external magnet, washed alternately with absolute ethanol and distilled water several times, and dried in the oven at 50 °C overnight.
2.2.2.2 Synthesis of core–shell hybrid nanoparticles of Fe3O4@M (M = Ag or Au). Fe3O4@M core–shell nanoparticles synthesized by the hydrothermal method were achieved by following the same procedures as in Section 2.2.2.1. Then 5 mL of 0.5 mmol aqueous AgNO3 0.5 mmol HAuCl4·3H2O, for the synthesis of Fe3O4@Ag or Fe3O4@Au, respectively, were dropped slowly into the formed solution until it had totally dissolved. The same molar ratio of noble metal precursor to iron precursor was taken into account, which was 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The remaining steps were followed exactly as previously.
2. The remaining steps were followed exactly as previously.
The chemical steps for the synthesis of Fe3O4@Ag and Fe3O4@Au core–shell nanoparticles by the two investigated methods are shown in Scheme 1.
2.3 Catalytic performance of the synthesized NPs
The catalytic behavior of all synthesized nanostructures has been demonstrated toward the reduction of Congo red dye, CR. CR was not reduced readily in aqueous media by addition of NaBH4 as a reducing agent and the reaction was not kinetically feasible. The reaction mixture consisted of 0.5 mL of dye solution of 1 × 10−3 mol L−1, 5 mL of aqueous solution of 0.05 mol L−1 NaBH4 and the solution mixture was brought up to 10 mL using distilled water and then finally 20 mg of nanocatalyst was added. The time-dependent UV/Vis absorption spectra were recorded at 496 nm immediately after the addition of the nanocatalyst into the reaction mixture to follow the reduction process. The decrease in the absorbance of unreduced CR was recorded, until the steady-state was reached.To follow the progress of CR dye degradation under these conditions, the efficiency percentage was calculated as a function of time. The degradation efficiency was calculated as;34
2.4 Instrumentation
An FT-IR-4100 (JASCO, Japan) spectrophotometer was used to record the FT-IR spectra within the range 4000–400 cm−1 with a resolution of 2 cm−1 using KBr pellets. The crystalline structure of all synthesized nanostructures was analyzed by X-ray powder diffraction (XRD) with a GNR APD 2000 PRO diffractometer in the angular range 2θ = 5°–90° at 293 K using nickel-filtered Cu Kα (λ = 1.5405 Å) radiation operating at 40 kV and 30 mA. The morphology was examined by transmission electronic microscopy (TEM) measurements using a JEOL (Jem-2100) electron microscope (HT 200 eV; resolution 0.1432 nm; option 1.5 million). An energy-dispersive X-ray spectrometer (EDX) IT100LA operating at an accelerating voltage of 20.00 keV was attached to a transmission electron microscope (TEM). Magnetic properties were measured by a vibrating sample magnetometer (VSM, Lake Shore, 7410 model) at room temperature. The thermal analysis, TGA, was carried out in the range 30–800 °C using a Shimadzu TG-50 thermogravimetric analyzer under a nitrogen atmosphere with a heating rate of 20 °C min−1. The UV-Vis absorption spectra were recorded using a Shimadzu double beam UV-Vis Scanning Spectrophotometer (UV-3101 PC), using matched quartz cuvettes.3. Results and discussion
3.1 Mechanism of formation of Fe3O4@M core–shell nanostructures
A possible mechanism for the formation of the Fe3O4@M core–shell from the iron–urea complex is depicted in Schemes 2 and 3. It was proposed that cyanuric acid is formed along with iron oxide nanoparticles on the thermal decomposition of the iron–urea complex.35 The cyanuric acid molecules present on the surface of the iron oxide nanoparticles are hydrogen-bonded to sodium citrate molecules and coat them completely. Citrate and PAM play important roles in the formation of well-defined Fe3O4@M core–shell NPs. The polymer, PAM, played two roles in the synthetic route. Firstly, PAM with abundant amide groups is considered to be an efficient chelating agent for the preparation of supported Ag NPs through the coordination of N and O atoms with Ag+ ions.36 Secondly, it worked as a capping agent and stabilizer.37 Due to the large amount of amide ligands, PAM could be adsorbed on the surface of the product, consequently stabilizing the primary formed particles. The increased viscosity slows down the reaction rate as well as the rate of movement of the primary nanoparticles.38 Slowing down the rate allows primary nanoparticles to have enough time to aggregate into regular spheres.3.2 Structural characterization of the synthesized nanostructures
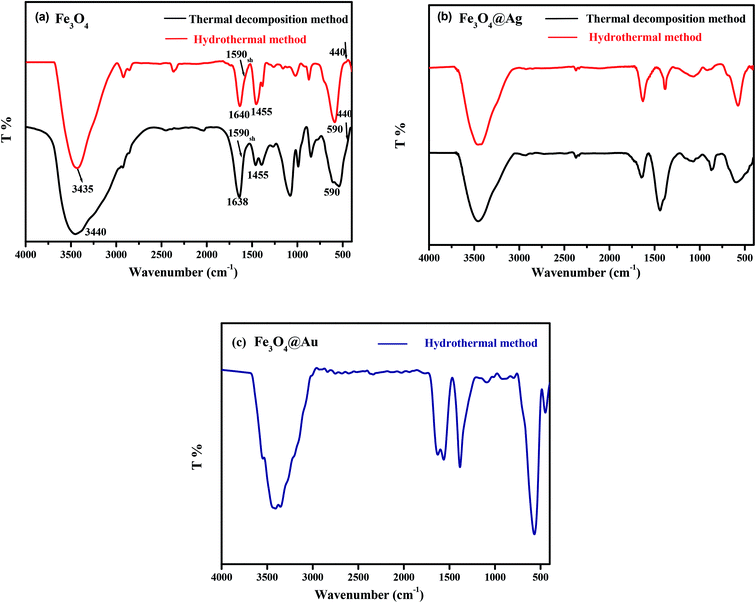
Different spectroscopic techniques were utilized to characterize and confirm the structure of the investigated nanostructured materials, Fe3O4, Fe3O4@Ag and Fe3O4@Au synthesized by the two methods.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH amide group, proving that an amount of –NH2 is embedded on the surface of Fe3O4 NPs. The characteristic absorption peaks at 1590 (sh) and 1455 cm−1 were assigned to the asymmetric and symmetric vibrations of the carboxylate ion COO− in trisodium citrate molecules. The two bands appearing around 590 and 440 cm−1 correspond to the Fe–O stretching vibrational mode in the tetrahedral site and the Fe–O stretching vibrational mode in an octahedral environment, which confirm the formation of the mixed Fe3O4 oxide. Actually, the characteristic IR peaks of Fe3O4 synthesized by the two methods shown in Fig. 1(a) are very similar. Fig. 1(b and c) illustrate the FTIR spectra of the Fe3O4@Ag core–shell obtained by the two methods and Fe3O4@Au by the hydrothermal method. By comparing Fig. 1(b and c) with Fig. 1(a), it was found that there is not much difference between them. The spectra of Fe3O4@M (M = Ag or Au) display the well-defined characteristic bands that appeared in the spectrum of Fe3O4. The bands were slightly shifted with different relative intensities.
C–NH amide group, proving that an amount of –NH2 is embedded on the surface of Fe3O4 NPs. The characteristic absorption peaks at 1590 (sh) and 1455 cm−1 were assigned to the asymmetric and symmetric vibrations of the carboxylate ion COO− in trisodium citrate molecules. The two bands appearing around 590 and 440 cm−1 correspond to the Fe–O stretching vibrational mode in the tetrahedral site and the Fe–O stretching vibrational mode in an octahedral environment, which confirm the formation of the mixed Fe3O4 oxide. Actually, the characteristic IR peaks of Fe3O4 synthesized by the two methods shown in Fig. 1(a) are very similar. Fig. 1(b and c) illustrate the FTIR spectra of the Fe3O4@Ag core–shell obtained by the two methods and Fe3O4@Au by the hydrothermal method. By comparing Fig. 1(b and c) with Fig. 1(a), it was found that there is not much difference between them. The spectra of Fe3O4@M (M = Ag or Au) display the well-defined characteristic bands that appeared in the spectrum of Fe3O4. The bands were slightly shifted with different relative intensities.
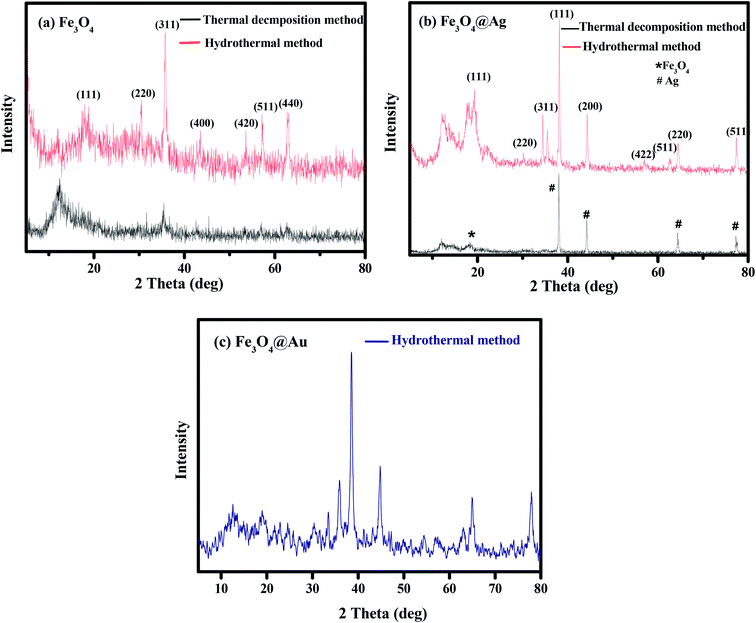 | ||
| Fig. 2 XRD patterns of (a) Fe3O4, (b) Fe3O4@Ag and (c) Fe3O4@Au synthesized by the investigated methods. | ||
The crystal size of the synthesized nanoparticles was determined from the width of the X-ray diffraction peaks using the Debye–Scherrer equation for the most intense diffraction peaks for the (220), (311), (400) faces of Fe3O4.40
The calculated average crystalline sizes of Fe3O4 NPs synthesized by thermal decomposition and hydrothermal methods were found to be 27.75 and 16.02 nm, respectively. While for Fe3O4@Ag, they were 69.82 and 29.19 nm for the two previous methods, respectively. The calculated particle size of Fe3O4@Au synthesized by the hydrothermal method was found to be 46.93 nm.
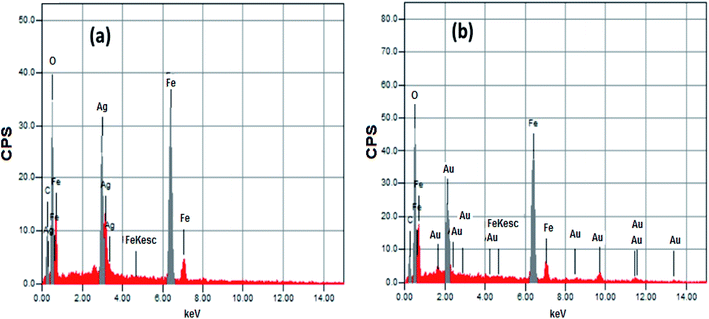
| Core–shell | Element mass% | ||||
|---|---|---|---|---|---|
| C | O | Fe | Ag | Au | |
| Fe3O4@Ag | 9.18 | 32.84 | 36.73 | 21.25 | — |
| Fe3O4@Au | 8.99 | 31.10 | 40.17 | — | 19.74 |
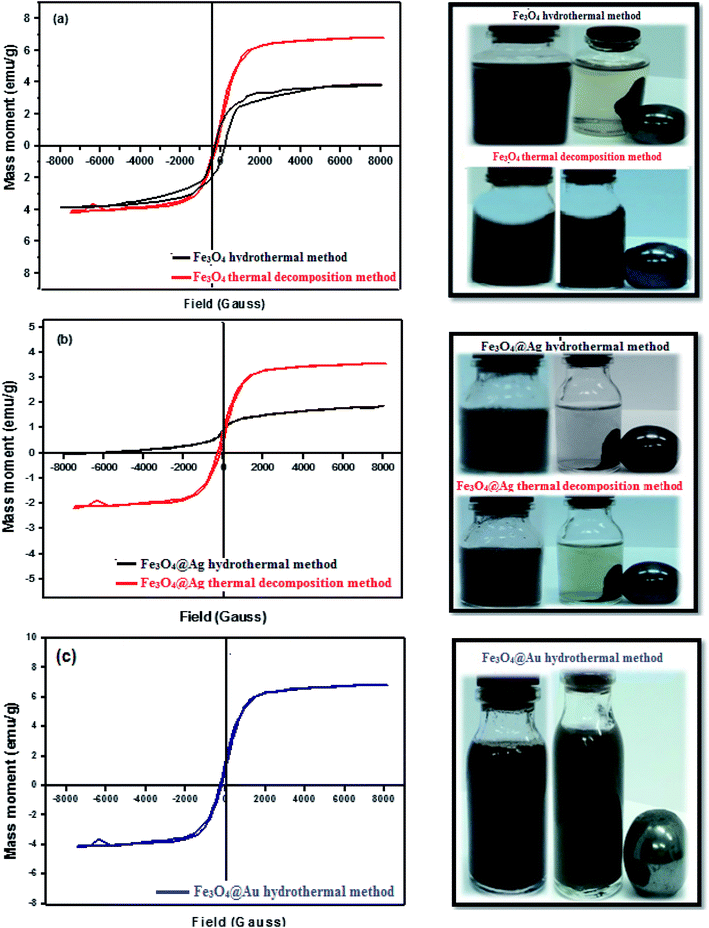
Fig. 5(a–c) depict the hysteresis loop of the nanocatalysts measured under a magnetic field varying from −8000 to 8000 Oe at room temperature. Generally, the saturation magnetization, Mg, values of magnetized Fe3O4, and Fe3O4@Ag nanoparticles decreased from 6.82, and 3.55 emu g−1 to 3.9, and 1.99 emu g−1 for the nanocatalysts synthesized by the thermal decomposition and hydrothermal methods, respectively. However, the saturation magnetization value of hydrothermal magnetized Fe3O4@Au was found to be 6.88 emu g−1. Obviously, the decrease in Ms in the case of Fe3O4@Ag relative to Fe3O4 is due to the shielding effect of nonmagnetic Ag NPs. However, the saturation magnetization value of Fe3O4@Au was greater than that of Fe3O4@Ag. This may be due to the liberation of Au NPs from their core–shell, leaving Fe3O4 NPs with the same value for its individual case, as will be discussed later in Section 3.3. Also, Fe3O4@Ag and Fe3O4@Au NPs could be magnetically separated easily from aqueous solution by an external magnet and re-dispersed in water, as shown in the images in Fig. 5(b and c). The outstanding magnetic properties of Fe3O4@Ag NPs rendered them economic and reusable for various applications, especially for treatment of wastewater.
3.3 Kinetics of reductive degradation of the investigated dye
The kinetics of the catalytic reduction of CR using the investigated Fe3O4, Fe3O4@Ag and Fe3O4@Au core–shell nanoparticles was studied using UV-Vis spectroscopy by following the disappearance of the absorbance of CR at λmax = 496 nm (Fig. 7). The absorption spectra indicated that in the presence of NaBH4, Fe3O4, Fe3O4@Ag and Fe3O4@Au core–shell nanoparticles reduce the intensity of the absorption bands of CR over time intervals, where the band disappeared completely within 35, 16, and 26 min for the mentioned nanocatalysts, respectively. The breakage of the azo group of the CR dye leads to the formation of aromatic amine products (Scheme 4).
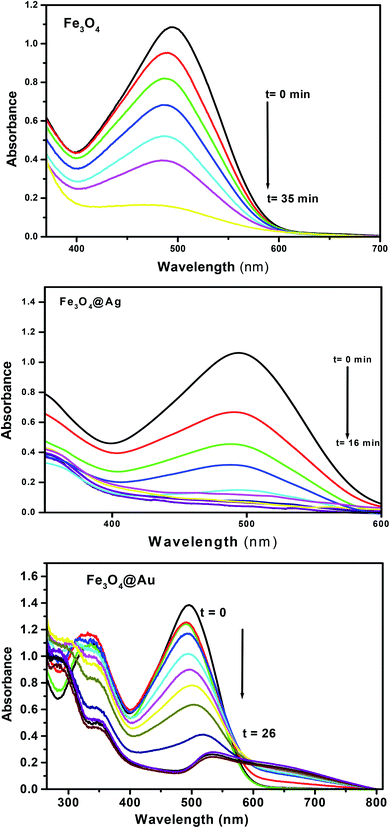 | ||
| Fig. 7 Absorption spectra of Congo red under the optimum conditions, [CR] = 5 × 10−5 M, [NaBH4] = 2.5 × 10−2 M, and the amount of nanocatalysts = 20 mg, recorded at different time intervals. | ||
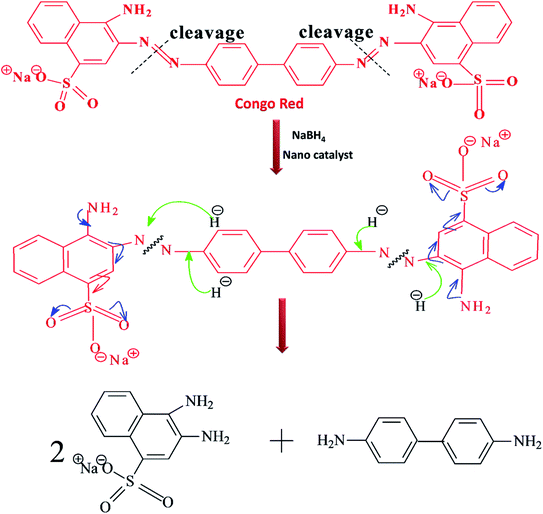 | ||
| Scheme 4 Proposed mechanism for the reduction of Congo red by NaBH4 catalyzed by the synthesized nanocatalyst. | ||
An interesting phenomenon occurred when tracking the degradation of the CR using Fe3O4@Au, where a bathochromic shift of the absorption band of CR dye by 36 nm was observed on increasing the reaction time, with the formation of an isopiestic point at 593 nm. This was assigned to the liberation of Au NPs in the solution, which has a high plasmonic band and leads to a noticeable overlap in the absorption spectra with increasing time. This observation confirms the higher value of saturation magnetization of Fe3O4@Au, as represented in Fig. 5(c).
The efficiencies of removal of CR were found to be 84.2, 96.2, and 91.2% within 35, 16, and 26 min in the presence of Fe3O4, Fe3O4@Ag and Fe3O4@Au NPs, respectively. This clearly shows the higher efficiency of Fe3O4@Ag core–shell NPs over those of Fe3O4 and Fe3O4@Au NPs.
The kinetics of the catalytic reaction were adapted to the pseudo-first-order reaction model, which was used to determine the catalytic rate constant, as expressed by the following equation;
| ln(A0/At) = kt |
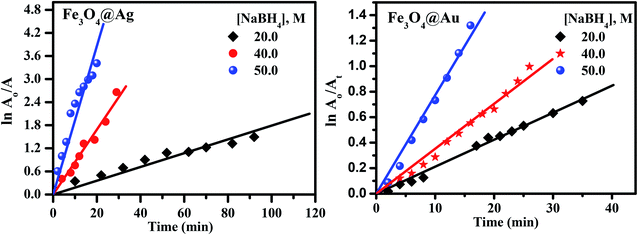
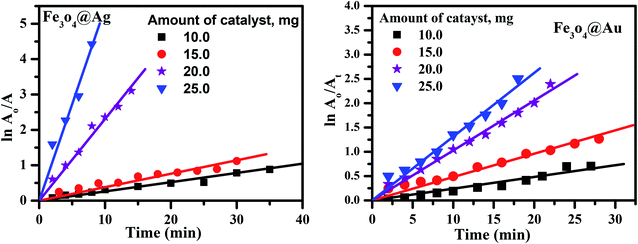
Examining the obtained results, it was found that the core–shells, Fe3O4@Ag and Fe3O4@Au were the most efficient nanocatalysts for the degradation of CR dye over Fe3O4 nanoparticles. So the reaction conditions, such as the initial concentration of the dye, the concentration of NaBH4, and the amount of catalyst have been studied considering the two nanocatalysts: Fe3O4@Ag and Fe3O4@Au core–shells.
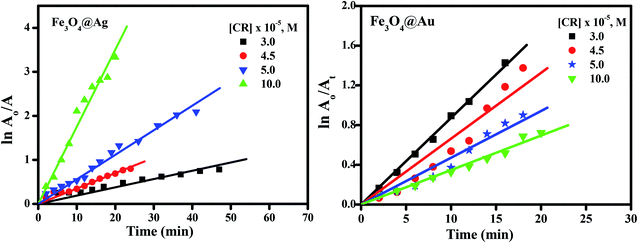
It is clear from Fig. 9(a) that the reaction rate increases with an increase in the dye concentration in the presence of Fe3O4@Ag as a nanocatalyst, reaching a maximum value of 0.17 min−1 at a dye concentration of 1 × 10−4 M. The increase in rate is due to an enhancement of the electron transfer process on the surface of the nanocatalyst, by the generation of electrons as a result of oxidation of Ag0 to Ag+ ions, in addition to the electrons of the NaBH4 which leads to an enrichment of the electrons on the surface of the nanocatalysts, and an enhancement in the degradation of CR dye.
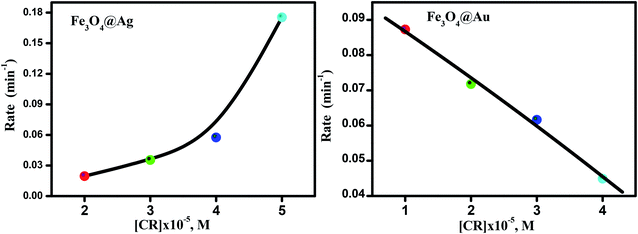 | ||
| Fig. 9 The effect of dye concentrations on reaction rate, [NaBH4] = 2.5 × 10−2 M, and the amount of catalyst = 20 mg. | ||
However, in the presence of Fe3O4@Au as a nanocatalyst, the reaction rate decreases with an increase in the dye concentration, reaching a minimum value of about 0.044 min−1 at a dye concentration of 1 × 10−4 M (Fig. 9(b)). The decrease in the rate of catalytic reaction upon increasing the concentration of CR dye may be due to competition between the reaction products or the generated intermediates and the free dye molecules which attach to the catalyst surface. Such interference of reaction products or intermediates will render the catalyst surface poisoned and consequently the dye degradation will ultimately be retarded. A further explanation of this phenomenon is that, when the initial concentration of the dye is increased, more and more dye molecules are adsorbed on the surface of the nanocatalysts. The existence of large amounts of adsorbed dye resulted in the lack of any direct contact with the active sites, thus causing an inhibition effect on the dye degradation.44
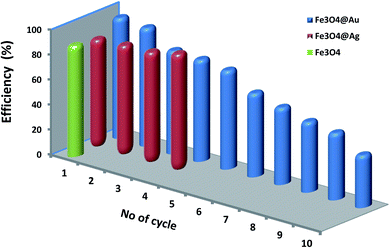 | ||
| Fig. 12 The relation between catalytic efficiency of Fe3O4, Fe3O4@Ag, and Fe3O4@Au nanocatalysts and the number of cycles of CR dye reduction. | ||
A quick review of the literature relevant to the degradation of CR by different methods is summarized in Table 2. This reveals that degradation of CR dye is carried out using Fe3O4 based nanocomposites in different systems, such as catalytic reduction, photo-degradation and adsorption using Fe3O4@Ag hydrothermally synthesized in the present study. As shown from this review, an excellent catalytic degradation for CR dye with fantastic recyclability reaching 10 cycles without any considerable loss of the catalyst efficiency was achieved using our catalyst.
| Catalyst | Method | Reaction time (min) | Efficiency% | Weight | No. of cycles | Reference |
|---|---|---|---|---|---|---|
| Fe3O4@PANI@Au | Reduction NaBH4 | 20 | 100 | 10 mg | 5 | 46 |
| Fe3O4/MgAl-LDH | Adsorption | 30 | 100 | 80 mg | — | 47 |
| Fe3O4–PbS | UV-photo degradation | 120 | 80 | — | — | 48 |
| Fe3O4@BTCA | Adsorption | 20 | 97 | 20 mg | 5 | 49 |
| 1,2,4,5-Benzenetetracarboxylic acid | ||||||
| BTCA | ||||||
| Fe3O4–Ag nanoalloys | Adsorption | 4 | 92 | 30 μg | — | 46 |
| Fe3O4@Ag (present work) | Reduction NaBH4 | 16 | 96 | 20 mg | 10 | — |
4. Conclusion
Herein, highly efficient and reusable nanocatalysts, Fe3O4@Ag and Fe3O4@Au, have been synthesized by utilizing a facile and economic synthesis using a hydrothermal method which showed excellent CR dye reduction ability in the presence of NaBH4 as a reducing agent via an electron-relaying process. The reduction followed pseudo-first-order kinetics and the rate of the reaction depends greatly on the concentration of the dye, NaBH4 and the nanocatalyst. The excellent catalytic activity and good reusability suggest that the Fe3O4@Ag, and Fe3O4@Au nanocatalysts could be used as potential candidates for the treatment of industrially discharged wastewater.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the Scientific Research Fund at Tanta University, Egypt for funding this work through research project code TU:02-19-03.References
- B. J. Kim, T. Park, H. C. Moon, S.-Y. Park, D. Hong, E. H. Ko, J. Y. Kim, J. W. Hong, P. S. Woo, Y.-G. Kim and I. S. Choi, Cytoprotective Alginate/Polydopamine Core/Shell Microcapsules in Microbial Encapsulation, Angew. Chem., Int. Ed., 2014, 53, 14443–14446, DOI:10.1002/anie.201408454.
- T. Ozel, G. R. Bourret, A. L. Schmucker, K. A. Brown and C. A. Mirkin, Hybrid semiconductor core-shell nanowires with tunable plasmonic nanoantennas, Adv. Mater., 2013, 25, 4515–4520, DOI:10.1002/adma.201301367.
- Z. Shan, D. Clayton, S. Pan, P. S. Archana and A. Gupta, Visible Light Driven Photoelectrochemical Properties of Ti@TiO2 Nanowire Electrodes Sensitized with Core–Shell Ag@Ag2S Nanoparticles, J. Phys. Chem. B, 2014, 118, 14037–14046, DOI:10.1021/jp504346k.
- C. R. Ghosh and S. Paria, Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications, Chem. Rev., 2012, 112, 2373–2433, DOI:10.1021/cr100449n.
- L. Yang, W. Luo and G. Cheng, Graphene-Supported Ag-Based Core–Shell Nanoparticles for Hydrogen Generation in Hydrolysis of Ammonia Borane and Methylamine Borane, ACS Appl. Mater. Interfaces, 2013, 5, 8231–8240, DOI:10.1021/am402373p.
- M. B. Gawande, A. Goswami, T. Asefa, H. Guo, A. V. Biradar, D.-L. Peng, R. Zboril and R. S. Varma, Core-shell nanoparticles: synthesis and applications in catalysis and electrocatalysis, Chem. Soc. Rev., 2015, 44, 7540–7590, 10.1039/c5cs00343a.
- R. Liu and R. D. Priestley, Rational design and fabrication of core–shell nanoparticles through a one-step/pot strategy, J. Mater. Chem. A, 2016, 4, 6680–6692, 10.1039/C5TA09607C.
- A. K. Gupta and M. Guptab, Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications, Biomaterials, 2005, 26, 3995–4021, DOI:10.1016/j.biomaterials.2004.10.012.
- W. Fang, J. Zheng, C. Chen, H. Zhang, Y. Lu, L. Mac and G. Chen, One-pot synthesis of porous Fe3O4 shell/silver core nanocomposites used as recyclable magnetic antibac-terial agents, J. Magn. Magn. Mater., 2014, 357, 1–6, DOI:10.1016/j.jmmm.2014.01.024.
- S. E. Lyshevski, Dekker encyclopedia of nanoscience and nanotechnology, CRC Press, Boca Raton, 3rd edn, 2014, p. 7 Search PubMed.
- L. R. Harivardhan, J. L. Arias, J. Nicolas and P. Couvreur, Magnetic Nanoparticles: Design andCharacterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications., Chem. Rev., 2012, 112, 5818–5878, DOI:10.1021/cr300068p.
- K. Ulbrich, K. Hol, V. Subr, A. Bakandritsos, J. Tucek and R. Zboril, Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications, Chem. Rev., 2016, 116, 5338–5431, DOI:10.1021/acs.chemrev.5b00589.
- S. Laurent, L. Vander Elst and R. N. Muller, Superparamagnetic iron oxide nanoparticles for MRI, The chemistry of contrast agents in medical magnetic resonance imaging, 2013, vol. 10, pp. 427–447 Search PubMed.
- S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. E. Vander and R. N. Muller, Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications, Chem. Rev., 2008, 108, 2064–2110, DOI:10.1021/cr068445e.
- H. Shokrollahi, Structure, synthetic methods, magnetic properties and biomedical applications of ferrofluids, Mater. Sci. Eng., C, 2013, 33, 2476–2487, DOI:10.1016/j.msec.2013.03.028.
- J.-M. Li, A. C. H. Huan, L. Wang, Y.-W. Du and D. Feng, Interface effects on magnetoresistance and magnetic-field-reduced Raman scattering in magnetite, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 6876, DOI:10.1103/PhysRevB.61.6876.
- R. R. Castillo and M. Vallet-Regí, Functional Mesoporous Silica Nanocomposites: Biomedical Applications and Biosafety, Int. J. Mol. Sci., 2019, 20, 929, DOI:10.3390/ijms20040929.
- J.-M. Li, X.-L. Zeng and Z.-A. Xu, Partial cationic inversion-induced magnetic hardening of densely packed 23-nm-sized nanocrystallite-interacting nickel ferrite electrospun nanowires, Appl. Phys. Lett., 2013, 103, 232410, DOI:10.1063/1.4840320.
- C. F. Bohren and D. R. Huffman, Absorption and scattering of light by small particles, Wiley-VCH, 2008 Search PubMed.
- M. Rai, A. P. Ingle, S. Birla, A. Yadav and C. A. Dos Santos, Strategic role of selected noble metal nanoparticles in medicine, Crit. Rev. Microbiol., 2016, 42, 696–719, DOI:10.3109/1040841x.2015.1018131.
- P. Gong, H. Li, X. He, K. Wang, J. Hu, W. Tan, S. Zhang and X. Yang, Nanotechnology Preparation and antibacterial activity of Fe3O4@Ag nanoparticles, Nanotechnology, 2007, 18, 285604, DOI:10.1088/0957-4484/18/28/285604.
- B. Chudasama, A. K. Vala, N. Andhariya, R. V. Upadhyay and R. V. Mehta, Enhanced antibacterial activity of bifunctional Fe3O4-Ag core-shell nanostructures, Nano Res., 2009, 2, 955–965, DOI:10.1007/s12274-009-9098-4.
- A. Amarjargal, L. D. Tijing, I. T. Im and C. S. Kim, Simultaneous preparation of Ag/Fe3O4 core–shell nanocomposites with enhanced magnetic moment and strong antibacterial and catalytic properties, Chem. Eng. J., 2013, 226, 243–254, DOI:10.1016/j.cej.2013.04.054.
- W. Q. Jiang, Y. F. Zhou, Y. L. Zhang, S. H. Xuan and X. L. Gong, Superparamagnetic Ag@Fe3O4core-shell nanospheres: fabrication, characterization and application as reusable nanocatalysts, Dalton Trans., 2012, 41, 4594–4601, 10.1039/c2dt12307j.
- J. Park, K. An, Y. Hwang, J.-G. Park, H.-J. Noh, J.-Y. Kim, J.-H. Park, N.-M. Hwang and T. Hyeon, Ultra-large-scale syntheses of monodisperse nanocrystals, Nat. Mater., 2004, 3, 891–895, DOI:10.1038/nmat1251.
- J. S. Salazar, L. Perez, O. D. Abril, L. P. Truong, D. Ihiawakrim, M. Vazquez, G. Jean-Marc, S. Begin-Colin and G. Pourroy, Magnetic Iron Oxide Nanoparticles in 10−40 nm Range: Composition in Terms of Magnetite/Maghemite Ratio and Effect on the Magnetic Properties, Chem. Mater., 2011, 23, 1379–1386, DOI:10.1021/cm103188a.
- S. Ge, X. Shi, K. Sun, C. Li, C. Uher, J. R. Baker, M. M. BanaszakHoll and B. G. Orr, Facile hydrothermal synthesis of iron oxide nanoparticles with tunable magnetic properties, J. Phys. Chem. C, 2009, 113, 13593–13599, DOI:10.1021/jp902953t.
- H. Cai, X. An, C. Jun, J. Li, S. Wen, K. Li, M. Shen, L. Zheng, G. Zhang and X. Shi, Facile hydrothermal synthesis and surface functionalization of polyethyleneimine-coated iron oxide nanoparticles for biomedical applications., ACS Appl. Mater. Interfaces, 2013, 5, 1722–1731, DOI:10.1021/am302883m.
- Y. B. Khollam, S. R. Dhage, H. S. Potdar, S. B. Deshpande, P. P. Bakare, S. D. Kulkarni and S. K. Date, Microwave hydrothermal preparation of submicron-sized spherical magnetite (Fe3O4) powders, Mater. Lett., 2002, 56, 571–577, DOI:10.1016/S0167-577X(02)00554-2.
- J. Li, X. Shi and M. Shen, Hydrothermal Synthesis and Functionalization of Iron Oxide Nanoparticles for MR Imaging Applications, Part. Part. Syst. Charact., 2014, 31, 1223–1237, DOI:10.1002/ppsc.201400087.
- S. A. M. Khawja Ansari, F. Eleonora, R. F. Alessandro, S. Ilaria, A. Monica, A. Ornella, C. Roberta, G. Caterina and D. Federico, Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Functionalization for Biomedical Applications in the Central Nervous System, Materials, 2019, 12, 465, DOI:10.3390/ma12030465.
- Á. D. Jesús Ruz-Baltazar, Green synthesis assisted by sonochemical activation of Fe3O-Ag nano-alloys: Structural characterization and studies of sorption of cationic dyes, Inorg. Chem. Commun., 2020, 120, 108148, DOI:10.1016/j.inoche.2020.108148.
- N. Mizutani, T. Iwasaki and S. Watano, Response Surface Methodology Study on Magnetite Nanoparticle Formation under Hydrothermal Conditions, Nanomater. Nanotechnol., 2015, 5, 1–7, DOI:10.5772/60649.
- W. Wu, Z. Wu, T. Yu, C. Jiang and W. S. Kim, Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications, Sci. Technol. Adv. Mater., 2015, 16, 023501, DOI:10.1088/1468-6996/16/2/023501.
- G. Sharma and P. Jeevanandam, Synthesis of self-assembled prismatic iron oxide nanoparticles by a novel thermal decomposition route, RSC Adv., 2013, 3, 189, 10.1039/C2RA22004K.
- Y. Zhu, Y. Qian, X. Li and M. Zhang, A nonaqueous solution route to synthesis of polyacrylamide-silver nanocomposites at room temperature, Nanostruct. Mater., 1998, 10, 673–678, DOI:10.1016/S0965-9773(98)00096-8.
- Y. Peng, A. W. Xu, B. Deng, M. Antonietti and H. Colfen, Polymer-controlled crystallization of zinc oxide hexagonal nanorings and disks, J. Phys. Chem. B, 2006, 110, 2988–2993, DOI:10.1021/jp056246d.
- G. Z. Wang, R. Saeterli, P. M. Rorvik, A. T. J. van Helvoort, R. Holmestad, T. Grande and M. A. Einarsrud, Self-Assembled Growth of PbTiO3 Nanoparticles into Microspheres and Bur-like Structures, Chem. Mater., 2007, 19, 2213, DOI:10.1021/cm063047d.
- M. Yamaura and D. A. Fungaro, Synthesis and characterization of magnetic adsorbent preparedby magnetite nanoparticles and zeolite from coal fly ash, J. Mater. Sci., 2013, 48, 5093–5101, DOI:10.1007/s10853-013-7297-6.
- S.-J. Cho, J.-C. Idrobo, J. Olamit, K. Liu, N. D. Browning and S. M. Kauzlarich, Growth Mechanisms and Oxidation-Resistance of Gold-Coated Iron Nanoparticles, Chem. Mater., 2005, 17, 3181–3186, DOI:10.1021/cm0500713.
- Q. Li, C. W. Kartikowati, S. Horie, T. Ogi, T. Iwaki and K. Okuyama, Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles, Sci. Rep., 2017, 7, 9894, DOI:10.1038/s41598-017-09897-5.
- R. F. Butler and S. K. Banerjee, Theoretical single-domain grain-size range in magnetite and titanomagnetite, J. Geophys. Res., 1975, 80, 4049–4058, DOI:10.1029/JB080i029p04049.
- D. L. Leslie-Pelecky and R. D. Rieke, Magnetic Properties of Nanostructured Materials, Chem. Mater., 1996, 8, 1770–1783, DOI:10.1021/cm960077f.
- N. Shimizu, C. Ogino, M. F. Dadjour and T. Murata, Sonocatalytic degradation of methylene blue with TiO2 pellets in water, Ultrason. Sonochem., 2007, 14, 184–190, DOI:10.1016/j.ultsonch.2006.04.002.
- S. Gupta, C. Giordano, M. Gradzielski and S. K. Mehta, Microwave-assisted synthesis of small Ru nanoparticles and their role in degradation of congo red, J. Colloid Interface Sci., 2013, 411, 173–181, DOI:10.1016/j.jcis.2013.08.030.
- M. Chen, P. Liu, C. Wang, W. Ren and G. W. Diao, Fast catalytic reduction of an azo dye by recoverable and reusable Fe3O4@PANI@Au magnetic composites, New J. Chem., 2014, 38, 4566–4573, 10.1039/C4NJ00806E.
- R. Shan, L. Yan, K. Yang, S. Yu, Y. Hao, H. Yu and B. Du, Magnetic Fe3O4/MgAl-LDH composite for effective removal of three red dyes from aqueous solution, Chem. Eng. J., 2014, 15, 38–46, DOI:10.1016/j.cej.2014.04.105.
- K. Hedayati, M. Goodarzi and M. Kord, Green and facile synthesis of Fe3O4-PbS magnetic nanocomposites applicable for the degradation of toxic organic dyes, Main Group Met. Chem., 2016, 39, 183–194, DOI:10.1515/mgmc-2016-0027.
- S. Chatterjee, N. Guha, S. Krishnan, A. K. Singh, P. Mathur and D. K. Rai, Selective and Recyclable Congo Red Dye Adsorption by Spherical Fe3O4 Nanoparticles Functionalized with 1,2,4,5-Benzenetetracarboxylic Acid, Sci. Rep., 2020, 10, 111, DOI:10.1038/s41598-019-57017-2.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08230a |
| This journal is © The Royal Society of Chemistry 2021 |