Open Access Article
Open Access ArticleOrganic dots (O-dots) for theranostic applications: preparation and surface engineering
Amin Shiralizadeh Dezfuli *ah,
Elmira Kohanb,
Sepand Tehrani Fatehc,
Neda Alimirzaeid,
Hamidreza Arzaghi
*ah,
Elmira Kohanb,
Sepand Tehrani Fatehc,
Neda Alimirzaeid,
Hamidreza Arzaghi e and
Michael R. Hamblin
e and
Michael R. Hamblin fg
fg
aPhysiology Research Center, Iran University of Medical Sciences, Tehran, Iran. E-mail: amindezfuli@outlook.com
bDepartment of Science, University of Kurdistan, Kurdistan, Sanandaj, Iran
cSchool of Medicine, Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran
dInstitute of Nanoscience and Nanotechnology, University of Kashan, Kashan, Iran
eDepartment of Medical Biotechnology, Faculty of Allied Medical Sciences, Iran University of Medical Sciences (IUMS), Tehran, Iran
fWellman Center for Photomedicine, Massachusetts General Hospital, Harvard Medical School Boston, MA 02114, USA
gLaser Research Centre, Faculty of Health Science, University of Johannesburg, Doornfontein 2028, South Africa
hRonash Technology Pars Company, Tehran, Iran
First published on 11th January 2021
Abstract
Organic dots is a term used to represent materials including graphene quantum dots and carbon quantum dots because they rely on the presence of other atoms (O, H, and N) for their photoluminescence or fluorescence properties. They generally have a small size (as low as 2.5 nm), and show good photostability under prolonged irradiation. The excitation and emission wavelengths of O-dots can be tailored according to their synthetic procedure, where although their quantum yield is quite low compared with organic dyes, this is partly compensated by their large absorption coefficients. A wide range of strategies have been used to modify the surface of O-dots for passivation, improving their solubility and biocompatibility, and allowing the attachment of targeting moieties and therapeutic cargos. Hybrid nanostructures based on O-dots have been used for theranostic applications, particularly for cancer imaging and therapy. This review covers the synthesis, physics, chemistry, and characterization of O-dots. Their applications cover the prevention of protein fibril formation, and both controlled and targeted drug and gene delivery. Multifunctional therapeutic and imaging platforms have been reported, which combine four or more separate modalities, frequently including photothermal or photodynamic therapy and imaging and drug release.
1. Introduction
The complexity of physiopathology of diseases1–3 and the importance of their early and accurate diagnosis4–7 make new approaches to overcome these human health issues urgent. Currently, there is a trend toward not only designing multifunctional treatment systems for targeting diseases from various aspects, but also performing the diagnosis with the same system or track the treatment in real time, which is known as theranostics.8–12 Moreover, researchers are looking for safer and more accurate therapies to reduce the side effects and increase the therapeutic outcome of treatments.13 Due to various parameters in biological systems, which should be considered in drug/treatment design for suitable function, producing a new drug/treatment requires significant effort. Accordingly, a system as multifunctional as possible for controlling or considering a set of parameters is necessary.14From a diagnostic point of view, the system should be capable of not only detecting the intended disease with high sensitivity, but also should distinguish and differentiate the underlying disease from other possible diseases with similar characteristics and lab test results, which is known as specificity.15
Cancer and neurological diseases are some examples of complex diseases that require specific and sensitive diagnostic methods as the first step, and efficient and accurate treatment in the next step.2,16 Accordingly, theranostics can be a possible solution for these types of problems. For designing an efficient theranostic system, researchers have to collect enough knowledge about both physiopathological processes of the diseases and material sciences.
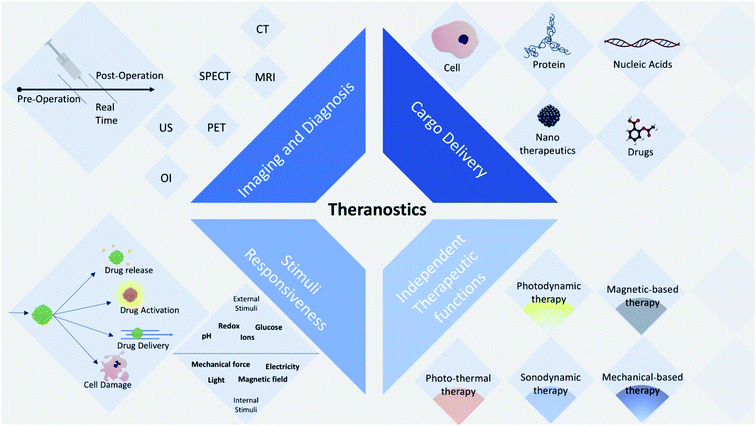
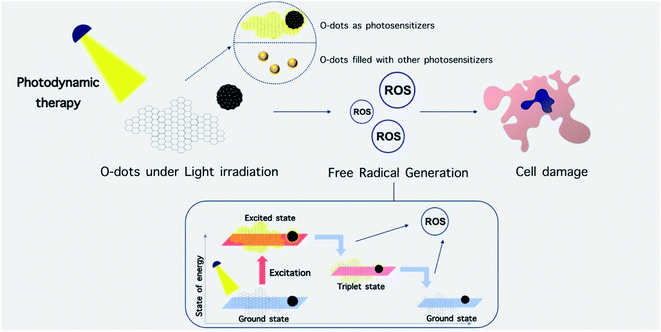
Theranostics is defined as a combination of imaging and treatment modalities within a single molecular targeted platform, which can be applied in the next generation of personalized medicine.17 In the diagnostic function, the role of a theranostic agent is to report the presence of a disease, its location and its extent, and to allow the monitoring of the response to the therapeutic agent.18 For instance, one application is to allow more accurate tumor resection via image-guided surgery, and to allow post-surgical appraisal of the success of removal. To improve the accuracy of surgery, intraoperative imaging of diseased tissue is helpful due to the fact that the extent of the tumor may have changed after the initial pre-surgical imaging and during the course of the resection.19–22 Also, to confirm the complete removal of diseased tissue, post-surgical imaging is also useful. The second role of theranostics is to deliver or release therapeutic agents precisely to the targeted location. The agents that are delivered can be chemotherapeutic drugs (including, cisplatin, doxorubicin, and paclitaxel), biologics (such as proteins and antibodies), nucleic acids for gene therapy (DNA, siRNA, and miRNA), nanotherapeutic agents, and even therapeutic cells.23–25 These agents can be fabricated to be responsive to certain stimuli. The generation of a reactive form of molecular oxygen (singlet oxygen, 1O2) with the capability to destroy the surrounding cells, which is triggered by light, is known as photodynamic therapy (PDT). In contrast, photothermal therapy is based on the absorption of photons by a chromophore, which creates heat from optical energy to kill cancer cells.26,27 The third role is the molecular alteration of a cellular or metabolic process.18 If particular cell surface receptors are engaged by theranostic agents, metabolic or cellular pathways may be disrupted, which can produce a therapeutic effect.28
Theranostic approaches combine multiple techniques into a single inclusive nanoplatform, often incorporating molecular imaging function.29 Molecular imaging can be based on optical imaging (fluorescence/bioluminescence/Raman), computed tomography (CT), magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT), ultrasound (US), and positron emission tomography (PET).30–32 This technique can be applied for surveying a wide range of biological samples, from cells and ex vivo tissue samples, to in vivo imaging of living organisms and small animals. Moreover, it is capable of covering a broad range of sizes of organisms, from sub micrometer-sized viruses and bacteria, to macroscopic living biological organisms33–35 (Fig. 1).
In theranostic approaches, nanomaterials are often used for drug delivery and cancer imaging due to the fact that they allow the synergistic combination of diagnosis and therapy in a single nanoplatform.36,37
Among the various groups of nanomaterials, carbon nanomaterials have strong absorption in the infrared (IR) and near infrared (NIR) region, and thus can be used for photothermal therapy (PTT) of cancer. Some organic nanomaterials such as carbon nanotubes and quantum dots show fluorescence in the visible and infrared regions for fluorescence imaging.32,38 Additionally, other carbon nanomaterials can convert the energy from a laser into acoustic signals, which makes them promising agents for photoacoustic imaging (PAI).39,40 Lastly, the inherent Raman vibration signals from carbon nanomaterials can also offer a method to track their distribution and metabolism in vivo.41,42
Among the carbon nanomaterials, carbon-based quantum dots (CBQDs) including graphene quantum dots (GQDs) and carbon quantum dots (CQDs) show beneficial properties of low toxicity, environmentally friendly nature, simple and cost effective synthetic routes, and comparable optical properties to conventional semiconductor quantum dots and organic dyes.43–45 Photoluminescent CBQDs are superior to other quantum dots in terms of solubility, biocompatibility, resistance to photobleaching, chemical inertness, and suitability for biological applications (one-photon and multiphoton bioimaging,46,47 biosensors46,48,49 and biomolecule or drug delivery48,50,51).
2. Definition of organic dots (O-dots)
In the last few years, CBQDs have been utilized in different biomedical applications, and many reports have discussed their synthesis, functionalization, combinations and applications.52–59 Although graphene quantum dots and carbon quantum dots have attracted significant attention, many researchers are still confused about the differences between these two sub-groups, and thus their correct and appropriate use in studies is challenging. GQDs are defined as graphene sheets with a size in the range of about 3–20 nm, which possess photoluminescence properties due to their physiochemical characteristics mostly because of their size,60 while CQDs are photoluminescence spherical nanoparticles.61 CQDs are also referred to as carbon dots in some studies. However, there are some significant chemical, physical and optical differences between GQDs and CQDs, which will be discussed below.As conventional in chemistry and biology, some materials can be tagged as organic materials, meaning that they are made of carbon, hydrogen and oxygen as the basic structure of the material, while they can be natural or synthetic.62 Accordingly, since GQDs and CQDs are materials with this composition of elements, we state that they can be categorized as organic materials and we intend to name them as “organic dots (O-dots)” or luminescent organic clusters (LOC). This categorization may cause some implications including attracting attention to their chemical and biological properties, expansion of their biomedical applications and facilitating the design and fabrication of other types of quantum dot fluorescence materials. Moreover, we hypothesize that due to the emergence of synthetic biology, the biological synthesis of these materials can be expected (for example via enzymes), and thus this designation may provide new ways and approaches toward it. Herein, different aspects of O-dots are discussed.
A semiconductor quantum dot is a single electronic oscillator, whereas an O-dot is a clustered pack of isolated oscillators (phosphors).63 Depending on the reaction conditions (temperature, duration of the procedure, and the ratio of precursors), two types of O-dots can be produced. The first type is obtained under mild conditions, e.g. at a moderate temperature. Here, each dot is comprised of only one type of phosphor, assembled into a particle mainly due to weak (physical) forces. Upon further heating, type-I dots can be converted into type-II dots; however, this step is not reversible. Type II dots are obtained through deep carbonization of pristine organic substances, and are more like elemental carbon. In this structure, different oscillators are linked together via strong bonds (for instance, σ-bonds between carbon atoms). The absorption spectra of type-II O-dots do not show discrete bands because they consist of multiple independent oscillators (in contrast to type-I O-dots). Another distinguishing characteristic of type II O-dots is that their emission wavelength depends on the excitation wavelength. However in type-I O-dots, the excitation wavelength just affects the intensity of the luminescence, and not the wavelength (color) of the emitted light (Fig. 2).62
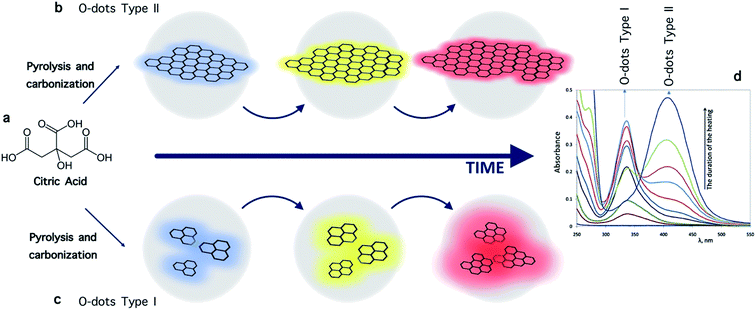 | ||
| Fig. 2 (a) Formation of type I and type II carbon dots starting from citric acid as the carbon precursor. The intermediate “primary fluorophores” is a nominal unit.62 (b and c) Schematic illustration of the luminescence of O-dots upon excitation, which is related to their size.62 (d) Typical absorption spectra of O-dots.62 | ||
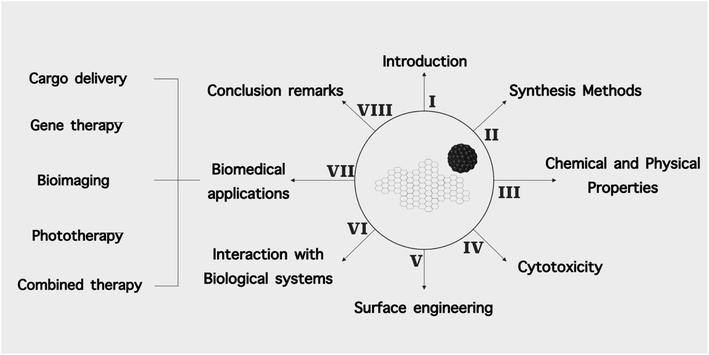
This review aims to summarize the recent advancements in the design and applications of O-dots in theranostics including bio-imaging, drug delivery, gene delivery and phototherapy. The organization and scope of this review are shown in Fig. 3.
3. Chemical and physical properties of organic dots
3.1. Chemical properties
The properties of O-dots including CQDs and GQDs depend mainly on their synthetic routes. Accordingly, the chemical structures of O-dots show large variability and chemical characterization is important to gain a better understanding of particular O-dots (Fig. 4).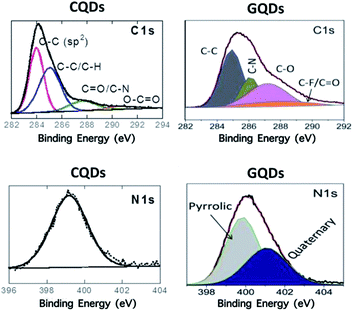 | ||
| Fig. 4 Characteristics of organic dots. XPS bonds of CQDs (hydrothermal synthesis using 4-aminophenylboronic acid)66 (reproduced from ref. 66 with permission from the American Chemical Society, Copyright 2016). | ||
For example, GQDs, are composed of a single or multiple graphene layers with chemical groups attached to the edges, and they are commonly anisotropic, with lateral dimensions much larger than their thickness. GQDs are synthesized from pristine few-layer-thick graphene flakes as a precursor.64 They have a narrow size distribution of 3–8 nm and small sheet-shaped morphology, as shown by transmission electron microscopy (TEM) imaging. Due to the presence of a carbon core, GQDs have a crystalline structure with a lattice spacing of 0.24 nm comparable to the (100) facet of graphite and a honeycomb lattice with zigzag edges of 7 nm GQDs. This is in contrast to the spherical CQDs that have been formed using ribonuclease A as a bimolecular templating agent under microwave irradiation, which have an interlayer distance of 0.34 nm, matching the (002) facet of graphite.65
X-ray diffraction (XRD) provides exact information about the crystalline structure of O-dots. The strong signal peak at 2θ = 26.51 shown in the XRD pattern of graphene flakes becomes weaker in GQDs, showing that GQDs are thinner than graphene flakes owing to the additional exfoliation of few-layered graphene. The broad peak positioned at 2θ ∼ 21.91 (d = 0.41 nm) may result from the p–p stacking of GQDs. No peaks are observed in the 2θ region of 5–20°, demonstrating that the GQDs are different from GO with fewer oxygen-containing groups.65 The XRD diffractogram of CQDs formed from 4-aminophenylbenzene displays a broad diffraction peak at around 21.73° with an interlayer spacing of 0.42 nm, which is larger than that of bulk graphite, indicating the poor crystallization and amorphous character of the structures.66
Using standard characterization methods such as X-ray photoelectron spectroscopy (XPS), Fourier transform infrared (FTIR) spectroscopy, and Raman spectroscopy, the different surface functionalities on O-dots can be characterized. The high-resolution C 1s XPS spectrum of CQDs prepared hydrothermally from 4-aminophenylboronic acid66 showed bands corresponding to C–C (283.9 eV), C–C/C–H (285.0 eV), CQO/C–N (287.3 eV) and O–CQO (290.3 eV). The band at 399.6 eV in the N 1s high-resolution XPS spectrum indicates the presence of amine groups. The C 1s XPS spectrum of GDQs formed by cutting pristine graphene flakes using an electrochemical redox reaction in an ionic liquid/water electrolyte under a reverse potential65 showed peaks related to C–C/C–H (285.0 eV), C–O (286.1 eV, such as C–OH or C–O–C), C–N (287.2 eV), and O–CQO (288.5 eV) (Fig. 4a). The N 1s band indicates the presence of two types of N atoms. The peak at 400.2 eV can be assigned to the N atom of a pyrrolic structure (imidazolium group) adsorbed or located at the graphitic edge. Quaternary N (401.2 eV) atoms may be incorporated into the graphene layer and take the place of carbon atoms within the graphene plane.65
The chemical composition of O-dots can be obtained from Fourier-transform infrared spectroscopy (FTIR). The three strong bands at around 3425, 1720 and 1645 cm−1 shown in the FTIR spectrum of the GQDs synthesized by an electrochemical method are associated with the vibrations of the hydroxyl (–OH), carbonyl (CQO) and graphitic (CQC) groups, respectively. The band at 1078 cm−1 is related to the alkoxy groups (O–C–O) present in the GQDs. The spectrum reveals that GQDs have many oxygenated functional groups on their surface. Their FTIR spectrum is also significantly different from that of the precursor graphene flakes, with a weak adsorption band at around 3425 cm−1. The FTIR spectrum of CQDs depicts similar features of distinct strong bands at 3465 cm−1 (OH vibration) and 1618 cm−1 (CQO) with additional weak bands attributed to graphitic CQC (1645 cm−1), O–C–O (1078 cm−1) and B–O (1090 cm−1), and stretching and deformation vibration modes of the boroxol bond of the boronic acid moieties.67 The presence of C–N is demonstrated by the peak at 1400 cm−1.
Raman analysis of these organic quantum dots displays the carbon characteristic D and G bands at around 1350 and 1570 cm−1, respectively. The intensity of the D band, which is related to the presence of sp3 defects, and the G band, related to the in-plane vibration of sp2 carbon (IG/ID), is a measure of the disorder in the nanostructure. The Raman spectrum of CQDs comprises a broad band at 2996 cm−1, corresponding to the 2D graphitic structures, with an ID/IG ratio of ≈1.24. In the case of graphene flakes used for the formation of GQDs, the weak D and strong G bands indicate the presence of slightly defective graphene flakes. The increase in the ID/IG ratio (IG/ID = 0.6) of the formed GQDs suggests the formation of even more defective materials.68
3.2. Optical properties
O-dots normally show strong optical absorption in the UV region (260–320 nm) due to the π–π* transition of their C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. The shoulder peak located at 270–390 nm is attributed to the n–π* transition of the C
C bonds. The shoulder peak located at 270–390 nm is attributed to the n–π* transition of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds.69 The location of the peak in the spectrum rather than the intensity is more affected by the preparation route.70–74 Under UV irradiation, different emission colors have been observed for GQDs, which is related to the synthetic routes employed. For example GQDs can emit bright UV,75,76 blue,73,77 green,78,79 yellow,80,81 red79 and near infra-red82 emissions. The PL emission in O-dots arises from quantum confinement effects, which can be excitation-dependent and excitation-independent PL83,84 and can be changed by altering the size of the quantum dots.68 The quantum yield (QY) is an important factor to evaluate the PL of O-dots. The QY of naked O-dots is very low, where GQDs have a higher QY compared to CQDs due to their special layered structure and crystallinity. However, despite the low QY commonly found in CQDs, some approaches have been investigated to improve their QY such as element doping, metal-enhanced fluorescence85,86 and surface passivation/modification.87
O bonds.69 The location of the peak in the spectrum rather than the intensity is more affected by the preparation route.70–74 Under UV irradiation, different emission colors have been observed for GQDs, which is related to the synthetic routes employed. For example GQDs can emit bright UV,75,76 blue,73,77 green,78,79 yellow,80,81 red79 and near infra-red82 emissions. The PL emission in O-dots arises from quantum confinement effects, which can be excitation-dependent and excitation-independent PL83,84 and can be changed by altering the size of the quantum dots.68 The quantum yield (QY) is an important factor to evaluate the PL of O-dots. The QY of naked O-dots is very low, where GQDs have a higher QY compared to CQDs due to their special layered structure and crystallinity. However, despite the low QY commonly found in CQDs, some approaches have been investigated to improve their QY such as element doping, metal-enhanced fluorescence85,86 and surface passivation/modification.87
Non-blinking PL and exceptional photostability are the main benefits of O-dots compared to conventional organic or inorganic fluorophores. Under continuous excitation with an Xe lamp, the PL intensity of O-dots is hardly changed for several hours, while the emission of organic fluorophores is changed within minutes (photobleaching).88 Moreover, in the presence of solvents or biological systems such as serum, the PL of O-dots hardly shows any changes.88 Variations in the pH value may alter the photoluminescence of O-dots, particularly for N-doped O-dots. The change is more obvious when basic/acidic sites are involved in the PL emission of the O-dots.89 When the pH value is increased, the surface charge is converted from positive to negative. This phenomenon occurs because of the ionization of amine, carboxyl and hydroxyl groups. Due to the alterations in the O-dots with a change in pH value, it is possible to create pH-responsive nanomaterials based on O-dots.90 These O-dots will be capable of being triggered via pH changes for temporal- and spatial-controlled cargo release and bio-imaging.91
Although the precise mechanism of fluorescence in O-dots is not yet completely understood, two models have been proposed to account for the PL in CQDs.91 Based on density-functional theory (DFT) and time-dependent DFT, the PL of GQDs arises from the quantum confinement of the conjugated π electrons in their sp2 carbon network, which can be governed by the size, edge configuration, shape, functional groups, heteroatom doping, and presence of defects.92 The PL process in O-dots mostly depends on their surface rather than the sp2 clusters inside their core. In fact, the recombination of electron–hole pairs creates PL in O-dots.93 This concept has been confirmed by introducing both electron donors and electron acceptors, which quench the PL of O-dots.87 Therefore, the optical properties of O-dots are governed by the interplay between the emissive sites and non-radiative trap sites on their surface, together with quantum confinement effects.94 CQDs have only a single excitation peak, which triggers the maximum emission, but GQDs regularly show two separate excitation peaks. The zigzag edges of GQDs are triplets like carbene, where they possess both σ–π and π–π* transitions, which have an energy difference of <1.5 eV between the two peaks.80 The different aspects of the properties of O-dots are illustrated in Fig. 5.
 | ||
| Fig. 5 Properties of organic dots. Both GQDs and CQDs possess various optical properties including high UV and NIR absorption, photosensitizing nature and high photo-stability. | ||
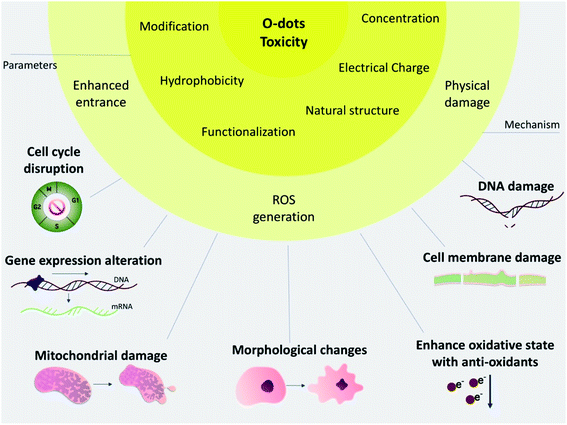
3.3. Effects of O-dots on cell viability and cytotoxicity
When nanomaterials are used to deliver exogenous imaging reporters or therapeutic molecules in living organisms, their in vivo and in vitro toxicity must be evaluated. In vitro cytotoxicity testing involves a few different biochemical and morphological indicators, including cell proliferation, apoptosis, necrosis, oxidative stress, and DNA damage. These studies can provide sufficient information about the biocompatibility of foreign substances. The evaluation of in vivo toxicity depends on how foreign molecules or materials are exploited. The physiological response may vary to a large extent. Therefore, the investigation of the fate of an externally introduced material involves absorption, distribution, interaction, metabolism, retention, and excretion in a living organism. Another difficulty in the in vivo assessment is the possible toxic effects prompted by the interactions between the nanomaterial and the living organism. To investigate this toxicity, monitoring of body weight, blood chemistry panel, and hematology profile, and histological analysis are carried out.95,96 For carbon-based quantum dots (O-dots), both their in vitro and in vivo toxicity have been widely studied, which are highly dependent on their shape, size, and surface coating96,97 (Fig. 6).Zboril and co-workers carried out an inclusive in vitro cytotoxicity study on mouse fibroblasts (NIH/3T3) using 3 types of CQDs, which were differed in their surface functionalization, providing overall negative, positive and neutral charges. The results suggested that the neutral carbon quantum dots had low toxicity and higher safety up to concentrations of 300 mg mL−1. However, the negatively charged carbon quantum dots caused morphological changes in the cells, and stimulated proliferation with higher levels of oxidative stress by interrupting the G2/M phase of the cell cycle; however, they did not enter the cell nucleus. In contrast, the positively charged CQDs showed the greatest toxicity to the cells due to the changes in the G0/G1 phase of the cell cycle, and they could also enter the cell nucleus, even at low concentrations.98
The effect of surface charge on the cytotoxicity and uptake of CQDs produced from different ratios of citrate and spermidine as starting materials in human umbilical cord-derived mesenchymal stem cells was investigated by Yan et al.99 All the nanoparticles at concentrations below 50 mg mL−1 were non-toxic. The slightly positively charged CQDs showed a higher cell uptake efficiency than the negatively charged CQDs. In vivo assessments were recently done to examine the toxicity of CQDs derived from glucose, and their surface was stabilized with ethylenediamine using zebrafish as a model.100
In another study, the toxicity of CQDs coated with hyper-branched polyglycerol (HPG) on red blood cells (RBC) was investigated (Fig. 7). Some CQDs show strong hydrophobic interaction with the RBC membrane, which can change the morphology of RBC and cause aggregation. Moreover, the rate of hemolysis with the use of these components was investigated. The cell viability was significantly lower than the control when treated with native CDs at a concentration as low as 0.5 mg mL−1. By contrast, the cell viability was not significantly different from the control when treated with CDs-g-HPG as high as 2 mg mL−1. Therefore, conjugation with HPG can inhibit the hydrophobic interaction between the CQDs and RBCs, and therefore improve the biosafety of CQDs.101
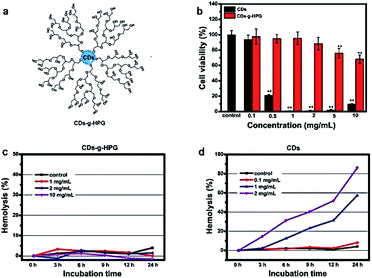 | ||
| Fig. 7 (a) Structure of carbon quantum dots coated with HPG. (b) Cell viability of A549 cells after incubation with CDs or CDs-g-HPG at different concentrations for 24 h (c) and (d) lysis rate of RBCs incubated with CD-g-HPG and CD respectively101 (figure reproduced from S. Li, Z. Guo, R. Feng, Y. Zhang, W. Xue and Z. Liu, RSC Adv., 2017, 7, 4975, Published by The Royal Society of Chemistry). | ||
In the study by Wei et al., CQDs were synthesized via a calcination method using the plant material Gynostemma as a precursor, which required no toxic reagents or surface passivation chemicals. The toxicity of different concentrations of these CQDs (up to 400 μg mL−1) was tested in zebra fish, looking at embryonic development, and the nervous and circulatory systems. Due to the excellent fluorescence stability and biocompatibility of the CQDs, bio-imaging in zebra fish was successfully achieved, showing that the CQDs could enter the zebra fish embryos by the chorion or the mouth. Moreover, the anti-oxidant effect of the CQDs was investigated both in vitro and in vivo using oxidative stress induced by H2O2. Biomarkers were measured including, the level of reactive oxygen species (ROS) content and malondialdehyde (MDA). The oxidative stress markers were lower after treatment with CQDs compared to the control groups, showing that the fluorescent CQDs could reduce the oxidative damage by controlling the generation of ROS. Furthermore, the presence of the CQDs promoted the mRNA expression of related genes, which encode antioxidant proteins to prevent oxidative damage in zebra fish. Therefore, fluorescent CQDs can be a potential candidate for the treatment of some diseases associated with oxidative damage.102
Possible toxicity to mitochondria and metabolic disturbances are other important issues that must be addressed. The results from previous studies indicated that the mitochondrial toxicity of carbon quantum dots, which ultimately leads to cell death, was very low at concentrations of up to 500 μg mL−1 over a period of 24 h, and the cell viability was higher than 82%. Nevertheless, after increasing the concentration to 1 mg mL−1 for longer than 24 h, there a cell death rate of about 40% was observed. Thus, the dose-dependent toxicity of O-dots towards mitochondria can be used to deliver drugs to cells at low doses and kill cancer cells at high doses.103
It is also important to assess the potential ability of GQDs to produce DNA damage since there is a close correlation between DNA damage and carcinogenesis. In a study reported by Wang et al., the genotoxicity of GQDs towards NIH- 3 T3 cells was investigated by flow cytometry analysis for DNA damage-related protein activation, while the GQD-induced ROS generation was studied as a potential explanation for DNA damage. The cellular uptake of GQDs and the cell death and proliferation of NIH-3T3 cells treated with GQDs were also studied to assess the cytotoxicity of GQDs.104 An analysis of GQD-mediated photodynamic cytotoxicity was performed by Markovic et al., demonstrating that photodynamic activation could induce oxidative stress and generate in vitro cytotoxicity, and subsequent activation of both apoptosis and autophagy programmed cell death pathways.105 However, studies on human breast cancer cells indicated that GQDs are non-toxic materials since they rapidly enter the cytoplasm but do not interfere with cell proliferation.
In another study using lung carcinoma A549 cells as a model, the cytotoxicity of three types of GQDs, including cGQDs (COOH-GQDs), hGQDs (OH-GQDs), and aGQDs (NH2-GQDs) was investigated. The results showed that hGQDs were the most toxic since significant cell death was induced at a concentration of 100 μg mL−1, as determined by the WST-1 assay as well as annexin-V-FITC/PI apoptosis analysis, whereas cGQDs and aGQDs were non-toxic in the tested concentration range (Fig. 8).106
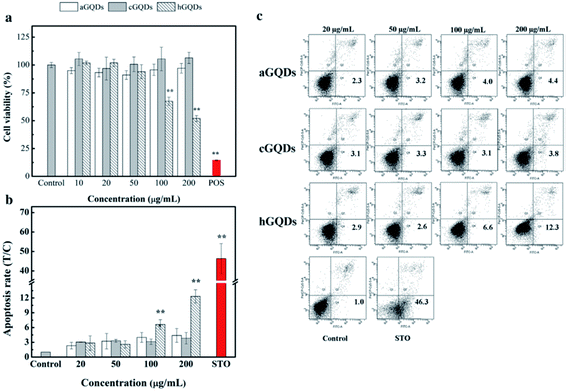 | ||
| Fig. 8 Cytotoxicity assays of GQDs. (a) Cell viability results via WST-1 assay under 24 h of exposure with GQDs at different concentrations. (b) Rate of apoptosis in the cells treated with GQDs, obtained from flow cytometry with annexin-V-FITC/PI staining. (c) Quadrant analysis of the flow (figure has been reproduced from ref. 106 with permission from Elsevier, Copyright 2019). | ||
The toxicity of GQDs to HeLa cells was tested using the CCK-8 assay, showing that the cell viability decreased with an increase in GQD concentration. More than 90% cell viability was observed at concentrations ranging from 21.5 to 50 μg mL−1 and nearly 80% cell viability was obtained at the highest concentration of 200 μg mL−1, which proves that a low concentration GQDs is biocompatible with low toxicity to HeLa cells. Furthermore, the lactate dehydrogenase (LDH) levels indicated the integrity of the cell membrane, confirming the low cytotoxicity of GQDs.107 In this work, the HeLa cells were exposed to different concentrations of GQDs for 24 h and then the LDH release was measured. The LDH release Hela cells incubated with various concentrations of GQDs for 24 h indicates that the LDH release levels were slightly higher than the control group at a low concentration of GQDs, suggesting that only a small fraction of the HeLa cell membrane was compromised by GQDs. However, compared to the control group, the LDH release level increased to about 50% with a high concentration of GQDs, suggesting that GQDs could enter the cells through endocytosis108 and cause corresponding membrane damage. The ROS assay is another effective technique to detect the oxidative stress levels in cells. Furthermore, the intracellular ROS level was low with GQDs at concentrations in the range of 0 to 50 μg mL−1, while the ROS level significantly increased at a GQD concentration of 200 μg mL−1. All these results suggest that a low concentration of GQDs displays relatively low cytotoxicity, while a high concentration (200 μg mL−1) shows more cytotoxicity.
Chong et al. reported a detailed and systematic study on the in vivo toxicity of GQDs.109 To simulate the drug administration in humans, 48 female mice were randomly divided into four groups, including a control group and GQDs functionalized with polyethylene glycol (PEG-GQDs) administered intraperitoneally (i.p.), PEG-GQDs administered intravenously (i.v.) and PEG-GO administered intraperitoneally (i.p.). There was no obvious difference between the various administration routes of PEG-GQDs, and all the mice injected with PEG-GQDs survived compared with the control group (Fig. 9e). However, 3/12 of the mice treated with PEG-GO died, while the remainder of the GO group displayed no significant loss in body weight (Fig. 9g). In addition, the weights (Fig. 9g) of the liver and spleen from the mice injected with PEG-GO were larger than that in the other groups, suggesting chronic damage to the liver and spleen caused by PEG-GO. In addition, dark spots were observed in the liver and spleen from the mice injected with PEG-GO, while nothing was found in the organs from mice injected with PEG-GQDs. The other toxicity assay results are shown in Fig. 9. All the above results showed that the PEG-GQDs possessed lower cytotoxicity than PEG-GO due to the size-effect of graphene materials.
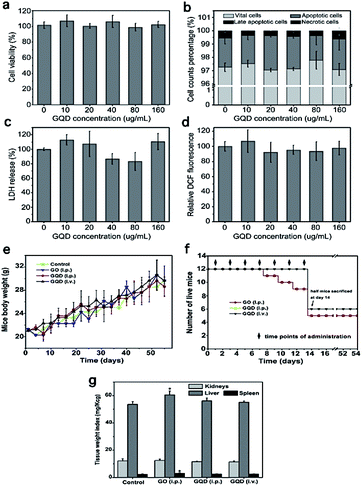 | ||
| Fig. 9 Toxicity assays of graphene quantum dots. (a) WST-1 assay, (b) cell apoptosis and necrosis results, (c) LDH assay, (d) ROS generation assay, (e) weight of surviving mice with no difference compared with the control group, (f) effect of PEG-GQD and PEG-GO injection seven times into the mice and their living status, which is lower in GO. (g) Weight indexes of the main organs collected on day 40 from the mice injected with PEG-GO and PEG-GQDs, which indicates abnormality in the PEG-GO group. All these assays together demonstrate the low toxicity of GQDs (Figure has been reproduced from ref. 109 with permission from Elsevier, Copyright 2014). | ||
In a summary, it can be said that toxicity is a state of a biological system malfunctioning after the administration of materials to the body under certain conditions. The interaction of these materials with different components of biological systems is a reason for this malfunctioning, which is related to the characteristics of materials. In the case of O-dots, their electrical charge, functional groups, modification, morphology, hydrophobicity and concentration define the quality of the interaction between them and biological systems. Carbon-based materials are active materials, and this property allows the possibility of their efficient functionalization and modification, but also allows them to take part in unwanted reactions with biological components. Thus, by manipulating these parameters, it will be possible to come up with a structure that possess a balance between maximum function and minimum toxicity. As it mentioned previously, biological systems are complex and their different parts react differently; therefore, more studies on the interaction of different levels of biological systems (organelles, cells, tissues and organs) of different types are necessary.
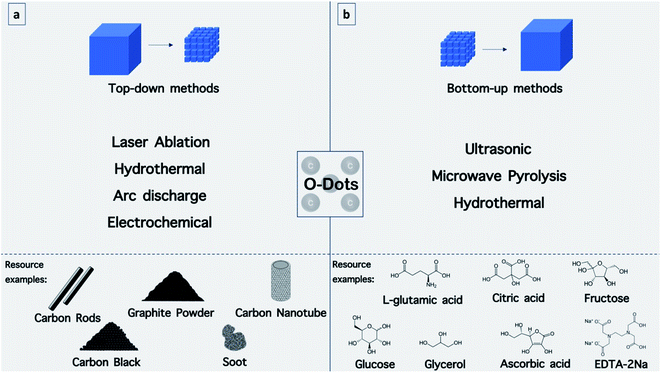
4. Synthesis of O-dots
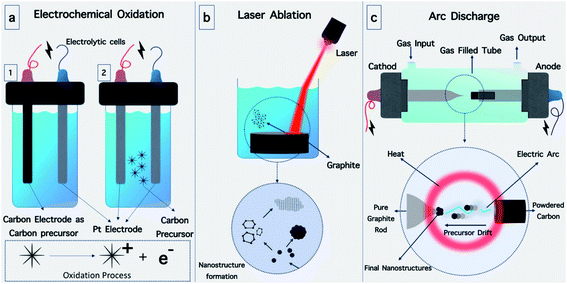
A variety of methods have been used for the preparation of O-dots (CQDs and GQDs) (Fig. 10). In this section, we summarize the different approaches for the production of O-dots. Regardless of the specific carbon nanostructure, the synthetic approaches can be categorized into two main categories, i.e. the top-down and bottom-up methods.Top-down methods are based on the progressive break down of larger carbon structures (e.g., graphite powder, carbon rods, carbon nanotubes, carbon black and even candle soot)110 by various methods including, laser ablation,111 hydrothermal,112 electrochemical oxidation113,114 and arc discharge115 (Fig. 8a). This method offers some advantages such as abundant raw materials for the fabrication of O-dots, large-scale production, and simple operation. The obtained O-dots possess a highly crystalline nature and good aqueous dispersibility. However, these nanomaterials commonly demonstrate a predominant sp2 hybridized carbon structure, and therefore often lack an efficient electron band gap to provide fluorescence. Therefore, the size and surface chemistry should be modified via oxidizing agents such as concentrated acids (HNO3 and H2SO4/HNO3 mixture). In this process, the bulk carbon materials are reduced into smaller fragments, while the surface is altered with oxygen-containing groups. This two-step route has become well-established for the formation of GQDs. In the first step, graphite is converted into graphene oxide (GO) sheets using the Hummers' method, while the second step involves cutting the GO into GQDs using various methods.59
The bottom-up approach employs certain molecular precursors that can form O-dots after dehydration and carbonization procedures. In general, the best precursors possess –OH, –COOH, –CQO, and NH2 groups, which can be dehydrated at higher temperatures. There are numerous approaches to perform the dehydration and carbonization processes, including hydrothermal,66 microwave-hydrothermal,116 plasma-hydrothermal approaches117 (Fig. 10b). These methods offer exciting opportunities to control the molecular size, and shape and fine-tune the physicochemical properties of O-dots.
4.1. Top-down approaches
The main idea of top-down methods is based on the fabrication of nanostructures from bulk materials. As will be discussed further in detail, in the top-down methods, bulk materials are broken down into their primary building blocks by applying an external source of energy and then become reconstructed into nanostructures with certain morphologies and atom configurations (Fig. 10a). An external source of energy, bulk carbon precursors and a suitable medium are the requirements for these methods, and based on these parameters, it is possible to invent new top-down fabrication methods (Fig. 11).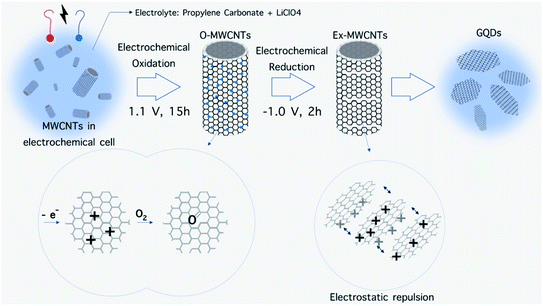 | ||
| Fig. 12 Electrochemical preparation of GQD from MWCNT. Electrochemical oxidation of MWCNTs and subsequent electrochemical reduction leads to break down of sheets and electrostatic repulsion, respectively.118 | ||
Alkali-assisted electrochemical etching allowed the preparation of CQDs with a controlled size.120 The electrochemical synthesis of photoluminescent CQDs from glycine under alkaline circumstances was recently reported by Wang et al.114 The application of +10 V between two Pt electrodes led to the oxidation of glycine (Fig. 9a) and resulted in the formation of ammonium ions, which further reacted with non-oxidized glycine through an amidation reaction. The ions produced allowed electro-polymerization, carbonization and passivation to form highly fluorescent CQDs with an average size of 2.4 ± 0.4 nm and a crystalline structure.
In another study, water-soluble red fluorescent GQDs with a uniform size of 3 nm and red emission were successfully prepared without any chemical modification via the electrochemical exfoliation of graphite in a K2S2O8 solution. The RF-GQDs were isolated sp2 domains with a diameter of 3 nm generated by the very active SO4− radicals produced from S2O82− as electrochemical “scissors” to precisely cut the graphene sheets into small intact sp2 structures.70
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was reported by Yogesh et al., which led to CQDs 4 nm in size.123 A one-step process based on the irradiation of a graphite target using a 532 nm second harmonic beam of an Nd:YAG laser in H2O/ethanol or diethylenetriamine penta acetic acid solution for the formation of 3 nm-sized CQDs was described by Tarasenka et al.124 The synthesized particles exhibited strong photoluminescence in the visible region. The laser ablation of a solid carbon material in a liquid environment with laser pulses of 1064, 532 and 355 nm at different irradiation times was recently investigated.111 A wide size distribution of the CQDs was observed for the 1064 nm laser due to the deeper penetration of the laser into the dielectric during the ablation process, and therefore this wavelength is less suitable for the formation of size-controlled CQDs.
2) was reported by Yogesh et al., which led to CQDs 4 nm in size.123 A one-step process based on the irradiation of a graphite target using a 532 nm second harmonic beam of an Nd:YAG laser in H2O/ethanol or diethylenetriamine penta acetic acid solution for the formation of 3 nm-sized CQDs was described by Tarasenka et al.124 The synthesized particles exhibited strong photoluminescence in the visible region. The laser ablation of a solid carbon material in a liquid environment with laser pulses of 1064, 532 and 355 nm at different irradiation times was recently investigated.111 A wide size distribution of the CQDs was observed for the 1064 nm laser due to the deeper penetration of the laser into the dielectric during the ablation process, and therefore this wavelength is less suitable for the formation of size-controlled CQDs.4.2. Bottom-up approaches
Since GQDs and CQDs are formed from carbon atoms organized in specific configurations, some carbon-based precursors can be used for the synthesis of these nanomaterials (Fig. 10b). Accordingly, these precursors act as carbon backbones, which can join together in a specific manner to form GQDs or CQDs under specific synthetic condition. For this purpose, a carbon precursor, a source of energy and a suitable medium are required. Specifically, the building blocks of GQDs and CQDs in the bottom-top methods are materials with small carbon chains that can merge under irradiation of an energy source and form honeycomb sheets of carbons or spherical carbon nanoparticles. The synthetic conditions can explain the difference between the formation of GQDs and CQDs since different bonds will be formed under different conditions.5. Surface engineering of O-dots
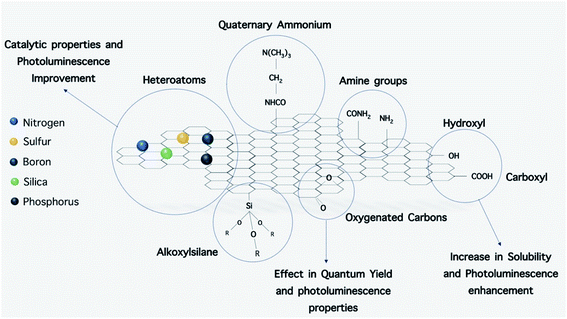
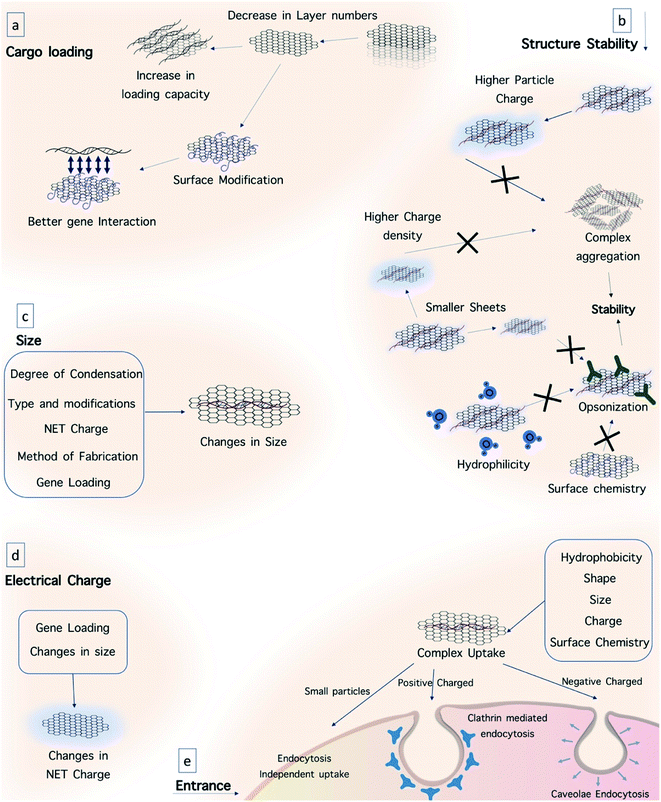
For better interacting with biological systems, modification of O-dots with different molecules and structures including biomolecules is necessary. Moreover, surface modification can alter the surface characteristics of a material to make it more suitable for a particular application.140,141 For this purpose, the addition of functional groups on O-dots will facilitate this process. Also, functionalization may change some of their physical and chemical characteristics.142The surface functional groups available on the surface of O-dots depend on the type of precursors and the reaction conditions.59 The functional groups present on the surface of O-dots include –OH and –COOH depending on the degree of oxidation. These groups readily form hydrogen bonds with water molecules, and their presence leads to reasonable solubility in water. Furthermore, these groups play a vital role in the enhancement of the PL efficiency. Therefore, alterations in the degree of oxidation can affect the optical properties of the O-dots. The QY of GQDs increases with a reduction in the oxygenation rate of GQDs, while the emission wavelength will be shifted towards longer wavelengths (red-shift) by their oxidation.92,143–145 In addition to these beneficial functional groups, additional functionalization with other materials such as polyethylene glycol (PEG) is necessary to improve the biocompatibility and also the QY of organic dots. The need for surface passivation offers some constraints in the synthetic procedure, which increases the overall particle size, resulting in a deleterious effects on the applications in of O-dots in different fields. PEG molecules, which are applied as surface passivation agents, can increase the inherent fluorescence emission.87,135 In addition to PEG, other small molecules such as ethylene diamine, octadecylamine, and 2-(2-aminoethoxy)-ethanol have been covalently linked to the surface of O-dots via an amide bond. Surface passivation leads to hydrophilicity and hydrophobicity in organic dots based on the nature of the functional groups.125,146 Furthermore, the addition of heteroatoms (especially nitrogen) can improve the PL and QY of carbon dots. N-doping of GQDs increases their QY and produces a blue-shifted emission due to the strong electron-withdrawing ability of the N atoms within the conjugated C plane.80,92,130,147 To add catalytic functions to organic dots or to improve their PL properties, other elements (e.g., Si,148 P,149 S150,151 and B108) have also been doped into CQDs and GQDs. S and N co-doped CQDs and GQDs can have a QY value as high as 73% and 71%, respectively.108,150 Thus far, different functional groups such as amine, carboxyl, quaternary ammonium and alkoxysilane have been coated onto the surface of O-dots (Fig. 13). The most common groups detected on the O-dot surface are amine and carboxyl, which allow the conjugation of organic, polymeric, inorganic or biological moieties.152–154 Herein, some approaches for surface modification will be discussed.
5.1. Amine capped CDs
Branched poly(ethylenimine) (BPEI), 2,2′-(ethylene-dioxy)bis (ethylamine) (EDBEA), 4,7,10-trioxa-1,13-tridecanediamine (TTDDA), poly(ethylene glycol)diamine (PEG1500N), ethylenediamine (EDA), polyenepolyamine (PEPA), tetraethylenepentamine (TEPA), urea and chitosan have been used to coat the surface of O-dots due to their amine groups. The synthesis of these types of coated O-dots is carried out using different approaches including microwave irradiation, hydrothermal carbonization, and pyrolysis, which convert carboxyl groups to amine groups.5.2. Carboxyl group-coated O-dots
Several approaches have been reported to produce carboxyl group (carboxylate)-coated C-dots, including pyrolysis, microwave irradiation, and chemical oxidation.166 Accordingly, O-dots coated with carboxyl groups were prepared using CA with pyrolysis at 180 °C for 150 min under normal air.167 This method was modified by Reisner et al.168 who carried out the reaction for 40 min. In this approach, further purification was not necessary because no residual citric acid was detected in the sample. A similar material was obtained using a microwave oven, in which glucose and poly(acrylatesodium) (PAAS) were dissolved in water and then heated for 4 min. After purification, carboxyl-capped C-dots were obtained.169 Chemical oxidation is another approach used for the preparation of O-dots capped with carboxyl groups. In this approach, different carbon sources, such as activated carbon or petroleum coke are employed. These materials are chemically oxidized in concentrated HNO3 or a mixture of concentrated H2SO4 and HNO3 at a temperature higher than 100 °C for more than 12 h.170,171 After centrifugation, the supernatant is removed and neutralized by NaOH. Then, the carboxylate-capped O-dot solution can be collected by dialysis. Callan et al.172 changed the surface coating of O-dots from amine to carboxyl groups via a reaction occurring between the amine groups and succinic anhydride at a basic pH. The synthesis procedure was based on two steps. Firstly, the amine-capped O-dots were prepared using carbon nanopowder refluxed in nitric acid for 12 h. Then, the dried oxidized carbon nanoparticles were refluxed in neat SOCl2 for 6 h. Finally, the product was reacted with bis-3-aminopropyl terminated poly(ethylene glycol), forming amino-capped O-dots. In the second step, the reaction between amine-capped C-dots and succinic anhydride led to the formation of carboxyl-capped dots. Succinic anhydride reacted with the amine capped O-dots in CH2Cl2 solution containing Et3N, and the reaction was continued for 15 h under argon at ambient temperature. Then, the mixture was neutralized with aqueous HCl, and the carboxyl coated O-dots were extracted with CHCl3. After dehydration of the organic phase over Na2SO4, the solvent was removed under reduced pressure, and dark yellow carboxyl terminated O-dots were finally obtained.5.3. Surface coating with quaternary ammonium
Several studies have reported the preparation of O-dots with a cationic surface coating,173–178 which are generally synthesized in three steps as follows. In the first step, a concentrated acidic solution of betaine hydrochloride was neutralized by the addition of tris(hydroxy methyl)-amino methane (Tris) at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After this step, the water-soluble organic salts were partially removed from the solution with isopropanol. A dry viscous white precursor was produced, and subjected to pyrolysis at 250 °C in air for 2 h. If the temperature was increased beyond this point, it may lead to the degradation of betaine, and thus 250 °C was adequate for the carbonization of Tris. The resultant product was extracted with water and precipitated from the colloid using acetone. After washing and drying, the surface quaternized O-dots were obtained as a dark brown powder.
1. After this step, the water-soluble organic salts were partially removed from the solution with isopropanol. A dry viscous white precursor was produced, and subjected to pyrolysis at 250 °C in air for 2 h. If the temperature was increased beyond this point, it may lead to the degradation of betaine, and thus 250 °C was adequate for the carbonization of Tris. The resultant product was extracted with water and precipitated from the colloid using acetone. After washing and drying, the surface quaternized O-dots were obtained as a dark brown powder.
5.4. Alkoxysilane coating
Amino-terminated alkoxy silanes including N-(β-aminoethyl)-γ-aminopropyl methyl dimethoxy silane (AEAPMS)179,180 and [3-(2-aminoethylamino)propyl]trimethoxy silane (AEATMS)158,181 have been used. These compounds were heated to 230–240 °C, and then anhydrous citric acid was added quickly under vigorous stirring. In the course of pyrolysis, citric acid was rapidly decomposed and carbonized in an oxygen-free environment, which led to the formation of carbon nanoparticles with residual carboxylic acid groups. Amide bonds were obtained via reaction of the terminal NH2 on the alkoxy silanes with the residual carboxylic acid groups. The carbon nanoparticles were attached to the alkoxy silane via amide bonds. The products were purified by precipitation with petroleum ether or a mixture of toluene/hexane repeated three times.6. O-dots and biological materials
As discussed previously, the reactive groups present in the surface coating of O-dots can be modified according to the requirements. Numerous organic, polymeric, inorganic or biological materials with specific functions can be attached on the surface of O-dots. Moieties can be attached to O-dots via several different interactions, including covalent bonds, electrostatic interactions and hydrogen bonds for specific applications.6.1. Interactions in functionalized O-dots
Callan et al.172 reported the preparation of a CQD-NO photo-releasable nanohybrid system for the two-photon phototherapy of hypoxic tumors. This nanohybrid was produced via an amide bond formation reaction using ethyl(dimethylaminopropyl)carbodiimide (EDC) plus N-hydroxysuccinimide (NHS). The carboxylic acid-coated CQDs were activated by EDC/NHS in PBS at room temperature for 20 min. Then a DMSO solution of the amine-terminated nitroaniline derivative NO photodonor was reacted with the activated carboxyl groups for 4 h at room temperature. The desired product was then obtained after purification. Sulfur-doped CQDs can be linked to dopamine via electrostatic interaction or hydrogen bond formation. The binding relies on the key role of NH2 groups and the aromatic ring, which occurs in plasma. This binding can be very useful for the detection of any abnormal dopamine that may be present in neurological disorders such as schizophrenia, Parkinson's disease and Huntington's disease.182 In another study, Liu et al.155 reported a multifunctional platform for serum-resistant gene delivery and bioimaging based on O-dots modified with a poly cationic-b-poly-zwitterion copolymer using an atom transfer radical polymerization (ATRP) reaction. In this method, the PDMA/O-dots were prepared according to the following procedure. Bromine-functionalized CQDs, 2-(dimethylamino)-ethyl methacrylate (DMAEMA), N,N,N′,N′′,N′′-pentamethyl-diethylenetriamine (PMDETA), and NaCl were added to a degassed ethanol suspension of CuCl2 under N2 protection, and the reaction was continued for 3 h at room temperature. An aqueous solution of MPDSAH was then injected into the mixture and the reaction was allowed to proceed at room temperature for 24 h. The solution was dialyzed against deionized water for 7 days to completely remove the impurities, and was subsequently freeze dried. Anhydrous polymer-modified CQDs (PDMA-PMPD/CQDs) were eventually obtained.Functional groups can also be attached to the surface of O-dots via hydrogen bonds. For instance, Ouyang et al. developed a novel turn-on fluorescence probe for the targeted imaging of cancer cells via the hydrogen bond interactions between folic acid (FA) and carboxyl-coated CQDs. The probe was synthesized by mixing FA and CQDs in an aqueous solution, which was dialyzed three times against pH 7.4 PBS buffer for 2 h.169 In another study, bi-functional protein nano fiber (PNF)–GQD nano-hybrids based on non-covalent interactions (π–π and electrostatic) between motif-designed PNFs and fluorescent GQDs were synthesized. Because these PNF–GQD nano-hybrids possessed both a recognition moiety (RGD peptide) and a fluorescent imaging probe (GQD), they could simultaneously target and image tumor cells.183
6.2. Interactions of O-dots with biomolecules
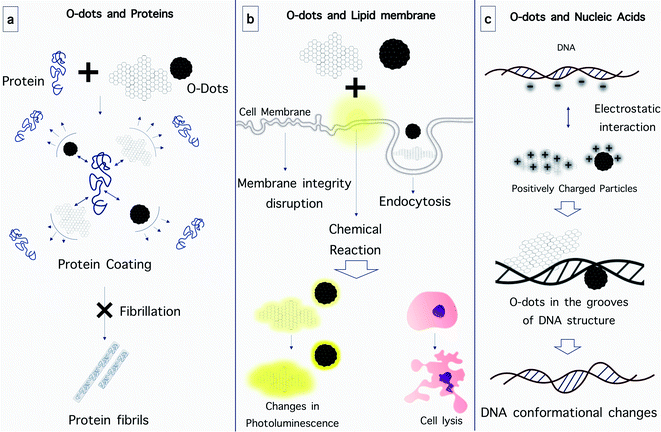
Fluorinated graphene quantum dots (F-GQDs) are a new type of carbon nanomaterials with unique physicochemical properties due to their highly electronegative fluorine atoms. Yousaf et al. prepared highly fluorescent and water-dispersible F-GQDs using a microwave-assisted hydrothermal method, and investigated their inhibitory effect on the aggregation and cytotoxicity of hIAPP in vitro. The efficient inhibition of amyloid aggregation by the addition of F-GQDs was confirmed. In the presence of F-GQDs, the morphology of the hIAPP aggregates changed from entangled long fibrils into short thin fibrils and amorphous aggregates. By employing fluorescence analysis using thioflavin T, inhibited aggregation with a prolonged lag time, and reduced fluorescence intensity at equilibrium were demonstrated after hIAPP was incubated with the F-GQDs. Based on the circular dichroism spectrum results, the F-GQDs could inhibit the conformational transition of the peptide from its native structure to β-sheets. F-GQDs could also rescue the cytotoxicity of INS-1 cells induced by hIAPP in a dose-dependent manner.198
Studies on the antibacterial activity of CQDs have also been reported recently.207 The antibacterial activity of these C-dots was attributed to the destructive interactions between CQDs with the bacterial cell membranes, which closely depended on the surface charge on the CQDs.208 To elucidate the cellular behavior of CQDs, Zhou and coworkers207 carried out a systematic study. In this study, it was found that the cellular uptake of CQDs was a dose-, time- and partially energy-dependent process, which also to some extent involved passive diffusion. These CQDs could penetrate the cell membranes by endocytosis via caveolae-mediated and clathrin-mediated pathways.
Similar to CQDs, graphene quantum dots can also alter the structure of cell membranes. Jiang and co-workers assessed the toxicity of graphene oxide (GO) and nitrogen-doped graphene quantum dots (N-GQDs) on red blood cells (RBCs) through analysis of hemolytic activity. The morphology of the RBCs changed and their ATP content was lower after being exposed to the different nanomaterials. The structural changes of the RBC lipid membranes were studied via surface-enhanced infrared absorption spectroscopy using model membranes. The analysis of the infrared spectra confirmed that the adsorption of GO perturbed the integrity of the membrane by extracting the lipid bilayer, resulting in hemolysis and aberrant-shaped cells. By contrast, when N-GQDs were tested, the disturbance of the structure and conformation of the lipid was less, which resulted in fewer abnormal cells.209
It is expected that the negative charge of nucleic acids caused by the phosphate backbone will interact strongly with positively-charged CQDs through electrostatic interactions.213 The interaction of CQDs with DNA may be so strong that it can alter the configuration of DNA. Sun and co-workers found that positively-charged CQDs selectively promoted the transformation of right-handed DNA (B-DNA) into left-handed DNA (Z-DNA).210 It is believed that in this interaction, CQDs bind inside the main groove of DNA, as verified by competitive binding experiments using ethidium bromide, daunomycin, and Hoechst 33258. It is worth noting that Z-DNA has been identified as a transient structure that is activated by normal biological processes such as transcription, DNA supercoiling and cytosine methylation.215,216 Based on this finding, some DNA-based logic gates have been designed to exploit the fluorescence resonance energy transfer between CQDs and DNA intercalators. With their high biocompatibility, superior optical properties and predictable interactions with nucleic acids, CQDs have also found important applications as delivery vehicles for nucleic acids in a highly efficient manner.214 A study was reported using fluorescent CQDs as an efficient nano-carrier for siRNA to enable gene knockdown in gastric cancer cells with promising results.215
As mentioned above, the phosphate groups of DNA provide a negative charge, and thus the positive charge on the surface of O-dots is very important for suitable electrostatic interaction. Without this modification, the interaction between O-dots and DNA is probably too weak, and thus will lead to rapid release of therapeutic genes during delivery. Thus, cationic polymers such as polyethylenimine (PEI) are widely used to provide a positive charge.216 In 2017, the Yang group prepared a gene delivery system using CQDs, equipped with PEI and folic acid (FA). They used gel electrophoresis to ensure the ability of CQDs with PEI to bind to DNA due to their positive charge. It was concluded that the composite could be used to transfer plasmid DNA into cells.217
A recent study on the interaction of GQDs with three different types of DNA (well-matched DNA (WM-DNA), base-impaired DNA (AB-DNA) and amino-modified DNA (AM-DNA)) showed that GQD could reduce the melting temperature of DNA, and thus reduce its stability. It was also observed that the WM-DNA structure and conformation were not changed, but AB-DNA changed after incubation with GQD.218
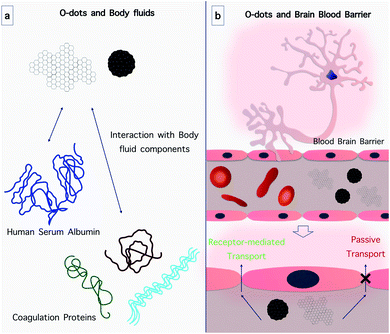
6.3. The interaction of O-dots with the blood–brain barrier (BBB)
In many applications, especially for drug and gene delivery systems, it is important to investigate the behavior of O-dots in biological environments. One of the important organs in the human body is the brain. In recent studies, scientists have concentrated on the ability of O-dots to cross the blood–brain barrier (BBB), the most important barrier in the central nervous system (CNS) (Fig. 15b). The BBB acts as a physical barrier and regulates the passage of designated molecules between the bloodstream and the brain by either paracellular or transcellular pathways. The BBB hinders the diffusion of many therapeutic agents from the blood into the brain. The tight junctions in the BBB have pores with a size of 4–6 nm. Therefore, nanoparticles that are 4 nm or less in size can cross the BBB by passing through these gaps.219,220The mechanisms of BBB penetration can be divided into active and passive transport routes.221 The passive transport route is an energy-independent process, for instance, simple diffusion. Frequently, the passive diffusion of drugs used against tumors occurs via the enhanced permeability and retention effect (EPR).222 The active transport routes include receptor-mediated and adsorption-mediated endocytosis and carrier-mediated transport, all of which require energy obtained by the hydrolysis of adenosine triphosphate (ATP).223 Besides, the transportation of drugs can be enhanced by different NPs and their specific mechanisms. For instance, the small stereospecific pores that are employed in the carrier-mediated transport system restrict the movement of large-molecule drugs.224 However most CDs have an ultra-small size (1–10 nm) and versatile surface functionalities,225 which can improve large molecule delivery if the CDs are covalently conjugated to the drug.226
Pediatric malignant glioma is one of the most common cancers among children, which is be fatal. Doxorubicin (Dox) is an effective drug that can hinder the replication of many different cancer cells. Nonetheless, Dox frequently causes severe side effects due to its non-specific attack on healthy cells, such as congestive heart failure. Thus, the delivery and release of drugs such as Dox across the BBB into the CNS can selectively target pediatric brain tumors. Li et al. covalently linked CQDs with transferrin to efficiently deliver Dox due to the over-expression of the transferrin receptor (TfR) on the BBB and cancer cells. CQDs were prepared from raw carbon powder using a “top-down” approach.227 Then SJGBM2 cells, a pediatric brain tumor cell line, were used to demonstrate the higher uptake of the conjugate compared Dox alone, which was confirmed by counterstaining with Hoechst. Four different tumor cell lines (CHLA-266, CHLA-200, SJGBM2 and Daoy) were exposed to 1, 10 and 100 nM of the conjugate and Dox aqueous solutions. The results showed that 10 nM conjugate solution gave the highest toxicity to all the pediatric tumor cell lines.
In the same year, based on publications that showed the importance of transferrin in transporting drugs to the brain parenchyma228 and brain glial cells,229,230 Li and coworkers employed zebrafish as a biological model to investigate whether transferring-conjugated CQDs could cross the BBB.231 Zebrafish is a vertebrate species with a high physiological similarity to humans and genetic homology.232 The advantages of zebrafish include their easy culture, transparent body, and the rapid formation of the BBB. Zebrafish are considered to be a superior model to mice for studying whether CQDs can pass across the BBB using transferrin-receptor mediated endocytosis.233,234
CQDs and the CQD/Trans/Dox conjugate were directly injected into the zebrafish heart. However, there was no fluorescence in the CNS when CQDs were injected alone. To show that the lack of fluorescence did not result from the low quantum yield of CQDs, a dye (5-(aminomethyl)fluorescein) was conjugated with the CQDs. The conjugated CQDs with 5-(aminomethyl)fluorescein also did not show any fluorescence in the CNS of the zebrafish. Therefore, it was concluded that CQDs alone could not cross the BBB. Similarly, the CQD/Trans/Dox conjugate was conjugated to a dye to enhance its PL emission. The CQDs/Trans/Dox conjugate labeled with the dye was successfully shown to reach the CNS by fluorescence spectroscopy, suggesting that the CQDs could cross the BBB, probably by TfR-mediated endocytosis.
Although CQDs are promising drug carriers, to some extent they possess the same limitations in crossing the blood–brain barrier as small molecule drugs. If CQDs can be prepared from a precursor that is known to be able to cross the BBB, there is a chance that the precursor molecules on the CQD surface can also allow CQDs to reach the brain. In the study by Mintz et al., tryptophan CQDs were produced from tryptophan, which is an amino acid that can cross the BBB via LAT1 transporter-mediated endocytosis.235 Two types of CQDs were synthesized using tryptophan and two additional nitrogen dopants, namely urea and 1,2-ethylenediamine. As mentioned previously, these CQDs were able to cross the BBB of zebrafish (Danio rerio) via the LAT1 transporter.
In another study, GQDs were used to hinder the fibrillization of α-synuclein (α-syn), and could interact directly with mature fibrils, causing their disaggregation. Moreover, GQDs could prevent neuronal cell death and synaptic loss, decrease the formation of Lewy bodies and Lewy neurites, reverse mitochondrial dysfunction, and prevent the neuron-to-neuron transmission of preformed α-syn fibrils.5,6 In vivo studies showed that GQDs could penetrate the BBB and protect against dopaminergic neuron loss induced by preformed α-syn fibrils, and reduce Lewy body/Lewy neurite pathology and behavioral deficits.236
6.4. O-dot interactions with biological fluids
The behavior of O-dots in various biological and body fluids is very important since most reactions in humans and other living organisms occur in a fluid environment (Fig. 15a). Injection of nanoparticles intravenously or subcutaneously is the most common route of administration, and therefore exhaustive testing is required to determine the fate of nanoparticles within the blood circulation (the main fluid within the body) and their impact on hematopoietic functions and cells.237 Moreover, studies in simulated physiological condition such as blood at pH ∼ 7.4 and temperature ∼ 37 °C have shown that CQDs can be efficient drug delivery systems. As an example, researchers increased the release rate of Dox in physiological fluid by linking carboxymethyl cellulose-hydroxyapatite to CQDs, compared to the composite without CQDs.238 Therefore, it seems that CQDs are effective drug delivery systems in physiological environments. The study of the interaction of CQDs with plasma components, especially coagulation-related proteins, is crucial in the field of drug delivery. In 2017, the effect of O-dots derived from Schizonepetae Herba Carbonisata (SHC) on a liver wound was investigated. The animals treated with O-dots had a shorter bleeding time compared with the control group. However, the study of O-dot behavior in plasma requires more research.239 The most abundant protein in plasma is serum albumin. In 2018 Guo and coworkers examined the behavior of O-dots in the presence of human serum albumin (HSA). The results showed that the O-dots interacted with HSA through hydrophobic interactions and hydrogen bonds. A bond was created between the oxygen or nitrogen atoms in the amino acids present in HSA and nitrogen and oxygen-containing functional groups in the O-dots. This binding could change the HSA secondary structure and disturb the normal function of the protein.2407. O-dots as a theranostic platform
The distinctive optical features of O-dots make them highly attractive candidates as imaging reporters combined with drug delivery and therapeutic applications. Their special properties allow them to overcome various problems associated with conventional imaging probes and to provide versatile nanoplatforms with both imaging and therapeutic capabilities. Herein, we survey the applications of O-dots for theranostic-enabled drug delivery and therapy (Fig. 16).7.1. Non-targeted cargo delivery
Although currently there is a trend toward targeted cargo delivery, non-targeted systems are still useful in some biomedical applications. Moreover, a need for systemic effects of drugs is another reason. Also, making cargo carriers responsive to certain stimuli can also be a suitable alternative to targeted cargo carriers.CQDs–Dox antitumor drug complexes were synthesized using a combination of CQDs and Dox through a hydrothermal method. The surface functional groups of CQDs were connected to Dox through electrostatic interactions, and the CQDs–Dox complexes could be transported into cells via both endocytosis and passive diffusion. Moreover, these complexes exhibited pH-sensitive Dox release behavior, and enhanced antitumor activity compared to Dox alone.241 In another study, hollow CQDs with a diameter of ca. 6.8 nm and pore size of ca. 2 nm were investigated as a delivery carrier for Dox. The Dox-hollow CQD drug delivery system (DDS) could be rapidly internalized by cells, and the release of the drug was regulated by pH. These hollow CQDs showed promise for both cell imaging and cancer therapy due to their nanostructure and photoluminescence properties.242 Li and co-workers prepared CQDs from ginger as a precursor, which were efficient without any drug cargo to kill hepatocellular carcinoma cells (HepG2) and showed lower toxicity towards normal mammary epithelial cells (MCF-10A) and normal liver cells (FL83B).243 They prepared the ginger CQDs via the simple hydrothermal treatment of ginger juice. Their CQDs selectively killed HepG2 cancer cells over other cancer cells, including human lung cancer cell line (A549), human breast cancer cell line (MDA-MB-231) and human cervical cancer cell line (HeLa). Western blot analysis revealed that the CQDs up-regulated the expression of p53 protein only in the case of HepG2 cells. In addition, they found that the uptake of CQDs by HepG2 cells increased the intercellular production of reactive oxygen species (ROS) up to 18.2-fold, which led to the induction of apoptosis, whereas in other cells, the ROS level was almost unchanged after treatment with 1.11 mg mL−1 CQDs. A subsequent in vivo study in a nude mice tumor model showed that the CQDs could effectively inhibit the growth of tumors (3.7 ± 0.2 vs. 104 ± 14 mg with and without treatment after 14 days).
Moreover, Yuan et al. reported that CQDs prepared from milk showed pH-dependent release behavior of Dox. Briefly, the negatively charged Dox was effectively bound to the positively charged CQDs. Compared with the free Dox, CQD–Dox demonstrated greater cytotoxicity towards cancer cells, whereas it showed lower toxicity towards a normal mouse fibroblast cell line.244
Khodadadei et al. produced 10 nm-sized nitrogen-doped GQDs (N-GQDs) with 10 graphitic layers, which could be loaded with methotrexate (MTX) to create a drug delivery system. The results showed that the GQDs were robust nanocarriers with higher anti-tumor activity, and could extend the cytotoxic effects of the loaded drug for a longer time.245 Some et al.246 used curcumin (Cur) as an anti-cancer drug. They showed that curcumin could attach to GQDs by interacting with the polar oxygen-containing groups at the edge of the GQDs. They found that the presence of more surface oxygen functional groups increased the loading capacity of the GQDs for curcumin. This occurred at pH 9, which is much higher than the physiological pH of 7.4, indicating that Cur was unlikely to dissociate in the extracellular environment or in normal healthy cells. They observed dissociation of Cur from the GQDs at the lower pH values typical of cancer cells. Up to 85% was released within 24 h at pH 5, compared to only 5% at pH 9 and 9.8% at pH 7.5. The GQD-Cur complex exhibited good ability to kill human colon adenocarcinoma HCT 116 cells, killing 90% at 100 μg mL−1, compared with 70% for Cur alone. The in vivo tests showed a much lower tumor volume and mass after 14 days in the mice with HCT tumors. The tumor volume for the mice treated with GQD-Cur was 200 mm3 (almost no increase in tumor size) compared to 1000 mm3 for the control mice.
7.2. Targeted cargo delivery
Some of the side effects of treatments are due to their unspecific functions and targets. Many drugs can be taken up by other cells rather than the intended cells. Moreover, this unspecific uptake also causes low concentrations of drugs in the intended sites, which would be below the therapeutic levels, and therefore, the real targets would not uptake a sufficient amount of drug. In this case, the primary concentration of the drug has to increase in order to provide a sufficient concentration at the diseased sites. This problem indicates a need for more specific therapy methods. Since biological systems demonstrate specific characteristics and properties, it is possible to target them for better therapies and treatments. Moreover, a suitable platform is needed for functionalization to become sensitive, specific and responsive to biological systems besides loading cargo. O-dots have shown promising properties in the field of targeted cargo delivery due to their capacities in being hybridized and functionalized properly with other nanoparticles, polymers, biomolecules, etc.Individual O-dots contain various functional groups on their surface. For example, the carboxyl groups of O-dots can be functionalized by N-hydroxysuccinimide (NHS) esters to conjugate with lysine residues in protein structures. O-dots have been used to label actin for actin polymerization studies, transferrin receptors were probed with fluorescent O-dot-labeled transferrin, and albumin-O-dot tags have been used for in vitro and in vivo signaling studies. This strategy is most suitable for CQDs with a high QY, which are prepared from citric acid with carboxyl functional groups. The conjugation of proteins with CQDs generally leads to an increase in fluorescence intensity and lifetime owing to the reduction of intramolecular dynamic fluctuations. Moreover, protein tagging can lead to less fluctuations in the fluorescence and more efficient use of excitation photons, which can be determined by single-molecule fluorescence measurements.247,248
The most common non-covalent interactions of CQDs occur between organic molecules or fluorophores. These interactions are hydrogen bonds, dipole–dipole interactions, hydrophobic interactions, van der Waals forces, electrostatic forces, and π–π stacking. Moreover, several factors such as pH, metal ions and organic solvent play a critical role in the attachment of organic molecules and fluorophores to CDs.249 P-CQDs/HA-Dox is one example of non-covalent interactions between positively charged polyethylene imine-modified CQDs (PEI-CQDs) and negatively charged hyaluronic acid (HA)-conjugated doxorubicin (Dox). These nano-hybrids were prepared via an electrostatic self-assembly method and used for the detection of hyaluronidase (HAase) activity in drug delivery.250 Moreover, PEI-CDs could also form a complex with folic acid (FA) via non-covalent binding, which was an effective approach for detecting and targeting folate-receptor (FR)-positive cancer cells. The fluorescent CQDs were produced using a hydrothermal method, and their surface was coated by positively-charged polyethylene imine (PEI), which allowed further conjugation with FA by electrostatic interaction. The FA-targeted PEI-modified CQDs (FA-PEI-CQDs) could act as a turn-on fluorescent nanoprobe in FR-positive cancer cells.251,252 Furthermore, the conjugation of PEI with CQDs led to positive charges on the CQD surface, which facilitated electrostatic interactions between the CQDs and DNA.253 Carboxyl-rich CQDs can be alkynylated using an amidation reaction with propargylamine in the presence of 1,1′-carbonyldiimidazole. Furthermore, Cu2−xSySe1−yNCs have been used as catalysts in the click reaction between alkynylated CQDs and azido-DNA for the preparation of modified CQDs. Covalently bonded DNA-CQDs can be produced by a copper(I)-catalyzed alkyne–azide cycloaddition click reaction (CuAAC) catalyzed by Cu2−xSySe1−yNCs. Research showed that DNA-modified CQDs are useful in biosensing and bioimaging approaches.88,254 Aptamer-based spectrofluorometric methods provide a sensitive approach for the detection of cancer cells. CQDs modified with the AS1411 aptamer act as a sensitive and selective signal enhancer for the spectrofluorometric detection of various target cancer cells. AS1411is a nucleolin-specific aptamer for the detection of nucleolin, which is over-expressed on the surface of cancer cells. The aptamer molecules are physically wrapped around CQDs. Incubation of target cancer cells in a CQD-aptamer solution led to an enhancement in the fluorescence intensity.255,256 The functionalization of CQDs with the nuclear localization signal peptide (NLS–CQDs) is a useful strategy for the transportation of Dox into cancer cells to improve the anticancer effect of this drug. Dox was covalently conjugated with NLS–CQDs (Dox–CQDs) via an acid-labile hydrazine bond, which was degraded in the weakly acidic intracellular milieu and showed superior suppression of tumor growth.257,258 A system of pH/redox dual-responsive CQDs (CQDs-RGD-Pt(IV)-PEG) could act as fluorescent imaging targeted nanocarriers. RGD peptide acted as an active targeting ligand and cisplatin(IV) as a prodrug. They were used for extracellular-triggered tumor targeting for improved anticancer drug delivery. The hydrolysis of the benzoic-imine bonds at the low extracellular pH led to exposure of the inner targeting RGD peptide, followed by effective uptake by interaction with the integrin αvβ3 (ligand–receptor) on the cancer cells.259–261 Polymer-coated CQDs (p-CQDs) are a new class of fluorescence nanoprobes with an ultra-small size, good biocompatibility, and high water solubility, which can be synthesized via direct solvothermal transformation. They can be covalently conjugated with I6P8, which is a fragment of interleukin-6 (IL-6), identified by the phage display technique, and can specifically bind to the IL-6 receptor. This receptor is expressed on both brain capillary endothelial cells and various cancer cells such as glioma. Conjugation of p-CQDs with I6P8 (cys-I6P7) led to improvements beyond that expected from passive targeting. The encapsulation of drugs such as Dox can be stabilized by these nanocarriers, allowing them to cross the BBB and deeper penetration of drugs into brain tumors. In summary, blocking the oncogenic function of IL-6 and the pH-responsive release of the loaded Dox are the main mechanisms for the improved anti-cancer effects.262,263 A nuclear-targeted drug delivery vehicle was fabricated by immobilizing a nuclear localization signal peptide (NLS) onto CQDs (NLS–CQDs). Through an acid-labile hydrazone bond, Dox was coupled onto NLS–CQDs (NLS–CQDs–Dox) and released in the weakly acidic intracellular compartment. NLS–CQD–Dox was mainly located in the nucleus and could efficiently induce apoptosis in human lung adenocarcinoma A549 cells. Moreover, NLS–CQD–Dox exhibited enhanced ability to inhibit tumor growth in vivo compared with free Dox in an A549 xenograft nude mouse model.264
Folic acid (FA)-conjugated GQDs could also be loaded with Dox. This nanoassembly could unequivocally distinguish cancer cells from normal cells, and efficiently deliver the drug to target cells. The inherently stable fluorescence of GQDs enabled real-time monitoring of the cellular uptake of the Dox–GQD–FA nanoassembly and the consequent release of Dox. The nanoassembly was rapidly internalized by HeLa cells via receptor-mediated endocytosis, while the Dox release and accumulation were improved.265
Justin et al.266 used GQDs as a non-toxic imaging agent to track the diffusion of drugs from microneedle arrays. Lidocaine hydrochloride (LH) was attached by π–π stacking to the GQDs and could passively diffuse through the microneedles due to its small molecular weight. They also coated GQDs with bovine serum albumin (BSA) as a large molecular weight cargo. GQD–LH also become loaded into chitosan to be used in a microneedle array for delivery. They used iontophoresis to stimulate the release of the BSA, which showed an increase in release rate from to 94.5% compared to 7.6% by passive diffusion.
Rare earth up-conversion nanoparticles and GQDs have attracted much interest in biomedicine, but they all have some inherent limitations as nanocarriers for in vivo drug delivery. One study reported a new concept for the fabrication of a GQD-rare earth up-conversion hybrid via coordination between rare earth metal ions and histidine, and attachment to octadecylamine-functionalized GQDs with fluoride ion replacement and subsequent hydrothermal treatment. The hybrid had a cage-like nanostructure with a mean particle diameter of 46.2 nm, comprising several layers of graphene sheets plus NaYF4:Yb,Tm nanocrystals. It showed high stability in physiological buffer and 140-fold enhancement of up-conversion luminescence. This biomimetic drug delivery system was composed of the hybrid plus gold nanoparticles as the core and MGC-803 cell membranes as the shell, with a Dox loading capacity of 461.2%. It showed both pH and light-responsive drug release and carried out chemo/photothermal therapy with higher anticancer activity than free Dox.267
In another study, a composite of GQDs and Fe3O4 was prepared and further conjugated with concanavalin A (a lectin protein) to produce GQD-ConA@Fe3O4 nanocomposites. These were employed for both the recognition of HeLa cancer cells and release of drugs. To study their capability to preferentially recognize HeLa cancer cells over normal endothelial cells, an electrochemical method was employed. These nanocomposites were deposited on a Pt electrode, which allowed the assessment of the dynamic linear range (5 × 102 to 1 × 105 cells per mL), and a detection limit of 273 cells per mL. Also, these nanocomposites could function as magnetically controlled nanocarriers for loading and releasing Dox. The in vitro results showed that the Dox concentration in HeLa cells was more than doubled in the presence of an external magnetic field due to the presence of Fe3O4 within the nanoplatform. The cytotoxicity in HeLa cells was 13% higher than normal cells because of the selective targeting by ConA. Thus, the GQD-ConA@Fe3O4 nanocomposites are a promising theranostic platform for cancer cell detection and targeted Dox release.268
7.3. Gene therapy
Gene therapy is considered a promising treatment for many diseases, but some barriers limit its clinical translation. Gene therapy involves the delivery of nucleic acid-based therapeutic agents into cells to modify the expression levels of various genes. Depending on the type of agent they can be activated in the cell nucleus or the cytoplasm. Genes, their expression, translation and transcription are a complicated system and their manipulations may cause serious implications on the functions of biological systems. Moreover, transferring a desired gene to the target properly without any damage to the gene is another limitation. Delivering other nucleic acids will also affect gene regulations in a complex manner. Therefore, gene delivery systems should be multifunctional besides being specific for certain targets and protective for their contents. Accordingly, GQDs and CQDs present a suitable function for this purpose due to their physiochemical properties. Gene delivery systems based on GQDs and CQDs should interact properly with nucleic acids, precisely deliver them to targeted cells, protect the nucleic acids from environmental conditions, not disrupt gene regulatory systems or other physiological process and cause proper gene expression or regulation. The parameters involved in efficient gene delivery are discussed below.269The success of gene therapy largely relies on the effectiveness of safe and efficient gene delivery into the target cell without any damage to the structure of the nucleic acids. A review of the literature from 2004 to date shows that the use of carbon-based nanomaterials for in vitro and in vivo gene delivery has steadily increased.270,271 Among them, O-dots can deliver numerous types of therapeutic nucleic acids in order to modulate the gene expression profile of cells. Two main approaches are used to carry out this goal. In the first approach, transcriptionally proficient DNA is delivered into cells to replace a copy of a defective or missing gene, and thus make up for the deficiency of the encoded protein. In the second approach, various therapeutic nucleic acids are used to regulate gene expression at different levels, which can be antisense oligonucleotides (AONs), small interfering RNA (siRNA), short hairpin RNA (shRNA) or microRNA (miRNA). These are used to inhibit or destroy the expression of unwanted genes.272
An efficient carrier for gene delivery should be capable of binding to nucleic acids properly and protecting them from environmental hazard conditions. These nanocarriers should be able to preserve the genetic materials during transportation, overcome extracellular and intracellular barriers, and deliver the functional form of the nucleic acid into the target cells in an intact manner.273
Several important physiochemical parameters, including the surface charge, size, shape and surface chemistry of the carrier should be optimized to produce a nano-gene delivery system (Fig. 17). By adjusting these factors, the fate of nanocarriers inside the body can be rationally tailored, and accordingly the final success of gene therapy can be realized.274
7.3.1.1. Surface charge. It is important to study the stability of nanocarriers, their cellular uptake mechanism, and their possible cytotoxicity. The overall surface charge probably has the most important role in facilitating these processes properly.275 Because nucleic acids (DNA, RNA, and ODN) possess an overall negative charge due to the phosphate groups in their backbone, they cannot pass across cellular membranes by simple diffusion, and therefore they require carrier systems, which can form complexes, leading to improved transportation across biological membranes. These polyplexes overcome the electrostatic repulsion between nucleic acids and the negatively charged cell membrane, and thereby expedite their internalization into cells.273 One simple approach to attach genetic materials to carriers is based on the electrostatic interactions between positively charged nanocarriers and nucleotides.276
Both charge polarity and charge density can affect the cellular uptake of nanoparticles, while simultaneously affecting their cytotoxicity. Positive surface charges combined with neutral or negative charges can improve the cellular uptake of nanoparticles allowing adhesion to the cell membrane in both phagocytes and non-phagocytic cell lines.277
Carriers with the highest positive charge density can be taken up to a higher extent at a faster rate.275 Additionally, the surface charge of nanocarriers determines the mechanism of cellular endocytosis. Nanocarriers can be internalized in cells via two pathways, i.e. clathrin-mediated endocytosis and caveolae-mediated pathways. Positively charged carriers use the first pathway, while carriers with a negative surface charge can be taken up through the second one.278
In comparison with the effects of negatively or neutral charged nanoparticles, positively charged nanoparticles exhibit greater overall cytotoxicity.277 Positively charged species can increase the Ca2+ influx into cells, and thereby inhibit their proliferation, disturb the structure of the lipid bilayer and its fluidity.279 Nanoparticles with a higher surface charge are more resistant to the formation of aggregates due to their strong self-repulsion.274 It is clear that the surface charge of the carriers will be affected by gene loading. Due to the electrostatic interaction between the positively charged carrier and negatively charged nucleic acids, the surface charge of the complex will be partly neutralized.280 The carrier/DNA ratio (known as the N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio) usually imposes a complex charge, which increases to more positive values with an increase in the N
P ratio) usually imposes a complex charge, which increases to more positive values with an increase in the N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio. However, at a lower N
P ratio. However, at a lower N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio, the formed complex may be incapable of effectively interacting with the cell membrane.281
P ratio, the formed complex may be incapable of effectively interacting with the cell membrane.281
7.3.1.2. Size. Another necessary factor in gene delivery is the size of nanocarriers, which can noticeably influence their colloidal stability, cellular uptake, transfection efficiency, residence in circulation, and the rate of clearance.274 In vitro experimental results revealed that nanoparticles are taken up by cells more efficiently as their size decreases.282 Nanoparticles with a size of around 20 nm can be taken up via cells in an endocytosis-independent manner. Longer circulation times in vivo can be achieved by the administration of nanoparticles with a size smaller than 200 nm. Smaller nanoparticles demonstrate lower clearance and better delivery to tumor tissues in comparison with larger nanoparticles, while they are not accumulated in other organs, such as the lungs, liver and spleen at this level. On the other hand, particles larger than 200 nm are rapidly removed by the liver, spleen and lungs.282 In the case of O-dots, size also has an important effect on amphiphilicity, and thus smaller sheets are more hydrophilic due to their higher edge-to-area ratio. Moreover, smaller graphene sheets show better colloidal stability because of their higher charge density.283 The layer number is another parameter that should be considered in the design of O-dots since this governs the specific surface area and bending stiffness.284 By decreasing the number of layers to one or more, the loading capacity of the cargo will be improved. The size of the carrier can also be strongly affected by the binding of nucleic acids.285 The electrostatic interaction between the carrier and nucleic acids leads to condensation or compaction of the genetic material. The degree of condensation affects the size variability. However, the size of the complex can be altered by changing the structure of the cationic carrier, the N
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio and also the fabrication method. For instance, by increasing the N
P ratio and also the fabrication method. For instance, by increasing the N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio during complex formation, the complex size will show a decreasing trend due to more efficient condensation of the loaded nucleotides.286 Therefore, the N
P ratio during complex formation, the complex size will show a decreasing trend due to more efficient condensation of the loaded nucleotides.286 Therefore, the N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio during complexation should be precisely optimized before transfection.287
P ratio during complexation should be precisely optimized before transfection.287
7.3.1.3. Shape. The shape of nanocarriers also governs their transportation across biological barriers and their fate inside cells.282 Spherical-shaped nanoparticles smaller than 100 nm are taken up more efficiently in contrast to rod-shaped nanoparticles, and therefore lower cell uptake occurs as the aspect ratio of nanorods increases.
7.3.1.4. Surface chemistry. It is known that the surface chemistry of nanocarriers affects their biocompatibility, circulation within the body, and their interaction with the cells. The blood half-life of nanocarriers is governed by their surface hydrophobicity. As the hydrophobicity of the surface increases, more proteins including opsonin bind to the surface and complexes would be taken up more rapidly, which subsequently leads to a shorter circulation time.277 Nanoparticles with a hydrophobic surface diffuse into cells and tissue more quickly than that with a hydrophilic surface due to the hydrophobic nature of the cell membrane.282 Many methods have been employed to modify the surface of nanoplatforms to improve the biocompatibility, circulation time, cellular uptake and thereby the transfection efficiency of nanocarriers.274 Since O-dots are intrinsically non-cationic, different modification approaches such as modification with PEI, chitosan, peptides, dendrimers, and PEG derivatives have been explored to provide cationic surfaces for a better interaction with anionic nucleic acids.
In another study, researchers prepared a multifunctional nanocomposite containing poly(L-lactide) (PLA), polyethylene glycol (PEG) and GQDs (f-GQDs) for the rapid imaging analysis of intracellular microRNAs (miRNAs), and combined gene delivery for improved therapeutic effects. The functionalization of GQDs with PEG and PLA enabled the f-GQDs to be stable in the physiological environment and also to have constant photoluminescence over a broad pH range, which is required for cell imaging. The f-GQDs possessed outstanding biocompatibility, low cytotoxicity, and protection of the cargo. The f-GQDs could efficiently transport a miRNA probe for intracellular miRNA imaging into HeLa cells. The large surface area of the GQDs could concurrently bind to the agents targeting miRNA-21, which led to the inhibition of cancer cell growth.289
Carbon quantum dots can also be applied as gene delivery platforms, especially N-doped CQDs, which generally possess a high quantum yield. For instance, N-CQDs fabricated using citric acid and tryptophan (Trp) were found to have good biocompatibility and capability to be functionalized with polyethyleneimine in order to deliver siRNA against the anti-apoptotic protein survivin into human gastric cancer cells. The siRNACQDs@PEI complex was rapidly taken up by cells and down-regulated the expression level of survivin mRNA. Flow cytometry showed that the nanocomposites could cause a mixture of early apoptosis, late apoptosis, and necrosis in the target cells.290
Furthermore, fluorescent CQDs were attached to siRNA against polo-like kinase-1 (Plk1) to strongly down regulate the expression of Plk1, an essential regulator of mitosis. The required amount of FCNs was only ∼1/30 of that of gold nanoparticles to deliver an equal amount of siRNA. They showed strong antitumor effects in vitro and in vivo by gene silencing, which was even greater than siRNA transfection by the positive control Lipo2000/siPlk1.291
Kim et al. (2017)292 reported the use of CQDs as a functional siRNA delivery system for effective gene knockdown in vitro and in vivo. They showed that CQDs increased the uptake of siRNA by tumor cells via endocytosis, accompanied by low cytotoxicity and interesting effects on the immune system. Fluorescence images verified the localization of the CQDs in the cytoplasm and release of siRNA within 12 h. The functional siRNA delivery system mediated by CQDs-PEI was used in an in vivo mouse model, with good gene knockdown efficacy and protection of the siRNA from degradation in vivo. Recently, Yang et al. (2017)293 reported a simple one-step hydrothermal carbonization procedure for the synthesis of positively charged CQDs using PEI and FA as carbon sources. The cytotoxicity experiments confirmed that the toxic effects of PEI were lower in the presence of CQDs, and the composite could be useful as a probe for the selective imaging of folate receptor (FR)-positive cancer cells compared to normal cells. The surface of CQD/pDNA had a positive charge, which facilitated the interaction with the weak negative charges of the cell membrane, suggesting that the surface of CQDs could efficiently capture pDNA molecules. To study gene expression in vitro, they used transfection with CQDs, and pDNA encoding enhanced green fluorescent protein (EGFP) in 293T and HeLa cells. These results showed that the positively-charged CQDs could efficiently transfect cells and could be useful for gene therapy.
Hyaluronic acid (HA) is a natural, biodegradable, biocompatible polymer that specifically recognizes and binds to CD44, a receptor expressed on the surface of cells, including tumor cells. HA was used in the construction of an O-dot-based nano-vehicle, which can be useful in tumor therapy.294 Wang et al.295 described the synthesis of CQD particles, in which HA was utilized as the carbon source and PEI as a passivation agent using a simple microwave (MW)-assisted pyrolysis procedure. In this study, the presence of HA improved the biocompatibility of the CQDs and the cationic amine groups on PEI helped DNA condensation and triggered endosomal release once internalized. Gel retardation and ethidium bromide (EtBr) exclusion assays suggested that the minimum C/P ratio required to inhibit DNA migration was equal to 1, while analysis of the particle size and zeta potential suggested that a C/P ratio above 6 was the most suitable for transfection. In comparison to PEI, these materials demonstrated lower cytotoxicity and up to 50-fold higher transfection efficiency in the presence of 10% serum. In vitro experiments using BSA protein binding, flow cytometry, and confocal microscopy also showed effective gene delivery. The fluorescent properties of these particles allowed the monitoring of the DNA internalization process, while an HA competition assay showed the specific ability of HA-QDs to identify cells overexpressing CD44. Different aspects of the use of GQD in gene delivery is shown in Fig. 17.
7.4. Bioimaging applications
Other major application of GQDs and CQDs is bio-imaging. This application is based on their photoluminescence properties,296,297 although they can be hybridized with other particles for use in other imaging modalities.298–300 Bioimaging can both provide basic knowledge about biological systems and used in diagnostic methods. Bioimaging is one of the pivots of theranostics, and since GQDs and CQDs are suitable cargo carriers and demonstrate other therapeutic activities including photothermal therapy and photodynamic therapy, showing bioimaging capabilities, they are promising theranostic agents.Luminescent nanoparticles have been widely used for bio-imaging applications and visualizing living cells. However, their potential toxicity is still a problem, which may limit the bio-imaging application of many luminescent nanoparticles. O-dots possess low toxicity and good biocompatibility, coupled with stable PL, and therefore they are ideal candidates for both in vitro and in vivo bio-imaging. In this section we discuss the recent progress in bio-imaging applications both in vitro and in vivo.301
The accumulation of CQDs inside cells can be improved if they are conjugated with TAT, a human immune deficiency virus-derived cell-penetrating peptide. TAT-conjugated CQDs were able to penetrate the cell nucleus.307 The surface passivation of CQDs for bio-imaging can affect their optical properties. For example, CQDs were prepared by microwave treatment of maltose, and passivated with dilute NaOH solution. The NaOH solution produced –OH groups on the sp2 hybridized carbon atoms, which increased the QY of the CQDs. It was found that these CQDs were taken up by the cells and produced a green fluorescence emission, while the cell viability was unaffected even after 24 h incubation.308
In other experiments,309 surface passivation could change both the fluorescence intensity and the emission wavelength. The presence of –OH groups can create hydrophilic CQDs, which are beneficial for bio-imaging applications. For example, CQDs with a particle size of 2–6 nm and a QY of 3% were produced by refluxing soot with nitric acid. The oxidation of the soot with nitric acid formed both –OH and –CO2H groups on the CQDs, making them negatively charged and hydrophilic. The in vitro experiments showed that the CQDs with no further passivation were taken up into the cells and released fluorescence. Under UV irradiation, the cells emitted blue-green fluorescence, and under blue excitation, a yellow emission was observed in the sample, whereas the sample without CQDs was colorless under the abovementioned excitations.310
When CQDs are purified by dialysis, the dialysis tube pore size or its molecular weight cut-off (MWCO) has a substantial influence on the emission characteristics of the CQD solution. For instance, in one study,311 the effect of the dialysis membrane molecular weight cut-off varying from 3500 to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da was examined, and it was found that the excitation wavelength for the oxidized CQDs changed for the different pore sizes, which emitted at different wavelengths in the range of 360 to 460 nm. The CQDs from membranes with MWCO > 3500 Da (up to 7000 Da) had similar emission intensities, while that collected by the membranes with an MWCO of 7000–14
000 Da was examined, and it was found that the excitation wavelength for the oxidized CQDs changed for the different pore sizes, which emitted at different wavelengths in the range of 360 to 460 nm. The CQDs from membranes with MWCO > 3500 Da (up to 7000 Da) had similar emission intensities, while that collected by the membranes with an MWCO of 7000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da had a reduced emission intensity. The CQDs that were collected by the membrane with an MWCO of less than 3500 Da had a low QY, and were contaminated with salts. The CQDs obtained from dialysis with an MWCO of 3500–7000 Da had the best optical properties.311
000 Da had a reduced emission intensity. The CQDs that were collected by the membrane with an MWCO of less than 3500 Da had a low QY, and were contaminated with salts. The CQDs obtained from dialysis with an MWCO of 3500–7000 Da had the best optical properties.311
Furthermore, oxidation has a strong effect on the PL features of CQDs. For instance, in chemically produced GO thin films in which isolated sp2 clusters were formed in an sp3 matrix of carbon–oxygen bonds, greater localization of the electron–hole pairs and better radiative recombination were observed, and the PL intensity was influenced by the reduction treatment. The reduction process altered the small sp2 clusters, and therefore, altered the PL intensity. The PL in graphene can be adjusted by engineering its sp2 clusters by controlled oxidation.312 Similar results were found by Hu et al.,313 who reported that the addition of oxidized branched polyethylene imine (BPEI) improved the band gap by modifying the oxygen–carbon matrix of the CQDs. The CQDs prepared through oxidation and hydrothermal treatment using BPEI, with a diameter of 3–4 nm could provide a QY as high as 54.3%. Incubation of the CQDs with MCF-7 cells and excitation at 405 nm showed luminescence inside the cells dispersed throughout the cytoplasm. The PL released from the CQDs did not decrease during 30 min excitation, indicating low photobleaching and good photostability.313
Larger carbon nanoparticles can also show fluorescence, for instance CQDs with a mean particle size of 70 nm can emit fluorescence and could be utilized for bio-imaging.314 The fluorescent CQDs were prepared via hydrothermal treatment of cocoon silk. During the hydrothermal treatment, the silk fibers were first cut into shorter ones, and then by heating at 200 °C were converted to asymmetrical polymeric fragments, and lastly to larger CQDs. The human cervical cancer (HeLa) cells incubated with the CQDs showed intracellular fluorescence. Confocal laser scanning microscopy images showed fluorescence in the perinuclear areas of the cytosol, confirming the effective uptake of the CQDs into the cells. These CQDs were used for in vivo tissue imaging of human tumor xenografts (MCF-7) in nude mice. The fluorescence emission observed in the tumor confirmed the permeability of the CQDs, while they showed low photobleaching and no blinking.314
Another valuable feature of CQDs for bio-imaging applications is their photostability. For example, the changes in PL intensity of the CQDs inside COS-7 cells were studied after incubation for 24 h. The COS-7 cells became bright, which revealed that the CQDs entered the cells, and the CQDs were able to label both the cell membrane and the cytoplasm of COS-7 cells. There was no apparent decrease upon continuous excitation for 10 min. This result was ascribed to the low photobleaching and high photostability of the CQDs.315
CQDs generally possess low cytotoxicity, even if they have been obtained from noxious components. For instance, CQDs prepared from halophenols (a group of industrial pollutants) were applied as fluorescent labeling agents. After incubation of the CQDs with HeLa cells and excitation with a 405 nm laser, the cell membrane and perinuclear cytoplasm showed fluorescence, while the signal inside the cell nucleus was weak. No morphological damage was observed in the cells after incubation with the CQDs, which signified their low cytotoxicity.316 Plants, fruits and food products such as orange juice,317 apple juice,318 gelatin,319 milk,320 soy milk,321 honey,322 and egg117 have all been used as a carbon source for the preparation of CQDs. It is thought that the use of natural products and foodstuffs may lead to lower toxicity of the resultant CQDs for biomedical applications. Mehta et al.323 used Saccharum officinarum juice as the carbon source for the fabrication of CQDs. These CQDs were used for cell imaging in E. coli (bacteria) and S. cerevisiae (yeast). The laser confocal fluorescence microscopic images showed that different colors (red, green and blue) could be observed in the labeled bacteria and yeast cells using 40 mg mL−1 of CQDs. In the confocal images of E. coli, the CQDs were well distributed in the membrane and cytoplasm. The CQDs were taken up by the yeast cells via an endocytosis mechanism. The CQDs were located in the yeast nucleus, but the yeast cells maintained >90% viability.323 Orange juice was used to make CQDs via a hydrothermal process, exhibiting a QY of 26%. These CQDs were used as imaging probes in human osteosarcoma (MG-63) cells, where they accumulated in the cytoplasm but not the nucleus. The PL intensity did not decay over a continuous excitation period.324
N-doped CQDs can be prepared by doping with various nitrogen-containing molecules such as diamine to increase their QY for bio-imaging probes. N-doping resulted in a QY as high as 36.3%. Fluorescent images (355 nm excitation) of HeLa cells showed that their cytoplasm was brighter than that with non-doped CQDs, indicating that labeling with N-doped CQDs can be performed at lower doses. Continuous UV exposure caused the fluorescence of the non-doped CQDs to be bleached after 15 min, while the fluorescence of the N-doped CQDs was still bright after the same period.325
Stem cells are fundamental to the understanding of embryonic development and tissue regeneration because they can give rise to several progenitor cells, which can in turn differentiate into cells of different tissue types.326 Due to the generally quiescent state of stem cells, their labeling processes face a substantial challenge.327 Bright yellow fluorescent GQDs328 showed direct and easy uptake into stem cells without affecting their viability, proliferation or differentiation capacity. This was the first use of stabilizer-free graphene as a fluorescent label in long-term stem cell imaging. Three different types of stem cells, namely neurosphere cells (NSCs), pancreatic progenitor cells (PPCs), and cardiac progenitor cells (CPCs) were incubated with GQDs at a concentration of 25 mg mL−1 for 24 h at 37 °C. The confocal fluorescent images showed that the GQDs were localized in the cytoplasm of the stem cells. The methyl thiazolyldiphenyl tetrazolium bromide (MTT) assay verified the low cytotoxicity of GQDs for stem cell imaging. In another study, the uptake mechanism and biocompatibility of GQDs interacting with human neural stem cells (hNSCs) were examined.329 TEM images proved that the GQDs were internalized into hNSCs via an endocytosis mechanism and were localized in the cytoplasm. The researchers reported that GQDs did not influence the self-renewal capacity of hNSCs. Single cells separated from GQD-labeled hNSCs still formed large neurospheres, and the cells expressed specific markers for hNSCs. In addition, no changes were detected in the viability, proliferation, metabolic activity or differentiation potential of NSCs after treatment with GQDs. After 14 days of differentiation, no differences were discovered in the hNSC proliferation rate between the control group and the 25 μg mL−1 GQDs treatment group. The cells from both groups exhibited an elongated cell morphology with neurite outgrowth, resulting in the formation of an interconnected neuronal network. Expression of glial fibrillary acidic protein (GFAP) and neuron-specific class III beta-tubulin (Tuj1) showed that the hNSCs could differentiate into glial cells and neurons, respectively. In addition to the bio-imaging of stem cells, other studies of GQD-based imaging have focused on tumor cells. Numerous studies have confirmed that fluorescent GQDs are taken up by tumor cells. In 2014, Yang et al. reported the use of B-GQDs for the imaging of HeLa cells.330 Zhu et al. cultured human osteosarcoma (MG-63) cells with solvothermally fabricated GQDs, which showed two-color imaging using different excitation wavelengths.331 Peng et al. incubated green luminescent GQDs with the T47D human breast cancer cell line and stained their nucleus with DAPI.332 The images included a phase contrast, where the nuclei were stained blue with DAPI, and high contrast green fluorescent GQDs were observed in the perinuclear region in the triple over-layered images.
Dong et al.333 incubated MCF-7 human breast cancer cells with GQDs synthesized via acidic oxidation, and a bright green emission could be detected using confocal laser scanning microscopy with excitation at 488 nm. The section analysis of single MCF-7 cells showed that GQDs not only labeled the cell membrane and the cytoplasm, but also the nucleus. This was the first time that luminescent carbon nanomaterials were shown to tag the cell nucleus. Moreover, Hela cells,334 A-549 cells,335 macrophages and hepatocellular cells335 have all been labeled with fluorescent GQDs by different groups. All these studies reported the low cytotoxicity and biocompatibility of GQDs using the MTT assay, and hence they could be used for bio-imaging. Fluorescent GQDs functionalized with folic acid (FA) were introduced as highly selective and specific tumor cell imaging agents by Wang et al.336 The fluorescence of the GQDs in FA over-expressing HeLa cells was noticeably stronger than in A549 and HEK293A cells, which both express the FA receptor (FR) at low levels.
CQDs have some advantages compared to other commercially available QDs for bio-imaging due to their high absorption coefficients. The higher absorptivity of CQDs can compensate for their lower fluorescence yield compared to heavy metal QDs. In one study, PEG surface-passivated CQDs were compared with CdSe/ZnS QDs. The PEG CQDs had better absorptivity and they also protected the dots for in vivo bio-imaging.305
The NIR emission of CQDs that were functionalized with a hyper branched polymer increased compared to the CQDs coated with a linear polymer (PEG). In vivo experiments in a mouse model led to visualization of lymph nodes due to the fluorescent emission of the CQDs.341 CQDs can also be doped with inorganic salts (e.g. ZnS) to increase their fluorescence yield for in vivo imaging. Injection of CQDs doped with ZnS (CQDs–ZnS) into mice (compared to the non-doped CQDs) showed a brighter emission. Green fluorescent CQD–ZnS was injected intradermally into the footpad and then transported along the leg via the lymphatic vessels. The migration of CQD–ZnS was slow in comparison to conventional semiconductor QDs. One reason was that the size of the CQDs–ZnS was smaller, while another reason could be due to the PEG functionalization. These two properties would result in decreased interactions with lymphatic cells.342
GQDs can also be exploited for in vivo imaging. Passivated nanographene sheet particles with attached PEG (NGS-PEG) with an average size of 30 nm were prepared by Yang et al. and administered into mice after tagging with the commonly used NIR fluorescent dye Cy7. In vivo fluorescence imaging showed the high tumor uptake of the NGS in several tumor xenograft mouse models.343 The effectiveness of GQDs fabricated via the one-step pyrolysis of L-glutamic acid was investigated for in vivo imaging in mice by Wu et al.344 After injecting the GQDs intradermally into the back of nude mice and intramuscularly into their right back leg, fluorescence images of the mice using different excitation and emission filters were captured. The detectable fluorescence from the intramuscular injection site in the leg was higher with longer excitation and emission wavelengths because of the better tissue penetration of light. Ge et al. examined in vivo fluorescence imaging using red fluorescent GQDs prepared via a hydrothermal method and modified with polythiophene derivatives.345 After injection of the GQD aqueous solution into the back of a nude mouse, fluorescent spots were observed against the background signal of the mouse skin. The fluorescent spots remained visible up to 1 week after injection.
7.5. Phototherapy
Surgery, radiotherapy and chemotherapy are the most common methods for the treatment of cancer; however, these methods exhibit undesirable side effects on other healthy tissues of the body. Therefore, many novel treatment methods have been developed to reduce the side effects of cancer therapy.346 In recent years, several methods based on the therapeutic use of light, such as photothermal therapy (PTT) and photodynamic therapy (PDT), have attracted attention for the treatment of cancer. PPT relies on the generation of heat from light absorption, while PDT is based on the photochemical generation of reactive oxygen species to destroy tumors.346,347Phototherapy is one of the independent therapeutic functions of GQDs and CQDs in synergism with light irradiation. The therapeutic function of phototherapy is based on the formation of lesions and causing damage to tissues. Accordingly, for more efficient function, besides the sonosensitizing properties of the material, targeting abilities are required for specialized therapy and reducing side effects, which can be provided by GQDs and CQDs due to their physiochemical properties.346
7.6. O-dots in phototherapy
The properties of O-dots include their chemical inertness, high water solubility, photostability, interplay between optoelectronic features and shape/size, fluorescence resonance energy transfer, high stability under physiological conditions, specific accumulation at target sites, facile surface functionalization, and high absorption coefficient due to their sp2 hybridized C–C bonds. Thus, these features make them an attractive chromophore for phototherapy.345,357,358One problem with many clinically employed PSs is that they are not specifically targeted to tumors, and consequently spatially controlled irradiation is utilized to target the site of treatment. Moreover, PS administration can cause ROS production in healthy cells, meaning that care needs to be taken after phototherapies to prevent light exposure to lessen the risk of skin photosensitivity.345 The QY of 1O2 production from NPs and quantum dots (QDs) alone are not as high as that from molecular PSs; however, O-dots can be applied as antennae to improve light harvesting and energy transfer to molecular PS owing to their high (size dependent345) light absorption cross-section.359 Furthermore, broad-wavelength excitable O-dots with tunable up-conversion and down-conversion properties can also be applied in PDT.360
The large number of mobile π-electrons in O-dots leads to comparatively strong electron–electron scattering and weak electron–electron interactions. It was predicted that the π-electrons in O-dots can act in a similar manner to the free electrons in metallic nano-clusters rather than that in inorganic semiconductor QDs. The limited QY of O-dots means that most of the absorbed light will be ultimately transformed into heat through several non-radiative relaxation pathways. This consideration suggests their potential use in PTT rather than PDT.361,362
Carbon dots attached to a platinum-coordinated porphyrin formed a composite (CQDs@PtPor) through the electrostatic interaction between four platinated porphyrins and the negatively charged CQDs in a complex (PtPor). This was utilized as a theranostic agent for PDT cancer therapy.366 They claimed that the composite combined the optical properties of CQDs and the anticancer activity of porphyrin into a distinctive new structure.
Pheophytin is a type of Mg-free chlorophyll derivative and is also a natural product with low toxicity, which was used as a raw carbon source for the synthesis of CQDs with the assistance of a microwave method by Weng and co-workers. The hydrophobic CQDs exhibited high potential to emit NIR light, with an excitation peak at around 680 nm, and high 1O2 production with a quantum yield of 0.62. The self-assembled CQDs from the precursor (DSPE-mPEG2000) showed efficient 1O2 generation. This carbon dot assembly was found to be useful as a fluorescence imaging agent and also as a smart PDT agent. Thus, these nanostructures can serve as a new phototheranostic agent for cancer therapy.367
Meng and co-workers reported the synthesis of a novel multifunctional two-photon excited nano probe based on GQDs, GQD@MnO2, with the capability for imaging glutathione (GSH) in biological systems and for improved PDT. GSH is a ubiquitous tri-peptide and the most common intracellular thiol, which has been reported to consume 1O2. Thus, GSH can decrease the effectiveness of PDT, as confirmed in vitro by Meng et al. Their prepared nanoprobe exhibited high sensitivity and selectivity toward GSH because the reduction of MnO2 nanosheets could be carried out by intracellular GSH, which led to its overall consumption. Therefore, GSH removal could improve the efficacy of PDT by GQDs. Researchers reported that the PDT efficiency of the prepared nanoprobes was better than that of GQDs alone by incubating HeLa cells with GQDs or GQD@MnO2 and irradiating with 560 nm light for 30 min. In addition, they found that nitrogen-doped GQDs had a good two-photon absorption cross-section for enhanced PDT. Doping GQDs with N can cause widening the light adsorption peak and prolongation of the PL lifetime. NGQDs also exhibited a good PTT effect when compared with other doped materials. Recently, Wen et al. confirmed this hypothesis.367,368
The combination of GQDs and methylene blue (MB) to destroy pathogenic bacteria by enhancing light-mediated 1O2 generation was reported by Monroe and co-workers. Moreover, their group also measured the ability of GQDS, MB and other GQD-MB combinations to kill MCF-7 breast cancer cells. They utilized the cell counting method to evaluate the cytotoxicity of GQDs, MB and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 GQD
1 GQD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MB mixture. The measurement of 1O2 generation in the medium was then checked by means of singlet oxygen sensor, in which the dye 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) was used to measure the production of reactive oxygen species. Another dye, H2DCFDA, was used to examine the total ROS generated during the process. Monroe and co-workers confirmed that GQDs and MB could be taken up into MCF-7 cells, and the most appropriate proportion for the combination was found to be 1
MB mixture. The measurement of 1O2 generation in the medium was then checked by means of singlet oxygen sensor, in which the dye 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) was used to measure the production of reactive oxygen species. Another dye, H2DCFDA, was used to examine the total ROS generated during the process. Monroe and co-workers confirmed that GQDs and MB could be taken up into MCF-7 cells, and the most appropriate proportion for the combination was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which led to superior cytotoxicity and the highest amount of 1O2 and ROS. In general, they found that the effects on MCF-7 cells were not dependent on the GQD concentration or the intensity of light.369
1, which led to superior cytotoxicity and the highest amount of 1O2 and ROS. In general, they found that the effects on MCF-7 cells were not dependent on the GQD concentration or the intensity of light.369
 | ||
| Fig. 19 (a) Confocal laser-scanning microscopy (CLSM) images of HeLa cells incubated with N–O-CDs (100 μg mL−1) excited by 405 nm. (b). In vivo fluorescence imaging of the tumor site from a mouse intratumor injected with N–O-CDs at 0 h, 5 min, 1 h, and 3 h post-treatment. The color bars represent the fluorescence intensity. Red circles indicate the position of the implanted tumor. (c) Synthetic procedure of N–O-CQDs (this figure has been reproduced from ref. 371 with permission from Elsevier, Copyright 2018). | ||
In another study, Thakut and co-workers developed highly crystalline GQDs with good physicochemical and NIR optical properties via the carbonization of Ficus racemosa leaves, which is a cluster fig tree native to India.372 The production yield of GQDs was 18% with a QY of about 14.16%. The synthesized GQDs exhibited good compatibility and photostability in both organic and aqueous media. The potential of these GQDs to act as in vivo fluorescent probes was confirmed via a cytotoxicity analysis. The generation of ROS in a concentration-dependent manner combined with PTT activity was observed after excitation with 808 nm laser. The GQDs were stable for 30 min under continuous laser irradiation. These highly fluorescent GQDs were also used for PTT of MDA-MB-231 breast cancer cells.
Tian and co-workers synthesized a composite derived from metal–organic frameworks combined with GQDs. They used zeolitic imidazolate framework-8 (ZIF-8) as a drug nanocarrier plus embedded GQDs as the PTT chromophore. The drug release performance, PTT efficacy and synergistic therapeutic efficacy were systematically investigated using Dox as a model anticancer drug. The Dox-loaded ZIF-8/GQD nanoparticles could transform NIR irradiation into heat. Using 4T1 breast cancer cells as a model, they showed the synergistic effect of the combined chemotherapy and PTT compared with either chemotherapy or PTT used alone (Fig. 20a).373
 | ||
| Fig. 20 (a and d) Schematic illustration of the ZIF-8/GQD nanoparticles with encapsulation of Dox molecules and synergistic Dox delivery and photo thermal therapy.373 (b and d) Dox-CuS@GQDs nanoparticles and their therapeutic function.375 (c and d) Schematic illustration of drug loading and release from mesoporous silica nanoparticles capped with GQDs and their function.376 | ||
Liu and co-workers reported the fabrication of GQDs with strong absorption at 1070 nm in the NIR-II region. They used a one-step solvothermal process with phenol as the only precursor. Their synthesis was controlled by the decomposition of hydrogen peroxide under a high magnetic field (9 T). Uniform size (3.6 nm), tunable quantum yield (16.67%), and high PTT conversion efficiency (33.45%) were the most important advantages reported by Liu and co-workers.374
Zheng et al. investigated a multifunctional platform composed of GQDs modified with hollow copper sulfide nanoparticles (CuSNPs) for controlled intracellular drug release and enhanced PTT-chemotherapy. Initially, the CuSNPs were synthesized and used as an encapsulating agent for Dox. Subsequently, GQDs were decorated on the surface of the CuSNPs. The beneficial properties of the nanoplatform include its good crystal structure, constant nanoscale size, colloidal stability, high drug encapsulation ability, and improved optical absorbance. Drug release could be triggered by high temperature and/or NIR laser irradiation. Confocal images and flow cytometry showed a significant level of NIR-triggered Dox release inside MDA-MB-231 cells. The combination of PTT and chemotherapy by Zheng et al. is illustrated in Fig. 20b.375
The synthesis of mesoporous silica nanoparticles (MSNs) capped with GQDs was reported by Gao and colleagues as a novel PTT and redox-responsive drug delivery system. An amidation reaction between cysteine and amino-functionalized MSNs was used to create disulfide bonds and Rhodamine B (RhB), a red fluorescent dye, was then incorporated into the mesoporous structure of MSNs as a model drug. Capping MSNs with GQDs acted as a blocking agent on the pores of mesoporous structure in order to control the release of the RhB cargo. The prepared nanocomposite demonstrated redox responsiveness to the presence of glutathione (GSH), which cleaved the disulfide bonds and released the loaded drug under redox control (Fig. 20c).376
In this section, we summarize the recent reports of O-dot-based nanocomposites that have combined imaging with one or more other modalities, including phototherapy, chemotherapy, and computed tomography (CT) (Fig. 21).
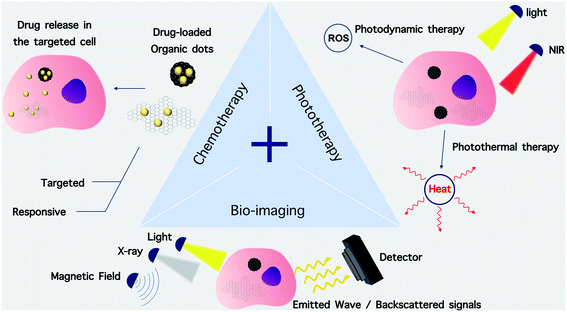 | ||
| Fig. 21 Schematic illustration of combined therapy. Combined therapeutic systems possess 2 or 3 of these properties. | ||
Lan et al. synthesized CQDs with an absorption of up to 1100 nm via the hydrothermal treatment of 1,3,6-trinitropyrene and Na2SO3.377 The CQDs could simultaneously provide strong fluorescence and generate 1O2 through a two-photon excitation mechanism. An outstanding PTT conversion ability under 800 nm femtosecond pulsed laser irradiation was also observed. The wide absorption peak of the CQDs enabled them to be used as a photoacoustic (PA) imaging probe. In vitro and in vivo experiments showed that the fluorescent CQDs had good biocompatibility and they could be used as a versatile phototheranostic probe for PA/fluorescence imaging, and as a PDT/PTT dual photoactive material with synergistic effects for killing cancer cells using a single NIR laser.377
Zhang and co-workers synthesized magneto-fluorescent Fe3O4/CQDs coated with single-walled carbon nanotubes (SWNTs) as a multifunctional theranostic material for targeted imaging plus chemo/photodynamic/photothermal triple-mode therapy. In this study, Dox as a model chemotherapy drug, was encapsulated in the porous structure of the SWCNTs-PEG-Fe3O4@CQD nanocarriers with high efficiency. These magneto-fluorescent structures were then conjugated to the sgc8c DNA aptamer, which specifically recognizes the receptor tyrosine-protein kinase-like 7 (also known as colon carcinoma kinase 4, CCK4) for targeted dual mode fluorescence/magnetic resonance (MR) imaging. The multifunctional porous structure demonstrated the synergistic killing of lung cancer cells via PDT, PTT and rapid release of Dox under simultaneous NIR laser irradiation and low pH.
In another study, Wo and co-workers synthesized GQD-coated hollow magnetic nanospheres (HMNSs) as a versatile system with a synergistic combination of magneto-mechanical, photothermal, photodynamic and chemotherapy of cancer. These mixed modalities structurally and physically destroyed cancer cells, where their morphology was visibly different from the cells killed by other therapies. HMNSs were coated with silica shells and linked to carboxylated GQDs in a core–shell structure. The synthesized composite was then loaded with Dox and stabilized with liposomes. The system was able to destroy cancer cells using four different therapeutic modalities in a synergistic manner including magnetic field-mediated mechanical stimulation, PTT damage, PDT toxicity, and chemotherapy.378
Xuan and colleagues functionalized the surface of GQDs with gold nanosphere clusters. They performed this process via a simple procedure, in which the reduction and stabilization of gold were both carried out by the GQDs themselves. They showed good dispersion, stability, excellent performance for photoacoustic imaging (PAI) and computed tomography (CT) imaging, low cytotoxicity, and PTT conversion efficiency of up to 51.31%. Cellular and animal experiments showed that the targeted PAI/CT imaging of tumors could be enhanced by modification with folic acid (FA). The combination chemo-PTT therapy could be carried out through controlled Dox release from GQD under the influence of heat and the acidic environment of the tumor. Furthermore, the observed therapeutic effect was superior compared to all the single modes alone.
Body weight monitoring, hematoxylin and eosin (H&E) staining of organs, and blood biochemical indicators demonstrated the good safety and low toxicity of the probe after injection.379
Badrigilan et al. synthesized nanoparticles from super paramagnetic iron oxide (SPIO) and bismuth oxide (Bi2O3) and they coated them with GQDs for use as in vitro CT/MR dual-modal biomedical imaging probes and for cancer-specific PTT. These nanocomposites showed strong light absorbance with a wide-band in the NIR region. The photo-thermal conversion efficacy (η) was 31.8%, with high photostability upon irradiation with 808 nm laser. The results of in vitro PTT of cancer cells showed a dose-dependent loss of viability of ∼53.4% in comparison to the laser alone group (3.0%). The presence of Bi with a high atomic number (Z = 83) had good X-ray attenuation capability (175%) and strong shortening of the T2-relaxation time.380
8. Conclusions and future perspectives
In this review, we summarized the recent progress in the design, properties, and theranostic applications of O-dots including graphene quantum dots and carbon quantum dots and introduce this new concept in order to facilitate new possibilities in the synthesis, preparation and application of these materials. These small-sized quantum dots have attracted much attention in theranostics due to their exceptional optical and chemical properties such as non-blinking, low-toxicity and photostability. They are now being investigated in medical diagnostics and imaging, bio-sensing, drug/gene delivery, photoactivation, and light-activated therapy. Here, we highlight some future directions that can produce further advances in this field.One of the reasons for the interest in O-dots is that their synthetic process is relatively simple, and their integration with other nanomaterials is easy. Despite the wide variety of approaches and precursors that have been employed for their fabrication, none of these nanostructures have been transitioned into a marketable product to date. Better controlled synthetic approaches, more precise characterization, and more extensive nanotoxicology studies will be required for effective translation. Because the fluorescence properties and the quantum yield are related to the overall composition and the presence of chemical groups on the surface of O-dots, standardization of the preparation procedures is urgently required.
Surface engineering is used to further functionalize O-dots by tailoring their surface coating with reactive groups for subsequent chemical modification. The reactive groups on the surface of O-dots can be reacted with the terminal groups of organic, polymeric, inorganic and biological materials via covalent bonds, electrostatic interactions and hydrogen bonds. Unfortunately, precisely controlled surface passivation has been challenging to date. The number of reactive groups capping the surface and the ratio of reactive groups on modifying molecules are difficult to be exactly controlled. In the future, scientists should continue to develop rational strategies for the preparation of O-dots with bright fluorescence emission in the red/near-IR spectral regions to allow deeper optical imaging in tissue and decrease the interference from background auto-fluorescence. Therefore, efficient one-pot procedures for producing surface-passivated O-dots with high quantum yields need further attention.
O-dots can act as carriers for many pharmaceutical applications. Once functionalized with various polymers, they can serve as versatile biocompatible gene delivery vehicles. Moreover, they can be designed to have extended therapeutic lifetimes within the body. Despite some safety concerns about O-dots, many studies have reported their use in hybrid forms for biological applications, particularly in gene delivery. Hybridization approaches using biocompatible polymers have been tested in order to lower their potential toxicity and to increase their biocompatibility and applicability as multi-functional imaging probes and delivery vehicles. However, more systematic toxicology studies are needed to confirm the safety and understand the pharmacokinetics of O-dots.
Multifunctional combinational therapeutic applications, including PPT, PDT, photoacoustic therapy, MRI, chemotherapy, and computed tomography can increase therapeutic efficacy, overcome tumor resistance, and eradicate undesirable side effects. However, the practical applications of O-dots that rely on their optical properties, size and shape, surface chemistry will need more work before there is any hope of clinical applications. The ability to combine a number of imaging and therapeutic modalities within a single platform may still allow theranostics to become a reality in the coming years.
Conflicts of interest
There are no conflicts to declare.References
- A. L. Rivas, A. L. Hoogesteijn, A. Antoniades, M. Tomazou, T. Buranda, D. J. Perkins, J. M. Fair, R. Durvasula, F. O. Fasina, G. P. Tegos and M. H. V. Van Regenmortel, Front. Immunol., 2019 DOI:10.3389/fimmu.2019.01258.
- J. Visvader and G. J. Lindeman, Cell Stem Cell, 2012 DOI:10.1016/j.stem.2012.05.007.
- J. Zugazagoitia, C. Guedes, S. Ponce, I. Ferrer, S. Molina-Pinelo and L. Paz-Ares, Clin. Ther., 2016, 38, 1551–1566 CrossRef.
- W. H. Organization, Guide to Cancer Early Diagnosis, 2017 Search PubMed.
- P. Bhatkoti and M. Paul, IEEE Xplore, 2016 DOI:10.1109/IVCNZ.2016.7804459.
- S. Kaur, M. J. Baine, M. Jain, A. R. Sasson and S. K. Batra, Biomarkers Med., 2012, 6, 597–612 CrossRef CAS.
- T. Goh, L. G. Goh, C. H. Ang and C. H. Wong, Br. J. Surg., 2014, 101, e119–e125 CrossRef CAS.
- G. Chen, H. Qiu, P. N. Prasad and X. Chen, Chem. Rev., 2014, 114, 5161–5214 CrossRef CAS.
- T. Peng, Z. Deng, J. He, Y. Li, Y. Tan, Y. Peng, X. Wang and W. Tan, Coord. Chem. Rev., 2020, 403 DOI:10.1016/j.ccr.2019.213080.
- N. Gupta, D. Rai, A. Jangid and H. Kulhari, Methods Microbiol., 2019, 46, 143–172 CAS.
- X. Le Hu, N. Kwon, K. C. Yan, A. C. Sedgwick, G. R. Chen, X. P. He, T. D. James and J. Yoon, Adv. Funct. Mater., 2020, 30 DOI:10.1002/adfm.201907906.
- Y. Zhang, K. Huang, J. Lin and P. Huang, Biomater. Sci., 2019 10.1039/C8BM00642C.
- B. Katzung, S. B. Masters, A. J. Trevor, Basic and clinical pharmacology, Mc Grow-Hill Medical, 12th edn, 2012 Search PubMed.
- E. Butcher, E. Berg and E. J. Kunkel, Nat. Biotechnol., 2004, 22, 1253–1259 CrossRef CAS.
- R. Parikh, A. Mathai, S. Parikh, G. C. Sekhar and R. Thomas, Indian J. Ophthalmol., 2008, 56, 45–50 CrossRef.
- M. Rovaris, C. Confavreux, R. Furlan, L. Kappos, G. Comi and M. Filippi, Lancet Neurol., 2006, 5, 343–354 CrossRef.
- X. Chen, S. S. Gambhir and J. Cheon, Acc. Chem. Res., 2011, 44, 841 CrossRef CAS.
- J. V. Jokerst and S. S. Gambhir, Acc. Chem. Res., 2011, 44, 1050–1060 CrossRef CAS.
- M. F. Kircher, U. Mahmood, R. S. King, R. Weissleder and L. Josephson, Cancer Res., 2003, 63, 8122–8125 CAS.
- D. McDonald and P. L. Choyke, Nat. Med., 2003, 9, 713–725 CrossRef CAS.
- J.-C. G. Bünzli, Chem. Rev., 2010, 110, 2729–2755 CrossRef.
- J.-C. G. Buenzli and S. V. Eliseeva, Chem. Sci., 2013, 4, 1939–1949 RSC.
- A. Malek, O. Merkel, L. Fink, F. Czubayko, T. Kissel and A. Aigner, Toxicol. Appl. Pharmacol., 2009, 236, 97–108 CrossRef CAS.
- M. E. Davis, J. E. Zuckerman, C. H. J. Choi, D. Seligson, A. Tolcher, C. A. Alabi, Y. Yen, J. D. Heidel and A. Ribas, Nature, 2010, 464, 1067 CrossRef CAS.
- G. B. Jacobson, E. Gonzalez-Gonzalez, R. Spitler, R. Shinde, D. Leake, R. L. Kaspar, C. H. Contag and R. N. Zare, J. Pharm. Sci., 2010, 99, 4261–4266 CrossRef CAS.
- D. E. Dolmans, D. Fukumura and R. K. Jain, Nat. Rev. Cancer, 2003, 3, 380 CrossRef CAS.
- P. K. Jain, X. Huang, I. H. El-Sayed and M. A. El-Sayed, Acc. Chem. Res., 2008, 41, 1578–1586 CrossRef CAS.
- J.-H. Lee, Y.-M. Huh, Y. Jun, J. Seo, J. Jang, H.-T. Song, S. Kim, E.-J. Cho, H.-G. Yoon and J.-S. Suh, Nat. Med., 2007, 13, 95 CrossRef CAS.
- J. Xie, S. Lee and X. Chen, Adv. Drug Delivery Rev., 2010, 62, 1064–1079 CrossRef CAS.
- X. Gao, Y. Cui, R. M. Levenson, L. W. K. Chung and S. Nie, Nat. Biotechnol., 2004, 22, 969 CrossRef CAS.
- I. L. Medintz, H. T. Uyeda, E. R. Goldman and H. Mattoussi, Nat. Mater., 2005, 4, 435 CrossRef CAS.
- K. Welsher, Z. Liu, S. P. Sherlock, J. T. Robinson, Z. Chen, D. Daranciang and H. Dai, Nat. Nanotechnol., 2009, 4, 773 CrossRef CAS.
- P. N. Prasad, Introduction to biophotonics, John Wiley & Sons, 2004 Search PubMed.
- P. N. Prasad, Nanophotonics, John Wiley & Sons, 2004 Search PubMed.
- P. N. Prasad, Introduction to nanomedicine and nanobioengineering, John Wiley & Sons, 2012, vol. 7 Search PubMed.
- A. Schroeder, D. A. Heller, M. M. Winslow, J. E. Dahlman, G. W. Pratt, R. Langer, T. Jacks and D. G. Anderson, Nat. Rev. Cancer, 2012, 12, 39 CrossRef CAS.
- A. S. Thakor and S. S. Gambhir, Ca-Cancer J. Clin., 2013, 63, 395–418 CrossRef.
- C. H. Lee, R. Rajendran, M.-S. Jeong, H. Y. Ko, J. Y. Joo, S. Cho, Y. W. Chang and S. Kim, Chem. Commun., 2013, 49, 6543–6545 RSC.
- A. De La Zerda, C. Zavaleta, S. Keren, S. Vaithilingam, S. Bodapati, Z. Liu, J. Levi, B. R. Smith, T.-J. Ma and O. Oralkan, Nat. Nanotechnol., 2008, 3, 557 CrossRef CAS.
- J.-W. Kim, E. I. Galanzha, E. V. Shashkov, H.-M. Moon and V. P. Zharov, Nat. Nanotechnol., 2009, 4, 688 CrossRef CAS.
- Z. Liu, X. Li, S. M. Tabakman, K. Jiang, S. Fan and H. Dai, J. Am. Chem. Soc., 2008, 130, 13540–13541 CrossRef CAS.
- Z. Liu, C. Davis, W. Cai, L. He, X. Chen and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 1410–1415 CrossRef CAS.
- S. Y. Lim, W. Shen and Z. Gao, Chem. Soc. Rev., 2015, 44, 362–381 RSC.
- P. Miao, K. Han, Y. Tang, B. Wang, T. Lin and W. Cheng, Nanoscale, 2015, 7, 1586–1595 RSC.
- J. Zhang and S. H. Yu, Mater. Today, 2016, 19, 382–393 CrossRef CAS.
- S. Zhu, Q. Meng, L. Wang, J. Zhang, Y. Song, H. Jin, K. Zhang, H. Sun, H. Wang and B. Yang, Angew. Chem., 2013, 125, 4045–4049 CrossRef.
- L. Cao, X. Wang, M. J. Meziani, F. Lu, H. Wang, P. G. Luo, Y. Lin, B. A. Harruff, L. M. Veca and D. Murray, J. Am. Chem. Soc., 2007, 129, 11318–11319 CrossRef CAS.
- C. Ding, A. Zhu and Y. Tian, Acc. Chem. Res., 2013, 47, 20–30 CrossRef.
- B. Kong, A. Zhu, C. Ding, X. Zhao, B. Li and Y. Tian, Adv. Mater., 2012, 24, 5844–5848 CAS.
- X. Sun, Z. Liu, K. Welsher, J. T. Robinson, A. Goodwin, S. Zaric and H. Dai, Nano Res., 2008, 1, 203–212 CrossRef CAS.
- J. Tang, B. Kong, H. Wu, M. Xu, Y. Wang, Y. Wang, D. Zhao and G. Zheng, Adv. Mater., 2013, 25, 6569–6574 CrossRef CAS.
- M. Bacon, S. J. Bradley and T. Nann, Part. Part. Syst. Charact., 2014, 31, 415–428 CrossRef CAS.
- H. Sun, L. Wu, W. Wei and X. Qu, Mater. Today, 2013, 16, 433–442 CrossRef CAS.
- Z. Fan, S. Li, F. Yuan and L. Fan, RSC Adv., 2015, 5, 19773–19789 RSC.
- P. Tian, L. Tang, K. Teng and S. Lau, Mater. Today Chem., 2018, 10, 221–258 CrossRef CAS.
- M. Li, T. Chen, J. J. Gooding and J. Liu, ACS Sens., 2019, 4, 1732–1748 CrossRef CAS.
- S. Campuzano, P. Yáñez-Sedeño and J. M. Pingarrón, Nanomaterials, 2019 DOI:10.3390/nano9040634.
- M. J. Molaei, RSC Adv., 2019, 9, 6460–6481 RSC.
- K. Nekoueian, M. Amiri, M. Sillanpää, F. Marken, R. Boukherroub and S. Szunerits, Chem. Soc. Rev., 2019, 48, 4281–4316 RSC.
- X. Zhao, W. Gao, H. Zhang, X. Qiu and Y. Luo, Modern Trends in Analysis, 2020, 493–505 CAS.
- R. Wang, K. Lu, Z. Tang and Y Xu, J. Mater. Chem. A, 2017, 5, 3717–3734 RSC.
- N. M. Zholobak, A. L. Popov, A. B. Shcherbakov, N. R. Popova, M. M. Guzyk, V. P. Antonovich, A. V. Yegorova, Y. V. Scrypynets, I. I. Leonenko and A. Y. Baranchikov, Beilstein J. Nanotechnol., 2016, 7, 1905–1917 CrossRef CAS.
- M. O. Dekaliuk, O. Viagin, Y. V. Malyukin and A. P. Demchenko, Phys. Chem. Chem. Phys., 2014, 16, 16075–16084 RSC.
- R. Yan, H. Wu, Q. Zheng, J. Wang, J. Huang, K. Ding, Q. Guo and J. Wang, RSC Adv., 2013, 4, 23097–23106 RSC.
- R. Yan, H. Wu, Q. Zheng, J. Wang, J. Huang, K. Ding, Q. Guo and J. Wang, RSC Adv., 2014, 4, 23097–23106 RSC.
- A. Barras, Q. Pagneux, F. Sane, Q. Wang, R. Boukherroub, D. Hober and S. Szunerits, ACS Appl. Mater. Interfaces, 2016, 8, 9004–9013 CrossRef CAS.
- N. Wang, Y. Wang, T. Guo, T. Yang, M. Chen and J. Wang, Biosens. Bioelectron., 2016, 85, 68–75 CrossRef CAS.
- K. Nekoueian, M. Amiri, M. Sillanpää, F. Marken, R. Boukherroub and S. Szunerits, Chem. Soc. Rev., 2019, 48, 4281–4316 RSC.
- Z. L. Wu, M. X. Gao, T. T. Wang, X. Y. Wan, L. L. Zheng and C. Z. Huang, Nanoscale, 2014, 6, 3868–3874 RSC.
- X. Tan, Y. Li, X. Li, S. Zhou, L. Fan and S. Yang, Chem. Commun., 2015, 51, 2544–2546 RSC.
- S. Qu, X. Wang, Q. Lu, X. Liu and L. Wang, Angew. Chem., 2012, 124, 12381–12384 CrossRef.
- S. Zhu, S. Tang, J. Zhang and B. Yang, Chem. Commun., 2012, 48, 4527–4539 RSC.
- Z. Fan, Y. Li, X. Li, L. Fan, S. Zhou, D. Fang and S. Yang, Carbon, 2014, 70, 149–156 CrossRef CAS.
- D. Pan, L. Guo, J. Zhang, C. Xi, Q. Xue, H. Huang, J. Li, Z. Zhang, W. Yu and Z. Chen, J. Mater. Chem., 2012, 22, 3314–3318 RSC.
- L. Tang, R. Ji, X. Cao, J. Lin, H. Jiang, X. Li, K. S. Teng, C. M. Luk, S. Zeng and J. Hao, ACS Nano, 2012, 6, 5102–5110 CrossRef CAS.
- H. Qin, T. Gong, Y. Jin, Y. Cho, C. Shin, C. Lee and T. Kim, Carbon, 2015, 94, 181–188 CrossRef CAS.
- H. Tetsuka, R. Asahi, A. Nagoya, K. Okamoto, I. Tajima, R. Ohta and A. Okamoto, Adv. Mater., 2012, 24, 5333–5338 CrossRef CAS.
- M. D. Nurunnabi, Z. Khatun, G. R. Reeck, D. Y. Lee and Y. Lee, Chem. Commun., 2013, 49, 5079–5081 RSC.
- F. Yuan, L. Ding, Y. Li, X. Li, L. Fan, S. Zhou, D. Fang and S. Yang, Nanoscale, 2015, 7, 11727–11733 RSC.
- Y. Li, Y. Zhao, H. Cheng, Y. Hu, G. Shi, L. Dai and L. Qu, J. Am. Chem. Soc., 2011, 134, 15–18 CrossRef.
- Y. Dong, H. Pang, S. Ren, C. Chen, Y. Chi and T. Yu, Carbon, 2013, 64, 245–251 CrossRef CAS.
- J. Ge, M. Lan, B. Zhou, W. Liu, L. Guo, H. Wang, Q. Jia, G. Niu, X. Huang and H. Zhou, Nat. Commun., 2014, 5, 4596 CrossRef CAS.
- X. Li, H. Wang, Y. Shimizu, A. Pyatenko, K. Kawaguchi and N. Koshizaki, Chem. Commun., 2010, 47, 932–934 RSC.
- Y. H. Yuan, Z. X. Liu, R. S. Li, H. Y. Zou, M. Lin, H. Liu and C. Z. Huang, Nanoscale, 2016, 8, 6770–6776 RSC.
- J. Zong, X. Yang, A. Trinchi, S. Hardin, I. Cole, Y. Zhu, C. Li, T. Muster and G. Wei, Nanoscale, 2013, 5, 11200–11206 RSC.
- L. Deng, L. Liu, C. Zhu, D. Li and S. Dong, Chem. Commun., 2013, 49, 2503–2505 RSC.
- Y.-P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang and H. Wang, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS.
- Z. L. Wu, Z. X. Liu and Y. H. Yuan, J. Mater. Chem. B, 2017, 5, 3794–3809 RSC.
- L. Shen, L. Zhang, M. Chen, X. Chen and J. Wang, Carbon, 2013, 55, 343–349 CrossRef CAS.
- X. Jia, J. Li and E. Wang, Nanoscale, 2012, 4, 5572–5575 RSC.
- L. Zhou, Z. Li, Z. Liu, J. Ren and X. Qu, Langmuir, 2013, 29, 6396–6403 CrossRef CAS.
- M. A. Sk, A. Ananthanarayanan, L. Huang, K. H. Lim and P. Chen, J. Mater. Chem. C, 2014, 2, 6954–6960 RSC.
- L. I. Cao, M. J. Meziani, S. Sahu and Y.-P. Sun, Acc. Chem. Res., 2012, 46, 171–180 CrossRef.
- H. Li, Z. Kang, Y. Liu and S.-T. Lee, J. Mater. Chem., 2012, 22, 24230–24253 RSC.
- Y. Zhang, Y. Zhang, G. Hong, W. He, K. Zhou, K. Yang, F. Li, G. Chen, Z. Liu and H. Dai, Biomaterials, 2013, 34, 3639–3646 CrossRef CAS.
- A. Nel, T. Xia, H. Meng, X. Wang, S. Lin, Z. Ji and H. Zhang, Acc. Chem. Res., 2012, 46, 607–621 CrossRef.
- Y. Zhang, G. Hong, Y. Zhang, G. Chen, F. Li, H. Dai and Q. Wang, ACS Nano, 2012, 6, 3695–3702 CrossRef CAS.
- M. Havrdova, K. Hola, J. Skopalik, K. Tomankova, M. Petr, K. Cepe, K. Polakova, J. Tucek, A. B. Bourlinos and R. Zboril, Carbon, 2016, 99, 238–248 CrossRef CAS.
- J. Yan, S. Hou, Y. Yu, Y. Qiao, T. Xiao, Y. Mei, Z. Zhang, B. Wang, C. C. Huang, C. H. Lin and G. Suo, Colloids Surf., B, 2018, 1171, 241–249 CrossRef.
- Y. Kang, Y. Li, Y. Fang, Y. Xu, X. Wei and X. Yin, Sci. Rep., 2015, 5 DOI:10.1038/srep11835.
- S. Li, Z. Guo, R. Feng, Y. Zhang, W. Xue and Z. Liu, RSC Adv., 2017, 7, 4975–4982 RSC.
- X. Wei, L. Li, J. Liu, L. Yu, H. Li, F. Cheng, X. Yi, J. He and B. Li, ACS Appl. Mater. Interfaces, 2019, 11, 9832–9840 CrossRef CAS.
- X. W. Hua, Y. W. Bao, Z. Chen and F. G. Wu, Nanoscale, 2017, 9, 10948–10960 RSC.
- D. Wang, L. Zhu, J.-F. Chen and L. Dai, Nanoscale, 2015, 7, 9894 RSC.
- Z. M. Markovic, B. Z. Ristic, K. M. Arsikin, D. G. Klisic, L. M. Harhaji-Trajkovic, B. M. Todorovic-Markovic, D. P. Kepic, T. K. Kravic-Stevovic, S. P. Jovanovic and M. M. Milenkovic, Biomaterials, 2012, 33, 7084–7092 CrossRef CAS.
- Y. Yang, C. Xuejing and Y. Xin, Artic. J. Environ. Sci., 2019, 77, 198–209 CrossRef.
- D. Jiang, Y. Chen, N. Li, W. Li, Z. Wang, J. Zhu, H. Zhang, B. Liu and S. Xu, PLoS One, 2015 DOI:10.1371/journal.pone.0144906.
- Z. Fan, Y. Li, X. Li, L. Fan, S. Zhou, D. Fang and S. Yang, Carbon, 2014, 70, 149–156 CrossRef CAS.
- Y. Chong, Y. Ma, H. Shen, X. Tu, X. Zhou, J. Xu, J. Dai, S. Fan and Z. Zhang, Biomaterials, 2014, 35, 5041–5048 CrossRef CAS.
- H. Liu, T. Ye and C. Mao, Angew. Chem., Int. Ed., 2007, 46, 6473–6475 CrossRef CAS.
- D. Reyes, M. Camacho, M. Camacho, M. Mayorga, D. Weathers, G. Salamo, Z. Wang and A. Neogi, Nanoscale Res. Lett., 2016, 11 DOI:10.1186/s11671-016-1638-8.
- C. Hu, Y. Liu, Y. Yang, J. Cui, Z. Huang, Y. Wang, L. Yang, H. Wang, Y. Xiao and J. Rong, J. Mater. Chem. B, 2013, 1(1), 39–42 CAS.
- D. B. Shinde and V. K. Pillai, Chem.–Eur. J., 2012, 18, 12522–12528 CrossRef CAS.
- C. Wang, W. Wu, A. Periasamy and H. Chang, Green Chem., 2014, 16, 2509–2514 RSC.
- S. Dey, A. Govindaraj, K. Biswas and C. Rao, Chem. Phys. Lett., 2014, 596, 203–208 CrossRef.
- L. Tang, R. Ji, X. Cao, J. Lin, H. Jiang, X. Li, K. S. Teng, C. M. Luk, S. Zeng, J. Hao and S. P. Lau, ACS Nano, 2012, 6, 5102–5110 CrossRef CAS.
- J. Wang, C. F. Wang and S. Chen, Angew. Chem., Int. Ed., 2012, 51, 9297–9301 CrossRef CAS.
- J. Zhou, C. Booker, R. Li, X. Zhou, T. K. Sham, X. Sun and Z. Ding, J. Am. Chem. Soc., 2007, 129, 744–745 CrossRef CAS.
- Q. L. Zhao, Z. L. Zhang, B. H. Huang, J. Peng, M. Zhang and D. W. Pang, Chem. Commun., 2008, 41, 5116–5118 RSC.
- H. Li, X. He, Z. Kang, H. Huang, Y. Liu, J. Liu, S. Lian, C. H. A. Tsang, X. Yang and S. T. Lee, Angew. Chem., Int. Ed., 2010, 49, 4430–4434 CrossRef CAS.
- N. Arora and N. Sharma, Diamond Relat. Mater., 2014, 50, 135–150 CrossRef CAS.
- H. Liu, T. Ye and C. Mao, Angew. Chem., Int. Ed., 2007, 46, 6473–6475 CrossRef CAS.
- G. K. Yogesh, E. P. Shuaib and D. Sastikumar, Mater. Res. Bull., 2017, 91, 220–226 CrossRef CAS.
- N. Tarasenka, A. Stupak, N. Tarasenko, S. Chakrabarti and D. Mariotti, ChemPhysChem, 2017, 18, 1074–1083 CrossRef CAS.
- A. B. Bourlinos, A. Stassinopoulos, D. Anglos, R. Zboril, M. Karakassides and E. P. Giannelis, Small, 2008, 4, 455–458 CrossRef CAS.
- P.-C. Hsu, Z.-Y. Shih, C.-H. Lee and H.-T. Chang, Green Chem., 2012, 14, 917–920 RSC.
- C.-W. Lai, Y.-H. Hsiao, Y.-K. Peng and P.-T. Chou, J. Mater. Chem., 2012, 22, 14403–14409 RSC.
- X. Wu, F. Tian, W. Wang, J. Chen, M. Wu and J. X. Zhao, J. Mater. Chem. C, 2013, 1, 4676–4684 RSC.
- Y. Dong, J. Shao, C. Chen, H. Li, R. Wang, Y. Chi, X. Lin and G. Chen, Carbon, 2012, 50, 4738–4743 CrossRef CAS.
- J. Ju and W. Chen, Biosens. Bioelectron., 2014, 58, 219–225 CrossRef CAS.
- Y. Deng, D. Zhao, X. Chen, F. Wang, H. Song and D. Shen, Chem. Commun., 2013, 49, 5751–5753 RSC.
- J. Wang, C. Wang and S. Chen, Angew. Chem., 2012, 124, 9431–9435 CrossRef.
- J. Kim and J. S. Suh, ACS Nano, 2014, 8, 4190–4196 CrossRef CAS.
- J.-Y. Yin, H.-J. Liu, S. Jiang, Y. Chen and Y. Yao, ACS Macro Lett., 2013, 2, 1033–1037 CrossRef CAS.
- X. T. Zheng, A. Ananthanarayanan, K. Q. Luo and P. Chen, Small, 2015, 11, 1620–1636 CrossRef CAS.
- M. Larhed, C. Moberg and A. Hallberg, Acc. Chem. Res., 2002, 35, 717–727 CrossRef CAS.
- F. Li, C. Li, X. Liu, Y. Chen, T. Bai, L. Wang, Z. Shi and S. Feng, Chem.–Eur. J., 2012, 18, 11641–11646 CrossRef CAS.
- H. Zhu, X. Wang, Y. Li, Z. Wang, F. Yang and X. Yang, Chem. Commun., 2009, 5118–5120 RSC.
- H. Liu, Z. He, L.-P. Jiang and J.-J. Zhu, ACS Appl. Mater. Interfaces, 2015, 7, 4913–4920 CrossRef CAS.
- Z. Qian, J. Ma, X. Shan, L. Shao, J. Zhou, J. Chen and H. Feng, RSC Adv., 2013, 3, 14571–14579 RSC.
- X. T. Zheng, A. Ananthanarayanan, K. Q. Luo and P. Chen, Small, 2015, 11, 1620–1636 CrossRef CAS.
- J. Feng, H. Dong, L. Yu and L. Dong, J. Mater. Chem. C, 2017, 5, 5984–5993 RSC.
- S. Zhu, J. Zhang, S. Tang, C. Qiao, L. Wang, H. Wang, X. Liu, B. Li, Y. Li and W. Yu, Adv. Funct. Mater., 2012, 22, 4732–4740 CrossRef CAS.
- S. Hu, R. Tian, L. Wu, Q. Zhao, J. Yang, J. Liu and S. Cao, Chem.–Asian J., 2013, 8, 1035–1041 CrossRef CAS.
- H. Sun, L. Wu, N. Gao, J. Ren and X. Qu, ACS Appl. Mater. Interfaces, 2013, 5, 1174–1179 CrossRef CAS.
- H. Jiang, F. Chen, M. G. Lagally and F. S. Denes, Langmuir, 2009, 26, 1991–1995 CrossRef.
- C. Hu, Y. Liu, Y. Yang, J. Cui, Z. Huang, Y. Wang, L. Yang, H. Wang, Y. Xiao and J. Rong, J. Mater. Chem. B, 2013, 1(1), 39–42 RSC.
- Z. Qian, X. Shan, L. Chai, J. Ma, J. Chen and H. Feng, ACS Appl. Mater. Interfaces, 2014, 6, 6797–6805 CrossRef CAS.
- K. S. Prasad, R. Pallela, D. Kim and Y. Shim, Part. Part. Syst. Charact., 2013, 30, 557–564 CrossRef CAS.
- D. Qu, M. Zheng, P. Du, Y. Zhou, L. Zhang, D. Li, H. Tan, Z. Zhao, Z. Xie and Z. Sun, Nanoscale, 2013, 5, 12272–12277 RSC.
- Y. Dong, H. Pang, H. Bin Yang, C. Guo, J. Shao, Y. Chi, C. M. Li and T. Yu, Angew. Chem., Int. Ed., 2013, 52, 7800–7804 CrossRef CAS.
- M. Zheng, S. Liu, J. Li, D. Qu, H. Zhao, X. Guan, X. Hu, Z. Xie, X. Jing and Z. Sun, Adv. Mater., 2014, 26, 3554–3560 CrossRef CAS.
- X. Zhai, P. Zhang, C. Liu, T. Bai, W. Li, L. Dai and W. Liu, Chem. Commun., 2012, 48, 7955–7957 RSC.
- F. Du, Y. Ming, F. Zeng, C. Yu and S. Wu, Nanotechnology, 2013, 24, 365101 CrossRef.
- L. Cheng, Y. Li, X. Zhai, B. Xu, Z. Cao and W. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 20487–20497 CrossRef CAS.
- D. Mazzier, M. Favaro, S. Agnoli, S. Silvestrini, G. Granozzi, M. Maggini and A. Moretto, Chem. Commun., 2014, 50, 6592–6595 RSC.
- S. Chandra, S. Pradhan, S. Mitra, P. Patra, A. Bhattacharya, P. Pramanik and A. Goswami, Nanoscale, 2014, 6, 3647–3655 RSC.
- Y. Wang, S. Kalytchuk, Y. Zhang, H. Shi, S. V. Kershaw and A. L. Rogach, J. Phys. Chem. Lett., 2014, 5, 1412–1420 CrossRef CAS.
- J. Bian, C. Huang, L. Wang, T. Hung, W. A. Daoud and R. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 4883–4890 CrossRef CAS.
- L. Zhu, X. Cui, J. Wu, Z. Wang, P. Wang, Y. Hou and M. Yang, Anal. Methods, 2014, 6, 4430–4436 RSC.
- Y. Yang, J. Cui, M. Zheng, C. Hu, S. Tan, Y. Xiao, Q. Yang and Y. Liu, Chem. Commun., 2012, 48, 380–382 RSC.
- Z. Ye, R. Tang, H. Wu, B. Wang, M. Tan and J. Yuan, New J. Chem., 2014, 38, 5721–5726 RSC.
- N. Gogoi and D. Chowdhury, J. Mater. Chem. B, 2014, 2, 4089–4099 RSC.
- F. Wang, Z. Xie, B. Zhang, Y. Liu, W. Yang and C. Liu, Nanoscale, 2014, 6, 3818–3823 RSC.
- X. Fan, N. Hua, J. Xu, Z. Wang, H. Jia and C. Wang, RSC Adv., 2014, 4, 16524–16527 RSC.
- W. Liu, C. Li, Y. Ren, X. Sun, W. Pan, Y. Li, J. Wang and W. Wang, J. Mater. Chem. B, 2016, 4, 5772–5788 RSC.
- C. X. Guo, D. Zhao, Q. Zhao, P. Wang and X. Lu, Chem. Commun., 2014, 50, 7318–7321 RSC.
- B. C. M. Martindale, G. A. M. Hutton, C. A. Caputo and E. Reisner, J. Am. Chem. Soc., 2015, 137, 6018–6025 CrossRef CAS.
- Q. Liu, S. Xu, C. Niu, M. Li, D. He, Z. Lu, L. Ma, N. Na, F. Huang, H. Jiang and J. Ouyang, Biosens. Bioelectron., 2015, 64, 119–125 CrossRef CAS.
- M. Wu, Y. Wang, W. Wu, C. Hu, X. Wang, J. Zheng, Z. Li, B. Jiang and J. Qiu, Carbon, 2014, 78, 480–489 CrossRef CAS.
- S. Zhang, Q. Wang, G. Tian and H. Ge, Mater. Lett., 2014, 115, 233–236 CrossRef CAS.
- C. Fowley, A. P. McHale, B. McCaughan, A. Fraix, S. Sortino and J. F. Callan, Chem. Commun., 2015, 51, 81–84 RSC.
- S. Maiti, K. Das and P. K. Das, Chem. Commun., 2013, 49, 8851–8853 RSC.
- Y. Hao, Z. Gan, J. Xu, X. Wu and P. K. Chu, Appl. Surf. Sci., 2014, 311, 490–497 CrossRef CAS.
- K. Das, S. Maiti and P. K. Das, Langmuir, 2014, 30, 2448–2459 CrossRef CAS.
- K. K. R. Datta, O. Kozak, V. Ranc, M. Havrdova, A. B. Bourlinos, K. Šafářová, K. Hola, K. Tomankova, G. Zoppellaro and M. Otyepka, Chem. Commun., 2014, 50, 10782–10785 RSC.
- K. Junka, J. Guo, I. Filpponen, J. Laine and O. J. Rojas, Biomacromolecules, 2014, 15, 876–881 CrossRef CAS.
- A. B. Bourlinos, R. Zbořil, J. Petr, A. Bakandritsos, M. Krysmann and E. P. Giannelis, Chem. Mater., 2011, 24, 6–8 CrossRef.
- X. Liu, N. Zhang, T. Bing and D. Shangguan, Anal. Chem., 2014, 86, 2289–2296 CrossRef CAS.
- W. Kong, H. Wu, Z. Ye, R. Li, T. Xu and B. Zhang, J. Lumin., 2014, 148, 238–242 CrossRef CAS.
- A. Quaranta, S. Carturan, A. Campagnaro, M. Dalla Palma, M. Giarola, N. Daldosso, G. Maggioni and G. Mariotto, Thin Solid Films, 2014, 553, 188–192 CrossRef CAS.
- A. Gupta and C. K. Nandi, Sens. Actuators, B, 2017, 245, 137–145 CrossRef CAS.
- Z. Su, H. Shen, H. Wang, J. Wang, J. Li, G. U. Nienhaus, L. Shang and G. Wei, Adv. Funct. Mater., 2015, 25, 5472–5478 CrossRef CAS.
- F. Chiti and C. M. Dobson, Annu. Rev. Biochem., 2006, 75 DOI:10.1146/annurev.biochem.75.101304.123901.
- M. Li, S. E. Howson, K. Dong, N. Gao, J. Ren, P. Scott and X. Qu, J. Am. Chem. Soc., 2014, 136, 11655–11663 CrossRef CAS.
- C. Glabe, Neurobiol. Aging, 2006, 27, 570–575 CrossRef CAS.
- X. Han, J. Park, W. Wu, A. Malagon, L. Wang, E. Vargas, A. Wikramanayake, K. N. Houk and R. Leblanc, Chem. Sci., 2017, 8, 2003–2009 RSC.
- Y. Song, P. N. Cheng, L. Zhu, E. G. Moore and J. S. Moore, J. Am. Chem. Soc., 2014, 136, 5233–5236 CrossRef CAS.
- S. Palmal, N. R. Jana and N. R. Jana, J. Phys. Chem. C, 2014, 118, 21630–21638 CrossRef CAS.
- J. A. Yang, B. J. Johnson, S. Wu, W. S. Woods, J. M. George and C. J. Murphy, Langmuir, 2013, 29, 4603–4615 CrossRef CAS.
- C. Cabaleiro-Lago, F. Quinlan-Pluck, I. Lynch, S. Lindman, A. M. Minogue, E. Thulin, D. M. Walsh, K. A. Dawson and S. Linse, J. Am. Chem. Soc., 2008, 130, 15437–15443 CrossRef CAS.
- C. Li and R. Mezzenga, Nanoscale, 2013, 5, 6207–6218 RSC.
- S. Li, L. Wang, C. C. Chusuei, V. M. Suarez, P. L. Blackwelder, M. Micic, J. Orbulescu and R. M. Leblanc, Chem. Mater., 2015, 27, 1764–1771 CrossRef CAS.
- S. Li, Z. Peng and R. M. Leblanc, Anal. Chem., 2015, 87, 6455–6459 CrossRef CAS.
- C. Frieden, Protein Sci., 2007, 16, 2334–2344 CrossRef CAS.
- S. Zhu, T. Lu, G. Zhang, J. Xu, Y. Song, Y. Li, L. Wang, B. Yang and F. Li, J. Mater. Chem. B, 2016, 4, 4913–4921 RSC.
- S. Radic, T. Davis, P. Ke and F. Ding, RSC Adv., 2015, 5, 105489–105498 RSC.
- M. Yousaf, H. Huang, P. Li, C. Wang and Y. Yang, ACS Chem. Neurosci., 2017, 8, 1368–1377 CrossRef CAS.
- G. Rusciano, A. De Luca, G. Pesce and A. Sasso, Carbon, 2009, 47, 2950–2957 CrossRef CAS.
- S. Nandi, R. Malishev, K. P. Kootery, S. Kolusheva and R. Jelinek, Chem. Commun., 2014, 50, 10299–10302 RSC.
- S. Nandi, R. Malishev, S. K. Bhunia, S. Kolusheva, J. Jopp and R. Jelinek, Biophys. J., 2016, 110, 2016–2025 CrossRef CAS.
- S. Nandi, M. Ritenberg and R. Jelinek, Analyst, 2015, 140, 4232–4237 RSC.
- T. K. Mandal and N. Parvin, J. Biomed. Nanotechnol., 2011, 7, 846–848 CrossRef CAS.
- S. K. Misra, I. Srivastava, I. Tripathi, E. Daza, F. Ostadhossein and D. Pan, J. Am. Chem. Soc., 2017, 139, 1746–1749 CrossRef CAS.
- M. J. Meziani, X. Dong, L. Zhu, L. P. Jones, G. E. LeCroy, F. Yang, S. Wang, P. Wang, Y. Zhao and L. Yang, ACS Appl. Mater. Interfaces, 2016, 8, 10761–10766 CrossRef CAS.
- W. Bing, H. Sun, Z. Yan, J. Ren and X. Qu, Small, 2016, 12, 4713–4718 CrossRef CAS.
- N. Zhou, S. Zhu, S. Maharjan, Z. Hao, Y. Song, X. Zhao, Y. Jiang, B. Yang and L. Lu, RSC Adv., 2014, 4, 62086–62095 CAS.
- T. Wang, S. Zhu and X. Jiang, Toxicol. Res., 2015, 4, 885–894 CrossRef CAS.
- A. H. Loo, Z. Sofer, D. Bouša, P. Ulbrich, A. Bonanni and M. Pumera, ACS Appl. Mater. Interfaces, 2016, 8, 1951–1957 CrossRef CAS.
- H. Sun, J. Ren and X. Qu, Acc. Chem. Res., 2016, 49, 461–470 CrossRef CAS.
- L. Feng, A. Zhao, J. Ren and X. Qu, Nucleic Acids Res., 2013, 41, 7987–7996 CrossRef CAS.
- V. Milosavljevic, H. V. Nguyen, P. Michalek, A. Moulick, P. Kopel, R. Kizek and V. Adam, Chem. Pap., 2015, 69, 192–201 CAS.
- A. Rich and S. Zhang, Nat. Rev. Genet., 2003, 4, 566–572 CrossRef CAS.
- P. Pierrat, R. Wang, D. Kereselidze, M. Lux, P. Didier, A. Kichler, F. Pons and L. Lebeau, Biomaterials, 2015, 290–302 CrossRef CAS.
- Z. Peng, X. Han, S. Li, A. O. Al-Youbi, A. S. Bashammakh, M. S. El-Shahawi and R. M. Leblanc, Coord. Chem. Rev., 2017, 343, 256–277 CrossRef CAS.
- L. Wang, X. Wang, A. Bhirde, J. Cao, Y. Zeng, X. Huang, Y. Sun, G. Liu and X. Chen, Adv. Healthcare Mater., 2014, 3, 1203–1209 CrossRef CAS.
- X. Yang, Y. Wang, X. Shen, C. Su, J. Yang, M. Piao, F. Jia, G. Gao, L. Zhang and Q. Lin, J. Colloid Interface Sci., 2017, 492, 1–7 CrossRef CAS.
- L. Lu, L. Guo, X. Wang, T. Kang and S. Cheng, RSC Adv., 2016, 6, 33072–33075 RSC.
- S. Lu, S. Guo, P. Xu, X. Li, Y. Zhao, W. Gu and M. Xue, Int. J. Nanomed., 2016, 11, 6325–6336 CrossRef CAS.
- E. Barbu, J. Tsibouklis and D. C. Górecki, Expert Opin. Drug Delivery, 2009 DOI:10.1517/17425240902939143.
- S. Bhaskar, F. Tian and T. Stoeger, Toxicology, 2010, 7 DOI:10.1186/1743-8977-7-3.
- H. Maeda, J. Wu, T. Sawa, Y. Matsumura and K. Hori, J. Control. Release, 2000, 65, 271–284 CrossRef CAS.
- K. K. Pulicherla and M. K. Verma, AAPS PharmSciTech, 2015, 16, 223–233 CrossRef CAS.
- A. R. Jones and E. V. Shusta, Pharm. Res., 2007, 24, 1759–1771 CrossRef CAS.
- S. Bhunia, A. Saha, A. Maity, S. Ray and N. Jana, Sci. Rep., 2013, 3 DOI:10.1038/srep01473.
- M. Zheng, S. Ruan, S. Liu, T. Sun, D. Qu, H. Zhao, Z. Xie, H. Gao, X. Jing and Z. Sun, ACS Nano, 2015, 9, 11455–11461 CrossRef CAS.
- S. Li, Z. Peng, A. M. Othman and R. M. Graham, Nanoscale, 2016, 8 10.1039/C6NR05055G.
- D. T. Wiley, P. Webster, A. Gale and M. E. Davis, Proc. Natl. Acad. Sci. U. S. A., 2013 DOI:10.1073/pnas.1307152110.
- S. Dixit, T. Novak, K. Miller, Y. Zhu, M. E. Kenney and A.-M. Broome, Nanoscale, 2014, 7, 1782–1790 RSC.
- H. Xie, D. Ghoorah, Y. Shang, H. Shi, F. Liu, X. Yang, H. Xu and W. Jiang, PLoS One, 2012, 7(5), e37376 CrossRef.
- S. Li, Z. Peng, J. Dallman, J. Baker, A. Othman. P. Blackwelder and R. Leblanc, Colloids Surf., B, 2016, 145, 251–256 CrossRef CAS.
- A. Kalueff, A. Stewart and R. Gerlai, Trends Pharmacol. Sci., 2014, 35, 63–75 CrossRef CAS.
- Z. Peng, E. Miyanji, Y. Zhou, J. Pardo, S. Hettiarachchi, S. Li, P. Blackwelder, I. Skromne and R. M. Leblanc, Nanoscale, 2017, 9, 17533–17543 RSC.
- X. Han, Z. Jing, W. Wu, B. Zou, Z. Peng, P. Ren, A. Wikramanayake, Z. Lu and R. M. Leblanc, Nanoscale, 2017, 9, 12862–12866 RSC.
- K. J. Mintz, G. Mercado, Y. Zhou, Y. Ji, S. D. Hettiarachchi, P. Y. Liyanage, R. R. Pandey, C. C. Chusuei, J. Dallman and R. M. Leblanc, Colloids Surf., B, 2019, 176, 488–493 CrossRef CAS.
- D. Kim, J. Yoo, H. Hwang, J. Lee and S. H. Lee, Nat. Nanotechnol., 2018, 13, 812–818 CrossRef CAS.
- K. L. Li, Y. H. Zhang, R. Xing, Y. F. Zhou, X. D. Chen, H. Wang, B. Song, Y. H. Sima, Y. He and S. Q. Xu, RSC Adv., 2017, 7, 50317–50327 RSC.
- C. Sarkar, A. R. Chowdhuri, A. Kumar, D. Laha, S. Garai, J. Chakraborty and S. K. Sahu, Carbohydr. Polym., 2018, 181, 710–718 CrossRef CAS.
- M. Zhang, Y. Zhao, J. Cheng, X. Liu, Y. Wang, X. Yan, Y. Zhang, F. Lu, Q. Wang and H. Qu, Artif. Cells, Nanomed., Biotechnol., 2017, 1–10 Search PubMed.
- X. Guo, L. Xu, L. Zhang, H. Wang, X. Wang, X. Liu, J. Yao and A. Hao, J. Lumin., 2018, 196, 100–110 CrossRef CAS.
- Y. Yuan, B. Guo, L. Hao, N. Liu, Y. Lin, W. Guo, X. Li and B. Gu, Colloids Surf., B, 2017, 159, 349–359 CrossRef CAS.
- Q. Wang, X. Huang, Y. Long, X. Wang, H. Zhang and R. Zhu, Carbon, 2013, 59, 192–199 CrossRef CAS.
- C.-L. Li, C.-M. Ou, C.-C. Huang, W.-C. Wu, Y.-P. Chen, T.-E. Lin, L.-C. Ho, C.-W. Wang, C.-C. Shih, H.-C. Zhou, Y.-C. Lee, W.-F. Tzeng, T.-J. Chiou and S.-T. Chu, J. Mater. Chem. B, 2014, 2, 4564–4571 RSC.
- Y. Yuan, B. Guo, L. Hao, N. Liu, Y. Lin, W. Guo, X. Lee and B. Gu, Colloids Surf., B, 2017, 159, 349–359 CrossRef CAS.
- F. Khodadadei, S. Safarian and N. Ghanbari, Mater. Sci. Eng. C, 2017, 79, 280–285 CrossRef CAS.
- S. Some, A. Gwon, E. Hwang, G. Bahn, Y. Yoon, Y. Kim and S. Kim, Sci. Rep., 2014, 4 DOI:10.1038/srep06314.
- C. Rao, S. Khan, N. C. Verma and C. K. Nandi, ChemBioChem, 2017, 18, 2385–2389 CrossRef CAS.
- T. García-Mendiola, I. Bravo, J. M. López-Moreno, F. Pariente, R. Wannemacher, K. Weber, J. Popp and E. Lorenzo, Sens. Actuators, B, 2018, 256, 226–233 CrossRef.
- W. Zhang, L. Shi, Y. Liu, X. Meng, H. Xu, Y. Xu, B. Liu, X. Fang, H.-B. Li and T. Ding, RSC Adv., 2017, 7, 20345–20353 RSC.
- N. Gao, W. Yang, H. Nie, Y. Gong, J. Jing, L. Gao and X. Zhang, Biosens. Bioelectron., 2017, 96, 300–307 CrossRef CAS.
- D. Lei, W. Yang, Y. Gong, J. Jing, H. Nie, B. Yu and X. Zhang, Sens. Actuators, B, 2016, 230, 714–720 CrossRef CAS.
- J. Zhang, X. Zhao, M. Xian, C. Dong and S. Shuang, Talanta, 2018, 183, 39–47 CrossRef CAS.
- A. Zhao, Z. Chen, C. Zhao, N. Gao, J. Ren and X. Qu, Carbon, 2015, 85, 309–327 CrossRef CAS.
- M. X. Gao, L. Yang, Y. Zheng, X. X. Yang, H. Y. Zou, J. Han, Z. X. Liu, Y. F. Li and C. Z. Huang, Chem.–Eur. J., 2017, 23, 2171–2178 CrossRef CAS.
- H. Motaghi, M. A. Mehrgardi and P. Bouvet, Sci. Rep., 2017, 7, 10513 CrossRef.
- D. Musumeci, C. Platella, C. Riccardi, F. Moccia and D. Montesarchio, Cancer, 2017, 9, 174 CrossRef.
- L. Yang, Z. Wang, J. Wang, W. Jiang, X. Jiang, Z. Bai, Y. He, J. Jiang, D. Wang and L. Yang, Nanoscale, 2016, 8, 6801–6809 RSC.
- J. Pardo, Z. Peng and R. M. Leblanc, Molecules, 2018, 23, 378 CrossRef.
- T. Feng, X. Ai, H. Ong and Y. Zhao, ACS Appl. Mater. Interfaces, 2016, 8, 18732–18740 CrossRef CAS.
- X.-W. Hua, Y.-W. Bao, H.-Y. Wang, Z. Chen and F.-G. Wu, Nanoscale, 2017, 9, 2150–2161 RSC.
- J. S. Sidhu, A. Singh, N. Garg, N. Kaur and N. Singh, Analyst, 2018, 143, 1853–1861 RSC.
- S. Wang, C. Li, M. Qian, H. Jiang, W. Shi, J. Chen, U. Lächelt, E. Wagner, W. Lu and Y. Wang, Biomaterials, 2017, 141, 29–39 CrossRef CAS.
- E. Pugliese, J. Q. Coentro and D. I. Zeugolis, Adv. Mater., 2018 DOI:10.1002/adma.201704324.
- L. Yang, Z. Wang, J. Wang, W. Jiang, X. Jiang, Z. Bai and Y. He, Nanoscale, 2016, 8, 6801–6809 RSC.
- X. Wang, X. Sun, J. Lao, H. He, T. Cheng, M. Wang, S. Wang and F. Huang, Colloids Surf., B, 2014, 122, 638–644 CrossRef CAS.
- R. Justin, S. Román, D. Chen, K. Tao, X. Geng and R. Grant, RSC Adv., 2015, 5 10.1039/C5RA04340A.
- L. Ruiyi, L. Zaijun, S. Xiulan, J. Jan, L. Lin, G. Zhigou and W. Guangli, Chem. Eng. J., 2020, 382 DOI:10.1016/j.cej.2019.122992.
- A. Chowdhury, A. Ganganboina, Y. Tsai, H. Chiu and R. Doong, Anal. Chim. Acta, 2018, 1027, 109–120 CrossRef.
- R. Imani, F. Mohabatpour and F. Mostafavi, Carbon, 2018, 140, 569–591 CrossRef CAS.
- D. Scherman, A. Rousseau, P. Bigey and V. Escriou, Gene Ther., 2017, 24 DOI:10.1038/gt.2017.6.
- H. Yin, R. Kanasty, A. Eltoukhy, A. Vegas, R. Dorkin and D. Anderson, Nat. Rev. Genet., 2014, 15, 541–555 CrossRef CAS.
- M. K. Riley and W. Vermerris, Nanomaterials, 2017 DOI:10.3390/nano7050094.
- X. Loh, T. Lee, Q. Dou and G. Roshan Deen, Biomater. Sci., 2016, 4, 70–86 RSC.
- J. Ageitos, J. Chuah and K. Numata, Nanomedicines, 2016 10.1039/9781782622536-00001.
- A. Adler and K. Leong, Nano Today, 2010, 5, 553–569 CrossRef CAS.
- S. Jin and K. Ye, Biotechnol. Prog., 2007, 23, 32–41 CrossRef CAS.
- R. Misra, M. Upadhyay and S. Mohanty, Pharm. Anal. Acta, 2014 DOI:10.4172/2153-2435.1000279.
- V. Mundra and R. I. Mahato, Front. Chem. Sci. Eng., 2014, 8, 387–404 CrossRef CAS.
- A. Albanese, P. S. Tang and W. C. W. Chan, Annu. Rev. Biomed. Eng., 2012, 14, 1–16 CrossRef CAS.
- D. Yin, Y. Li, H. Lin, B. Guo, Y. Du, X. Li, H. Jia, X. Zhao, J. Tang and L. Zhang, Nanotechnology, 2013 DOI:10.1088/0957-4484/24/10/105102.
- A. Paul, A. Hasan, H. Al Kindi, A. K. Gaharwar, V. T. S. Rao, M. Nikkhah, S. R. Shin, D. Krafft, M. R. Dokmeci, D. Shum-Tim and A. Khademhosseini, ACS Nano, 2014, 8, 8050–8062 CrossRef CAS.
- R. Agarwal and K. Roy, Ther. Delivery, 2013, 4, 705–723 CrossRef CAS.
- J. Kim, L. J. Cote and J. Huang, Acc. Chem. Res., 2012, 45, 1356–1364 CrossRef CAS.
- V. C. Sanchez, A. Jachak, R. H. Hurt and A. B. Kane, Chem. Res. Toxicol., 2012, 25, 15–34 Search PubMed.
- C. Xu, D. Yang, L. Mei, B. Lu, L. Chen, Q. Li, H. Zhu and T. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 2715–2724 CrossRef CAS.
- H. Tian, W. Xiong, J. Wei, Y. Wang, X. Chen, X. Jing and Q. Zhu, Biomaterials, 2007, 28, 2899–2907 CrossRef CAS.
- M. Ogris, P. Steinlein, M. Kursa, K. Mechtler, R. Kircheis and E. Wagner, The size of DNA/transferrin-PEI complexes is an important factor for gene expression in cultured cells, 1998, vol. 5 Search PubMed.
- S. Ghafary, M. Nikkhah, S. Hatamie and S. Hosseinkhani, Sci. Rep., 2017, 7 DOI:10.1038/s41598-017-09890-y.
- H. Dong, W. Dai, H. Ju, H. Lu, S. Wang, L. Xu, S. F. Zhou, Y. Zhang and X. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 11015–11023 CrossRef CAS.
- Q. Wang, C. Zhang, G. Shen, H. Liu, H. Fu and D. Cui, J. Nanobiotechnol., 2014, 12 DOI:10.1186/s12951-014-0058-0.
- L. Zhang, W. Zheng, R. Tang, N. Wang, W. Zhang and X. Jiang, Biomaterials, 2016, 104, 269–278 CrossRef CAS.
- S. Kim, Y. Choi, G. Park, C. Won, Y. J. Park, Y. Lee, B. S. Kim and D. H. Min, Nano Res., 2017, 10, 503–519 CrossRef CAS.
- X. Yang, Y. Wang, X. Shen, C. Su, J. Yang and M. Piao, J. Colloid Interface Sci., 2017, 492, 1–7 CrossRef CAS.
- G. Mattheolabakis, L. Milane, A. Singh and M. M. Amiji, J. Drug Targeting, 2015, 23, 605–618 CrossRef CAS.
- H. J. Wang, J. Zhang, Y. H. Liu, T. Y. Luo, X. He and X. Q. Yu, RSC Adv., 2017, 7, 15613–15624 RSC.
- P. G. Luo, S. Sahu, S. T. Yang, S. K. Sonkar, J. Wang, H. Wang, G. E. Lecroy, L. Cao and Y. P. Sun, J. Mater. Chem. B, 2013, 1, 2116–2127 RSC.
- A. B. Bourlinos, A. Bakandritsos, A. Kouloumpis, D. Gournis, M. Krysmann, E. P. Giannelis, K. Polakova, K. Safarova, K. Hola and R. Zboril, J. Mater. Chem., 2012, 22, 23327–23330 RSC.
- M. Nafiujjaman, H. Khan and Y. Lee, J. Nanosci. Nanotechnol., 2017, 17 DOI:10.1166/jnn.2017.12825.
- K. Li, X. Zhao, G. Wei and Z. Su, Curr. Med. Chem., 2017, 25, 2876–2893 CrossRef.
- Y. Zhao, X. Hao, W. Lu, R. Wang, X. Shan, Q. Chen, G. Sun and J. Liu, ACS Appl. Nano Mater., 2018, 1, 2544–2551 CrossRef CAS.
- A. Dehghani, S. Ardekani, M. Hassan and V. Gomes, Carbon, 2018, 131, 238–245 CrossRef CAS.
- N. Vasimalai, V. Vilas-Boas and J. Gallo, Beilstein J. Nanotechnol., 2018, 9, 530–544 CrossRef CAS.
- G. Gao, Y. W. Jiang, H. R. Jia, J. Yang and F. G. Wu, Carbon, 2018, 134, 232–243 CrossRef CAS.
- X. Chen, F. Gong, Z. Cao, W. Zou and T. Gu, Anal. Bioanal. Chem., 2018, 410, 2961–2970 CrossRef CAS.
- S. T. Yang, X. Wang, H. Wang, F. Lu, P. G. Luo, L. Cao, M. J. Meziani, J. H. Liu, Y. Liu, M. Chen, Y. Huang and Y. P. Sun, J. Phys. Chem. C, 2009, 113, 18110–18114 CrossRef CAS.
- S. V. Bozrova, M. A. Baryshnikova, Z. A. Sokolova, I. R. Nabiev and A. V. Sukhanova, KnE Energy, 2018, 58–63 CrossRef.
- L. Cao, X. Wang, M. J. Meziani, F. Lu, H. Wang, P. G. Luo, Y. Lin, B. A. Harruff, L. M. Veca, D. Murray, S. Y. Xie and Y. P. Sun, J. Am. Chem. Soc., 2007, 129, 11318–11319 CrossRef CAS.
- R. Shereema, V. Sankar, K. Raghu and T. Rao, Electrochim. Acta, 2015, 182, 588–595 CrossRef CAS.
- Y. Zhang, M. Wu, Y. Wang, X. He, W. Li and X. Feng, Talanta, 2013, 117, 196–202 CrossRef CAS.
- S. C. Ray, A. Saha, N. R. Jana and R. Sarkar, J. Phys. Chem. C, 2009, 113, 18546–18551 CrossRef CAS.
- Y. Xu, M. Wu, Y. Liu, X.-Z. Feng, X.-B. Yin, X.-W. He and Y.-K. Zhang, Chem.–Eur. J., 2013, 19, 2276–2283 CrossRef CAS.
- G. Eda, Y.-Y. Lin, C. Mattevi, H. Yamaguchi, H.-A. Chen, I.-S. Chen, C.-W. Chen and M. Chhowalla, Adv. Mater., 2010, 22, 505–509 CrossRef CAS.
- L. Hu, Y. Sun, S. Li, X. Wang, K. Hu, L. Wang and X. Liang, Carbon, 2014, 67, 508–513 CrossRef CAS.
- W. Li, Z. Zhang, B. Kong, S. Feng, J. Wang, L. Wang, J. Yang, F. Zhang, P. Wu and D. Zhao, Angew. Chem., Int. Ed., 2013, 52, 8151–8155 CrossRef CAS.
- Z. A. Qiao, Y. Wang, Y. Gao, H. Li, T. Dai, Y. Liu and Q. Huo, Chem. Commun., 2010, 46, 8812–8814 RSC.
- W. S. Zou, Y. J. Ji, X. F. Wang, Q. C. Zhao, J. Zhang, Q. Shao, J. Liu, F. Wang and Y. Q. Wang, Chem. Eng. J., 2016, 294, 323–332 CrossRef CAS.
- D. Kumar, K. Singh and V. Verma, J. Bionanosci., 2014, 8, 274–279 CrossRef CAS.
- V. Mehta, S. Jha, H. Basu, R. Singhal and S. Kailasa, Sens. Actuators, B, 2015, 213, 434–443 CrossRef CAS.
- Q. Liang, W. Ma, Y. Shi, Z. Li and X. Yang, Carbon, 2013, 60, 421–428 CrossRef CAS.
- L. Wang and H. S. Zhou, Anal. Chem., 2014, 86, 8902–8905 CrossRef CAS.
- C. Zhu, J. Zhai and S. Dong, Chem. Commun., 2012, 48, 9367–9369 RSC.
- X. Yang, Y. Zhuo, S. Zhu, Y. Luo, Y. Feng and Y. Dou, Biosens. Bioelectron., 2014, 60, 292–298 CrossRef CAS.
- V. Mehta, S. Jha, H. Basu, R. Singhal and S. Kailasa, Sens. Actuators, B, 2014, 213, 434–443 CrossRef.
- S. Sahu, B. Behera, T. Maiti and S. Mohapatra, Chem. Commun., 2012, 48, 8835–8837 RSC.
- Z. Qian, J. Ma, X. Shan, H. Feng, L. Shao and J. Chen, Chem.–Eur. J., 2014, 20, 2254–2263 CrossRef CAS.
- G. C. Parker, M. Anastassova-Kristeva, L. M. Eisenberg, M. S. Rao, M. A. Williams, P. R. Sanberg and D. English, Stem Cells Dev., 2005, 14, 463–469 CrossRef CAS.
- A. Solanki, J. D. Kim and K. B. Lee, Nanomedicine, 2008, 3, 567–578 CrossRef CAS.
- M. Zhang, L. Bai, W. Shang, W. Xie, H. Ma, Y. Fu, D. Fang, H. Sun and L. Fan, J. Mater. Chem., 2012, 22, 7461–7467 RSC.
- W. Shang, X. Zhang, M. Zhang, Z. Fan, Y. Sun, M. Han and L. Fan, Nanoscale, 2014, 6, 5799–5806 RSC.
- Z. Fan, Y. Li, X. Li, L. Fan, S. Zhou, D. Fang and S. Yang, Carbon, 2014, 70, 149–156 CrossRef CAS.
- S. Zhu, J. Zhang, C. Qiao, S. Tang, Y. Li, W. Yuan, B. Li, L. Tian, F. Liu, R. Hu, H. Gao, H. Wei, H. Zhang, H. Sun and B. Yang, Chem. Commun., 2011, 47, 6858–6860 RSC.
- J. Peng, W. Gao, B. K. Gupta, Z. Liu, R. Romero-Aburto, L. Ge, L. Song, L. B. Alemany, X. Zhan, G. Gao, S. A. Vithayathil, B. A. Kaipparettu, A. A. Marti, T. Hayashi, J. J. Zhu and P. M. Ajayan, Nano Lett., 2012, 12, 844–849 CrossRef CAS.
- Y. Dong, C. Chen, X. Zheng, L. Gao, Z. Cui, H. Yang, C. Guo, Y. Chi and C. M. Li, J. Mater. Chem., 2012, 22, 8764–8766 RSC.
- X. Zhu, X. Xiao, X. Zuo, Y. Liang and J. Nan, Part. Part. Syst. Charact., 2014, 31, 801–809 CrossRef CAS.
- P. S. Saxena., A. Sirvastava, V. Kumar, V. Singh, S. Umrao, V. Parashar, S. Abraham, A. K. Singh and G. Nath, RSC Adv., 2014, 4, 21101–21107 RSC.
- X. Wang, X. Sun, J. Lao, H. He, T. Cheng, M. Wang, S. Wang and F. Huang, Colloids Surf., B, 2014, 122, 638–644 CrossRef CAS.
- J. Wang, Q. Li, J. Zhou, Y. Wang, L. Yu, H. Peng and J. Zhu, Materials, 2017, 72, 15–19 CAS.
- C. Matea, T. Mocan, F. Tabaran, T. Pop and O. Mosteanu, Int. J. Nanomed., 2017, 12, 5421–5431 CrossRef CAS.
- G. Gu, C. Park, H. Cho, T. Ha, J. Bae and O. Kwon, Sci. Rep., 2018, 8 DOI:10.1038/s41598-018-22828-2.
- H. Tao, K. Yang, Z. Ma, J. Wan, Y. Zhang, Z. Kang and Z. Liu, Small, 2012, 8, 281–290 CrossRef CAS.
- L. Wu, M. Luderer, X. Yang, C. Swain and H. Zhang, Theranostics, 2013, 3, 677–686 CrossRef.
- S. T. Yang, L. Cao, P. G. Luo, F. Lu, X. Wang, H. Wang, M. J. Meziani, Y. Liu, G. Qi and Y. P. Sun, J. Am. Chem. Soc., 2009, 131, 11308–11309 CrossRef CAS.
- K. Yang, S. Zhang, G. Zhang, X. Sun, S. T. Lee and Z. Liu, Nano Lett., 2010, 10, 3318–3323 CrossRef CAS.
- X. Wu, F. Tian, W. Wang, J. Chen, M. Wu and J. Zhao, J. Mater. Chem. C, 2013, 1, 4676–4684 RSC.
- X. J. Ge, M. Lan, B. Zhou, W. Liu, L. Guo, H. Wang, Q. Jia, G. Niu, X. Huang, H. Zhou, X. Meng, P. Wang, C. S. Lee and X. Han, Nat. Commun., 2014, 5, 4596, DOI:10.1038/ncomms5596.
- A. C. V. Doughty, A. R. Hoover, E. Layton, C. K. Murray, E. W. Howard and W. R. Chen, Materials, 2019, 12 DOI:10.3390/ma12050779.
- R. Singh and S. V. Torti, Adv. Drug Delivery Rev., 2013, 65, 2045–2060 CrossRef CAS.
- M. Kováčová, E. Špitalská, Z. Markovic and Z. Špitálský, Part. Part. Syst. Charact., 2020, 37, 1900348 CrossRef.
- F. Wu, L. Yue, H. Su, K. Wang, L. Yang and X. Zhu, Nanoscale Res. Lett., 2018, 13 DOI:10.1186/s11671-018-2761-5.
- T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. Peng, J. Natl. Cancer Inst., 1998, 90, 889–905 CrossRef CAS.
- L. Yang, G. Xing, L. Wang, Y. Wu, S. Li, G. Xu, Q. He, J. Chen, M. Chen, X. Liu, Z. Zhu and L. Yang, Lancet, 2015, 1465–1471 CrossRef.
- M. Lyons, I. Phang, S. Eljamel and F. Ir, Photodiagn. Photodyn. Ther., 2012, 9, 40–45 CrossRef.
- C. Kennedy and R. H. Pottier, J. Photochem. Photobiol., B, 1992, 14(4), 275–292 CrossRef.
- A. P. Castano, T. N. Demidova and M. R. Hamblin, Photodiagn. Photodyn. Ther., 2004 DOI:10.1016/S1572-1000(05)00007-4.
- K. Yang, L. Hu, X. Ma, S. Ye, L. Cheng, X. Shi and C. Li, Adv. Mater., 2012, 24, 1868–1872 CrossRef CAS.
- S. Liao, X. Hu, Z. Liu, Y. Lin, R. Liang, Y. Zhang, Q. Li, Y. Li and X. Liao, Oncol. Lett., 2019, 17, 603–615 CAS.
- T. A. Tabish, C. J. Scotton, D. C. J. Ferguson, L. Lin, A. Van Der, S. Lowry, M. Ali, F. Jabeen, M. Ali, G. Paul and S. Zhang, Nanomedicine, 2018, 13(15), 1923–1937 CrossRef CAS.
- M. Hassan, V. G. Gomes, A. Dehghani and S. M. Ardekani, Nano Res., 2018, 11, 1–41 CrossRef CAS.
- S. Brown, E. Brown and I. Walker, Lancet Oncol., 2004, 5, 497–508 CrossRef CAS.
- C. A. Leatherdale, W. K. Woo, F. V. Mikulec and M. G. Bawendi, J. Phys. Chem. B, 2002, 106, 7619–7622 CrossRef CAS.
- T. Nann, Engineering, 2011, 3, 137–143 CAS.
- P. Yu, X. Wen, Y.-R. Toh and J. Tang, J. Phys. Chem. C, 2012 DOI:10.1021/jp307308z.
- C. J. Reckmeier, J. Schneider, A. S. Susha and A. L. Rogach, Opt. Express, 2016, 24, A312 CrossRef CAS.
- P. Huang, J. Lin, X. Wang, Z. Wang, C. Zhang, M. He, K. Wang, F. Chen, Z. Li, G. Shen, D. Cui and X. Chen, Adv. Mater., 2012, 24, 5104–5110 CrossRef CAS.
- D. W. Zheng, B. Li, C. X. Li, J. X. Fan, Q. Lei, C. Li, Z. Xu and X. Z. Zhang, ACS Nano, 2016, 10, 8715–8722 CrossRef CAS.
- F. Wu, L. Yue, H. Su, K. Wang, L. Yang and X. Zhu, Nanoscale Res. Lett., 2018, 13 DOI:10.1186/s11671-018-2761-5.
- Y. Wen, Q. Jia, F. Nan, X. Zheng and W. Liu, Chem.–Asian J., 2019, 14, 2162–2168 CrossRef CAS.
- H. Fan, X. Yu, K. Wang, Y. Yin, Y. Tang, Y. Tang and X. Liang, Eur. J. Med. Chem., 2019, 182, 111620 CrossRef CAS.
- J. D. Monroe, E. Belekov, A. O. Er and M. E. Smith, Photochem. Photobiol., 2019, 95(6), 1473–1481 CrossRef CAS.
- X. Bao, Y. Yuan, J. Chen, B. Zhang, D. Li, D. Zhou, P. Jing, G. Xu, Y. Wang, K. Holá, D. Shen, C. Wu, L. Song, C. Liu, R. Zbořil and S. Qu, Light: Sci. Appl., 2018, 7, 1–11 CrossRef CAS.
- B. Geng, D. Yang, D. Pan, L. Wang, F. Zheng, W. Shen, C. Zhang and X. Li, Carbon, 2018, 134, 153–162 CrossRef CAS.
- M. Thakur, M. K. Kumawat and R. Srivastava, RSC Adv., 2017, 7, 5251–5261 RSC.
- Z. Tian, X. Yao, K. Ma, X. Niu, J. Grothe, Q. Xu, L. Liu, S. Kaskel and Y. Zhu, ACS Omega, 2017, 2, 1249–1258 CrossRef CAS.
- H. Liu, C. Li, Y. Qian, L. Hu, J. Fang, W. Tong, R. Nie, Q. Chen and H. Wang, Biomaterials, 2020, 232 DOI:10.1016/j.biomaterials.2019.119700.
- S. Zheng, Z. Jin, C. Han, J. Li, H. Xu, S. Park, J. O. Park, E. Choi and K. Xu, J. Mater. Sci., 2020, 55, 1184–1197 CrossRef CAS.
- Y. Gao, S. Zhong, L. Xu, S. He, Y. Dou, S. Zhao, P. Chen and X. Cui, Microporous Mesoporous Mater., 2019, 278, 130–137 CrossRef CAS.
- M. Lan, L. Guo, S. Zhao, Z. Zhang, Q. Jia, L. Yan, J. Xia, H. Zhang, P. Wang and W. Zhang, Adv. Ther., 2018, 1 DOI:10.1002/adtp.201800077.
- F. Wo, R. Xu, Y. Shao, Z. Zhang, M. Chu, D. Shi and S. Liu, Theranostics, 2016, 6, 485–500 CrossRef CAS.
- Y. Xuan, R. Zhang, D. Zhao, X. Zhang, J. An and K. Cheng, Chem. Eng. J., 2019, 369, 87–99 CrossRef CAS.
- S. Badrigilan, B. Shaabani, N. Gharehaghaji and A. Mesbahi, Photodiagn. Photodyn. Ther., 2019, 25, 504–514 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |