Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Reversible fluorescence modulation through the photoisomerization of an azobenzene-bridged perylene bisimide cyclophane
Guanghui
Ouyang
ab,
David
Bialas
a and
Frank
Würthner
 *a
*a
aInstitut für Organische Chemie and Center for Nanosystems Chemistry, Universität Würzburg, Am Hubland, 97074 Würzburg, Germany. E-mail: wuerthner@uni-wuerzburg.de
bCAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, ZhongGuanCun, North First Street 2, 100190, Beijing, China
First published on 16th March 2021
Abstract
Correction for ‘Reversible fluorescence modulation through the photoisomerization of an azobenzene-bridged perylene bisimide cyclophane’ by Guanghui Ouyang et al., Org. Chem. Front., 2021, DOI: 10.1039/D0QO01635G.
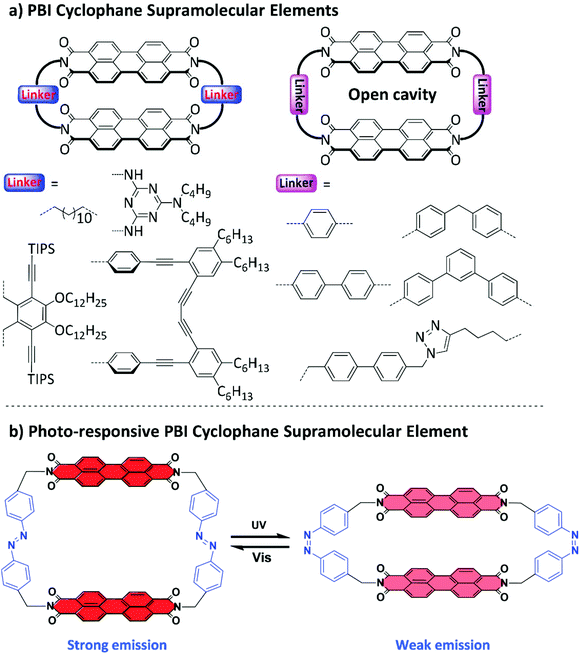
The authors regret that the structure of cis-azobenzene was incorrectly presented in Fig. 1b in the original article. The azobenzene is para-substituted instead of meta-substituted. The corrected Fig. 1 is presented here.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © the Partner Organisations 2021 |