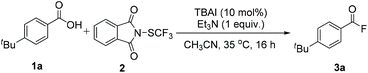Open Access Article
Open Access ArticleElectrophilic N-trifluoromethylthiophthalimide as a fluorinated reagent in the synthesis of acyl fluorides†
Chen
Zhu‡
 ,
Serik
Zhumagazy‡
,
Serik
Zhumagazy‡
 ,
Huifeng
Yue
,
Huifeng
Yue
 * and
Magnus
Rueping
* and
Magnus
Rueping
 *
*
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900, Saudi Arabia. E-mail: huifeng.yue@kaust.edu.sa; magnus.rueping@kaust.edu.sa
First published on 25th November 2021
Abstract
Herein we report the deoxygenated fluorination of readily available carboxylic acids. A series of acyl fluorides have been synthesized using shelf-stable N-trifluoromethylthiophthalimide as a fluorinated reagent for the first time. Scale-up reactions and sequential cross-couplings were performed successfully to demonstrate the practicability of this fluorination protocol.
Fluorine-containing molecules are one class of the most important organic compounds due to the unique character of the fluorine atom in modulating the chemical and biological properties such as metabolic stability, lipophilicity, and bioavailability.1 Therefore, the development of methods to access such compounds is always a research focus for organic chemists. In this context, acyl fluorides represent intriguing targets that play an important role in organic synthesis. Due to the outstanding balance between stability and reactivity, acyl fluorides were widely employed as versatile synthons in different types of organic transformations (Scheme 1a).2 Also, acyl fluorides could be used for the activation of silyl enol ethers or other silicon species in the presence of a Lewis base.3 Conventional methods to access acyl fluorides depend on the halogen exchange reaction of acyl chlorides with “F−” sources.4 Recent advances were mainly focused on the deoxyfluorination of readily available carboxylic acids with various fluorinated reagents (e.g., CF3SO2OCF3,5 PPh3/Selectfluor,6 Me4NSCF3,7 cyanuric fluoride,8 HF-Pyridine/DCC,9 sulphur tetrafluoride derivatives,10 and Carpino'salt TFFH11). Notably, Shibata and coworkers developed a general and practical method to prepare acyl fluorides, in which acids, aldehydes, and alcohols all could be transformed into acyl fluorides by using inexpensive reagents, TCCA and CsF.12 In addition, acyl fluorides were skillfully accessed via selective C–C bond cleavage using DAST or its derivatives as fluorination reagents.13 Although these achievements provide good alternatives, most of them suffered from some drawbacks, mainly including high reaction temperature or the use of fluorinated reagents that are toxic, unstable, or expensive. Thus, the development of new approaches for the preparation of acyl fluorides is still highly desirable.
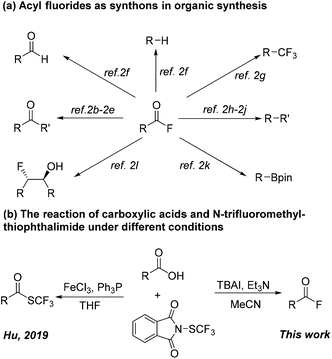 | ||
| Scheme 1 Acyl fluorides as synthons in organic synthesis and the reaction of N-trifluoromethylthiophthalimide and carboxylic acids. | ||
Shelf-stable N-trifluoromethylthiophthalimide has been developed as an easily synthesized and efficient trifluoromethylthiolation reagent, and an array of valuable compounds were obtained employing this versatile SCF3-reagent.14 In 2000, Munavalli and coworkers reported the reaction of SCF3-phthalimide with enamines, affording the α-trifluoromethyl-thiolated ketones.14a Our group realized the enantioselective trifluoromethylthiolation of β–ketoester14b and oxindoles,14c Cu-catalyzed cross-coupling of boronic acids and alkynes,14d as well as the metal-free ring-opening/trifluoromethylthiolation of cycloalkanols15 with this SCF3-reagent.
More recently, Hu and coworkers developed a protocol on deoxygenated trifluoromethylthiolation of carboxylic acids in the presence of FeCl3 and PPh3, wherein SCF3-phthalimide acted as a nucleophilic “SCF3” source.14e In contrast, we herein report that electrophilic N-trifluoromethylthiophthalimide acts as a fluorinated reagent in the reaction of carboxylic acids (Scheme 1b).
We started our investigation by evaluating the reaction of 4-(tert-butyl)benzoic acid 1a and N-trifluoromethylthiophthal-imide 2a (Table 1). After a series of screening, the optimal reaction conditions were assigned as follows: 10 mol% TBAI as catalyst, 1 equiv. of Et3N as reductant in 2 mL CH3CN at 35 °C. The use of DIPEA as base gave lower yield, while the application of other bases including 2,6-lutidine, pyridine, and nBnN led to no formation of the desired product. Other solvents such as DMF, DMA, DMSO, THF, acetone were also suitable for this transformation, albeit delivering the acyl fluoride product in low to moderate yields. Using NaI or KI instead of TBAI decreased the yields dramatically. Reversing the ratio of 1a and 2 from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 also damaged the yield. Control experiments showed that both TBAI and Et3N are essential for the high efficiency of this transformation.
2 also damaged the yield. Control experiments showed that both TBAI and Et3N are essential for the high efficiency of this transformation.
| Entry | Variables | Yieldb (%) |
|---|---|---|
| a Reaction conditions: 1a (0.40 mmol), 2 (0.20 mmol), TBAI (10 mol%), Et3N (1 equiv.) in CH3CN (2 mL) at 35 °C for 16 h. b GC Yields using dodecane as internal standard. | ||
| 1 | None | 91 |
| 2 | DIPEA as base | 57 |
| 3 | 2,6-Lutidine as base | 0 |
| 4 | Pyridine as base | 0 |
| 5 | n Bn3N as base | 0 |
| 6 | DMF as solvent | 55 |
| 7 | DMA as solvent | 60 |
| 8 | DMSO as solvent | 35 |
| 9 | THF as solvent | 42 |
| 10 | Acetone as solvent | 33 |
| 11 | 10 mol% NaI as additive | 60 |
| 12 | 10 mol% KI as additive | 42 |
| 13 | 0.5 mL CH3CN | 46 |
| 14 |
1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 = 1 2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
42 |
| 15 | No TBAI | 50 |
| 16 | NO Et3N | 0 |
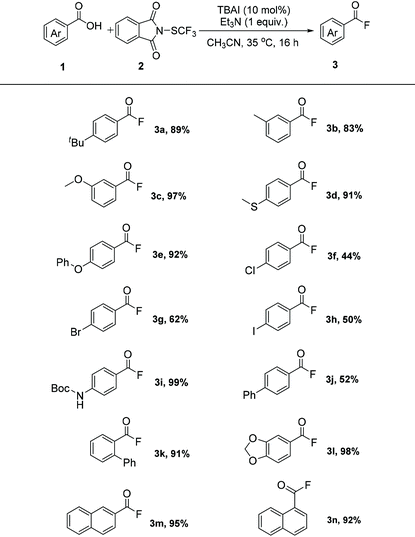
With the optimized reaction conditions in hand, the generality of the fluorination reaction was first examined. As shown in Table 2, a variety of aryl carboxylic acids could undergo this efficient fluorination transformation smoothly, affording the corresponding products in good to excellent yields. In addition to alkyl (3a and 3b), methoxyl (3c), methylthio (3d), and phenoxyl (3e) functional groups, reactive chloride (3f), bromide (3g), iodide (3h) were also tolerated in this fluorination protocol, allowing the further sequential functionalization of the generated acyl fluoride products. Aryl carboxylic acid bearing N-Boc protected amine was fluorinated in 99% yield (3i). Also, biphenyl carboxylic acids could undergo this transformation with good to high efficiency (3j and 3k). In addition, bicyclic carboxylic acid bearing acetal group (3l), as well as naphathyl carboxylic acid (3 m and 3n), could all give the corresponding product in high yield. Notably, steric hindrance seems to have no significant influence on the reactivity of the substrates (3b, 3c, 3k, and 3n).
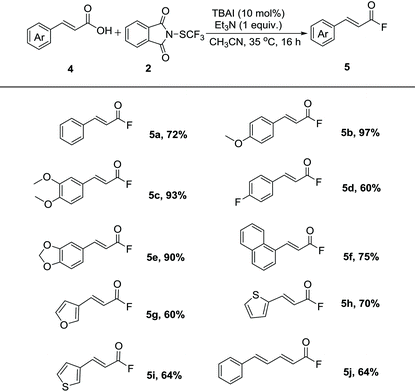
Gratefully, our fluorination protocol could be readily extended to vinyl carboxylic acids. As shown in Table 3, the reaction of cinnamic acid proceeded in good yield (5a). Vinyl carboxylic acids bearing methoxyl (5b and 5c) and fluoride (5d) on the aromatic ring underwent the protocol in good to excellent yields. The reactions of vinyl carboxyl acids containing benzodioxole and naphthyl groups (5e and 5f) with N-trifluoromethylthiophthalimide took place in good to high yields. Importantly, heterocycles such as furan and thiophene (5e–5i) were also tolerated in this protocol, providing the possibility for the synthesis of pharmaceutical-related molecules. Moreover, larger π-extended vinyl acid could also be converted to the corresponding acyl fluoride in good yield (5j).
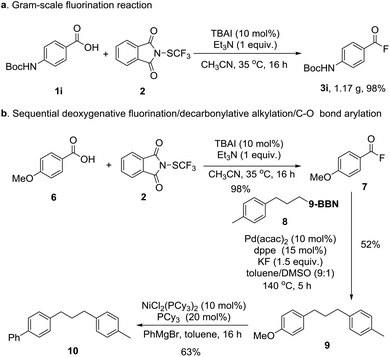
In order to demonstrate the practicality of this newly developed fluorination methodology from carboxylic acids, the gram-scale experiment of phenyl naphthalene-2-carboxylate 1i was conducted, and 98% yield of the desired product 3i was obtained (Scheme 2a). Furthermore, we also achieved the sequential deoxygenated fluorination/decarbonylative alkylation/C–O bond arylation, showing great advantages of this protocol (Scheme 2b).
Based on our results and previous studies,13,16 a mechanism for this fluorination protocol is proposed (Scheme 3). First, Et3N attacks the electrophilic sulfur center with the aid of TBAI, affording the phthalimide anion and intermediate I. Next, the phthalimide anion attacks one of the protons of the ethyl group at Et3N to generate the nucleophilic trifluoromethylthio group along with phthalimide and the aminium. Then the−SCF3 anion degrades to CSF2 with the release of one fluoride ion. The rapid reaction of CSF2 with carboxylic acid delivers the intermediate II that was attacked by a fluoride ion to afford the acyl fluoride product.
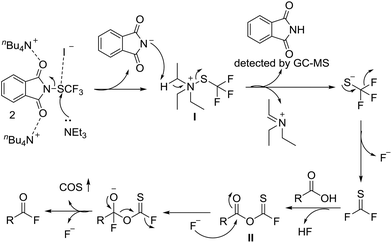 | ||
| Scheme 3 Proposed mechanism for the reaction of carboxylic acids and N-trifluoromethylthiophthalimide. | ||
In summary, we have developed an efficient deoxygenated fluorination of readily available carboxylic acids. In contrast to previous reports wherein bench-stable N-trifluoromethylthio-phthalimide was always used as a trifluoromethylthiolation reagent, this newly developed protocol employed it as a fluorinated reagent for the first time. A series of aryl and vinyl carboxylic acid could be converted to acyl fluorides with good to high efficiency. Gram-scale reaction and sequential synthesis, including deoxygenated fluorination/decarbonyl-ative alkylation/C–O bond arylation, were realized in good yield. This protocol provides a good alternative for the fluorination of carboxylic acids.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the King Abdullah University of Science and Technology (KAUST), Saudi Arabia, Office of Sponsored Research (URF/1/4384).Notes and references
- (a) P. Kirsch, Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, Wiley-VCH, Weinheim, 2004 CrossRef; (b) D. O'Hagan, Understanding organofluorine chemistry. An introduction to the C–F bond, Chem. Soc. Rev., 2008, 37, 308–319 RSC; (c) J. T. Welch, Fluorine in Medicinal Chemistry and Chemical Biology, ed. I. Ojima, Wiley-Blackwell, Chichester, 2009 Search PubMed; (d) M. C. Belhomme, T. Besset, T. Poisson and X. Pannecoucke, Recent progress toward the introduction of functionalized difluoromethylated building blocks onto C (sp2) and C (sp) centers, Chem. – Eur. J., 2015, 21, 12836–12865 CrossRef CAS PubMed; (e) M. V. Ivanova, A. Bayle, T. Besset, T. Poisson and X. Pannecoucke, Copper–Mediated Formation of Aryl, Heteroaryl, Vinyl and Alkynyl Difluoromethylphosphonates: A General Approach to Fluorinated Phosphate Mimics, Angew. Chem., Int. Ed., 2015, 54, 13406–13410 CrossRef CAS PubMed; (f) K. Grollier, A. De Zordo–Banliat, F. Bourdreux, B. Pegot, G. Dagousset, E. Magnier and T. Billard, (Trifluoromethylselenyl) methylchalcogenyl as Emerging Fluorinated Groups: Synthesis under Photoredox Catalysis and Determination of the Lipophilicity, Chem. – Eur. J., 2021, 27, 6028–6033 CrossRef CAS PubMed; (g) F. Brüning, C. R. Pitts, J. Kalim, D. Bornemann, C. Ghiazza, J. de Montmollin, N. Trapp, T. Billard and A. Togni, Difluoro (aryl)(perfluoroalkyl)–λ4−sulfanes and Selanes: Missing Links of Trichloroisocyanuric Acid/Potassium Fluoride Chemistry, Angew. Chem., Int. Ed., 2019, 58, 18937–18941 CrossRef PubMed; (h) A. Tlili, E. Ismalaj, Q. Glenadel, C. Ghiazza and T. Billard, Synthetic Approaches to Trifluoromethylselenolated Compounds, Chem. – Eur. J., 2018, 24, 3659–3670 CrossRef CAS PubMed; (i) X. Zhao, H.-Y. Tu, L. Guo, S. Zhu, F.-L. Qing and L. Chu, Intermolecular selective carboacylation of alkenes via nickel-catalyzed reductive radical relay, Nat. Commun., 2018, 9, 3488 CrossRef PubMed; (j) D. Chen, L. Xu, T. Long, S. Zhu, J. Yang and L. Chu, Metal-free, intermolecular carbopyridylation of alkenes via visible-light-induced reductive radical coupling, Chem. Sci., 2018, 9, 9012–9017 RSC.
- (a) Y. Ogiwara and N. Sakai, Acyl Fluorides in Late–Transition–Metal Catalysis, Angew. Chem., Int. Ed., 2020, 59, 574–594 CrossRef CAS PubMed; (b) Y. Zhang and T. Rovis, A unique catalyst effects the rapid room-temperature cross-coupling of organozinc reagents with carboxylic acid fluorides, chlorides, anhydrides, and thioesters, J. Am. Chem. Soc., 2004, 126, 15964–15965 CrossRef CAS PubMed; (c) Y. Ogiwara, Y. Maegawa, D. Sakino and N. Sakai, Palladium-catalyzed coupling of benzoyl halides with aryltrifluorosilanes leading to diaryl ketones, Chem. Lett., 2016, 45, 790–792 CrossRef CAS; (d) Y. Ogiwara, D. Sakino, Y. Sakurai and N. Sakai, Acid fluorides as acyl electrophiles in Suzuki–Miyaura coupling, Eur. J. Org. Chem., 2017, 4324–4327 CrossRef CAS; (e) Y. Ogiwara, Y. Iino and N. Sakai, Catalytic C− H/C− F coupling of azoles and acyl fluorides, Chem. – Eur. J., 2019, 25, 6513–6516 CrossRef CAS PubMed; (f) Y. Ogiwara, Y. Sakurai, H. Hattori and N. Sakai, Palladium-catalyzed reductive conversion of acyl fluorides via ligand-controlled decarbonylation, Org. Lett., 2018, 20, 4204–4208 CrossRef CAS PubMed; (g) S. T. Keaveney and F. Schoenebeck, Palladium–Catalyzed Decarbonylative Trifluoromethylation of Acid Fluorides, Angew. Chem., Int. Ed., 2018, 57, 4073–4077 CrossRef CAS PubMed; (h) Y. Okuda, J. Xu, T. Ishida, C.-a. Wang and Y. Nishihara, Nickel-catalyzed decarbonylative alkylation of aroyl fluorides assisted by Lewis-acidic organoboranes, ACS Omega, 2018, 3, 13129–13140 CrossRef CAS PubMed; (i) C. A. Malapit, J. R. Bour, C. E. Brigham and M. S. Sanford, Base-free nickel-catalysed decarbonylative Suzuki–Miyaura coupling of acid fluorides, Nature, 2018, 563, 100–104 CrossRef CAS PubMed; (j) S. Sakurai, T. Yoshida and M. Tobisu, Iridium-catalyzed decarbonylative coupling of acyl fluorides with arenes and heteroarenes via CH activation, Chem. Lett., 2019, 48, 94–97 CrossRef CAS; (k) Z. Wang, X. Wang and Y. Nishihara, Nickel-catalysed decarbonylative borylation of aroyl fluorides, Chem. Commun., 2018, 54, 13969–13972 RSC; (l) J. A. Kalow and A. G. Doyle, Enantioselective ring opening of epoxides by fluoride anion promoted by a cooperative dual-catalyst system, J. Am. Chem. Soc., 2010, 132, 3268–3269 CrossRef CAS PubMed; (m) Y. Liang, A. Taya, Z. Zhao, N. Saito and N. Shibata, Deoxyfluorination of acyl fluorides to trifluoromethyl compounds by FLUOLEAD®/Olah's reagent under solvent-free conditions, Beilstein J. Org. Chem., 2020, 16, 3052–3058 CrossRef CAS PubMed.
- (a) E. Bappert, P. Müller and G. C. Fu, Asymmetric [3 + 2] annulations catalyzed by a planar-chiral derivative of DMAP, Chem. Commun., 2006, 2604–2606 RSC; (b) T. Poisson, V. Dalla, C. Papamicael, G. Dupas, F. Marsais and V. Levacher, DMAP-organocatalyzed O-silyl-O-(or C-)-benzoyl interconversions by means of benzoyl fluoride, Synlett, 2007, 0381–0386 CAS; (c) T. Poisson, V. Dalla, F. Marsais, G. Dupas, S. Oudeyer and V. Levacher, Organocatalytic enantioselective protonation of silyl enolates mediated by Cinchona alkaloids and a latent source of HF, Angew. Chem., Int. Ed., 2007, 46, 7090–7093 CrossRef CAS PubMed; (d) S. J. Ryan, L. Candish and D. W. Lupton, N-heterocyclic carbene-catalyzed generation of α, β-unsaturated acyl imidazoliums: synthesis of dihydropyranones by their reaction with enolates, J. Am. Chem. Soc., 2009, 131, 14176–14177 CrossRef CAS PubMed; (e) S. J. Ryan, L. Candish and D. W. Lupton, N-heterocyclic carbene-catalyzed (4 + 2) cycloaddition/decarboxylation of silyl dienol ethers with α, β-unsaturated acid fluorides, J. Am. Chem. Soc., 2011, 133, 4694–4697 CrossRef CAS PubMed.
- (a) G. Olah and S. Kuhn, Organic Fluorine Compounds. XXVII. Preparation of Acyl Fluorides with Anhydrous Hydrogen Fluoride. The General Use of the Method of Colson and Fredenhagen, J. Org. Chem., 1961, 26, 237–238 CrossRef CAS; (b) C. Tullock and D. Coffman, Synthesis of fluorides by metathesis with sodium fluoride, J. Org. Chem., 1960, 25, 2016–2019 CrossRef CAS; (c) A. Pittman and D. Sharp, Fluoro Ketone—Metal Fluoride Adducts as Fluorinating Agents in the Preparation of Fluorosilanes and Fluorinated Acyl Fluorides, J. Org. Chem., 1966, 31, 2316–2318 CrossRef CAS.
- H. X. Song, Z. Y. Tian, J. C. Xiao and C. P. Zhang, Tertiary–Amine–Initiated Synthesis of Acyl Fluorides from Carboxylic Acids and CF3SO2OCF3, Chem. – Eur. J., 2020, 26, 16261–16265 CrossRef CAS PubMed.
- Z. Yang, S. Chen, F. Yang, C. Zhang, Y. Dou, Q. Zhou, Y. Yan and L. Tang, PPh3/Selectfluor–Mediated Transformation of Carboxylic Acids into Acid Anhydrides and Acyl Fluorides and Its Application in Amide and Ester Synthesis, Eur. J. Org. Chem., 2019, 5998–6002 CrossRef CAS.
- T. Scattolin, K. Deckers and F. Schoenebeck, Direct synthesis of acyl fluorides from carboxylic acids with the bench-stable solid reagent (Me4N) SCF3, Org. Lett., 2017, 19, 5740–5743 CrossRef CAS PubMed.
- S. Groß, S. Laabs, A. Scherrmann, A. Sudau, N. Zhang and U. Nubbemeyer, Improved syntheses of cyanuric fluoride and carboxylic acid fluorides, J. Prakt. Chem., 2000, 342, 711–714 CrossRef.
- C. Chen, C.-T. Chien and C.-H. Su, Preparation of acyl fluorides with hydrogen fluoride-pyridine and 1, 3-dicyclohexylcarbodiimide, J. Fluorine Chem., 2002, 115, 75–77 CrossRef CAS.
- (a) A. L'Heureux, F. Beaulieu, C. Bennett, D. R. Bill, S. Clayton, F. LaFlamme, M. Mirmehrabi, S. Tadayon, D. Tovell and M. Couturier, Aminodifluorosulfinium salts: selective fluorination reagents with enhanced thermal stability and ease of handling, J. Org. Chem., 2010, 75, 3401–3411 CrossRef PubMed; (b) F. Beaulieu, L.-P. Beauregard, G. Courchesne, M. Couturier, F. LaFlamme and A. L'Heureux, Aminodifluorosulfinium tetrafluoroborate salts as stable and crystalline deoxofluorinating reagents, Org. Lett., 2009, 11, 5050–5053 CrossRef CAS PubMed.
- (a) L. A. Carpino and A. El-Faham, Tetramethylfluoroformamidinium hexafluorophosphate: a rapid-acting peptide coupling reagent for solution and solid phase peptide synthesis, J. Am. Chem. Soc., 1995, 117, 5401–5402 CrossRef CAS; (b) L. A. Carpino, M. Beyermann, H. Wenschuh and M. Bienert, Peptide synthesis via amino acid halides, Acc. Chem. Res., 1996, 29, 268–274 CrossRef CAS.
- Y. Liang, Z. Zhao, A. Taya and N. Shibata, Acyl Fluorides from Carboxylic Acids, Aldehydes, or Alcohols under Oxidative Fluorination, Org. Lett., 2021, 23, 847–852 CrossRef CAS PubMed.
- I. Saidalimu, S. Suzuki, E. Tokunaga and N. Shibata, Successive C–C bond cleavage, fluorination, trifluoromethylthio-and pentafluorophenylthiolation under metal-free conditions to provide compounds with dual fluoro-functionalization, Chem. Sci., 2016, 7, 2106–2110 RSC.
- (a) S. Munavalli, D. Rohrbaugh, D. Rossman, F. Berg, G. Wagner and H. Durst, Trifluoromethylsulfenylation of masked carbonyl compounds, Synth. Commun., 2000, 30, 2847–2854 CrossRef CAS; (b) T. Bootwicha, X. Liu, R. Pluta, I. Atodiresei and M. Rueping, N–Trifluoromethylthiophthalimide: A Stable Electrophilic SCF3−Reagent and its Application in the Catalytic Asymmetric Trifluoromethylsulfenylation, Angew. Chem., Int. Ed., 2013, 52, 12856–12859 CrossRef CAS PubMed; (c) M. Rueping, X. Liu, T. Bootwicha, R. Pluta and C. Merkens, Catalytic enantioselective trifluoromethylthiolation of oxindoles using shelf-stable N-(trifluoromethylthio) phthalimide and a cinchona alkaloid catalyst, Chem. Commun., 2014, 50, 2508–2511 RSC; (d) R. Pluta, P. Nikolaienko and M. Rueping, Direct catalytic trifluoromethylthiolation of boronic acids and alkynes employing electrophilic shelf–stable N–(trifluoromethylthio) phthalimide, Angew. Chem., Int. Ed., 2014, 53, 1650–1653 CrossRef CAS PubMed; (e) R. Mao, S. Bera, A. Cheseaux and X. Hu, Deoxygenative trifluoromethylthiolation of carboxylic acids, Chem. Sci., 2019, 10, 9555–9559 RSC; (f) W. Xu, J. Ma, X. A. Yuan, J. Dai, J. Xie and C. Zhu, Synergistic catalysis for the umpolung trifluoromethylthiolation of tertiary ethers, Angew. Chem., Int. Ed., 2018, 57, 10357–10361 CrossRef CAS PubMed; (g) Q. Xiao, Q. He, J. Li and J. Wang, 1, 4-Diazabicyclo [2.2. 2] octane-Promoted Aminotrifluoromethylthiolation of α, β-Unsaturated Carbonyl Compounds: N-Trifluoromethylthio-4-nitrophthalimide Acts as Both the Nitrogen and SCF3 Sources, Org. Lett., 2015, 17, 6090–6093 CrossRef CAS PubMed; (h) S. Mukherjee, T. Patra and F. Glorius, Cooperative catalysis: a strategy to synthesize trifluoromethyl-thioesters from aldehydes, ACS Catal., 2018, 8, 5842–5846 CrossRef CAS.
- T. Ji, X.-Y. Chen, L. Huang and M. Rueping, Remote Trifluoromethylthiolation Enabled by Organophotocatalytic C–C Bond Cleavage, Org. Lett., 2020, 22, 2579–2583 CrossRef CAS PubMed.
- (a) R. Honeker, J. B. Ernst and F. Glorius, Transition–Metal–Free Trifluoromethylthiolation of N–Heteroarenes, Chem. – Eur. J., 2015, 21, 8047–8051 CrossRef CAS PubMed; (b) H. X. Song, Z. Y. Tian, J. C. Xiao and C. P. Zhang, Tertiary–Amine–Initiated Synthesis of Acyl Fluorides from Carboxylic Acids and CF3SO2OCF3, Chem. – Eur. J., 2020, 26, 16261–16265 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1qo01633d |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2022 |