Open Access Article
Open Access ArticleDeoxygenative nucleophilic difluoromethylselenylation of carboxylic acids and alcohols with BT-SeCF2H†
Matteo
Tironi
,
Stefan
Dix
and
Matthew N.
Hopkinson
 *
*
Institut für Chemie und Biochemie, Freie Universität Berlin, Fabeckstrasse 34-36, 14195 Berlin, Germany. E-mail: matthew.hopkinson@fu-berlin.de
First published on 25th August 2021
Abstract
The benzothiazolium salt BT-SeCF2H is introduced as an efficient nucleophilic reagent for transferring difluoromethylselenyl groups onto organic molecules. SeCF2H-Containing selenoesters could be prepared upon deoxygenative substitution of readily available carboxylic acids, while silver catalysis allowed for efficient formation of (difluoromethyl)selenoethers, including the established electrophilic reagent BnSeCF2H, directly from simple alcohols. To the best of our knowledge, these deoxygenative reactions represent the first reported nucleophilic difluoromethylselenylation processes and thus open up new approaches to prepare valuable fluorinated compounds.
Introduction
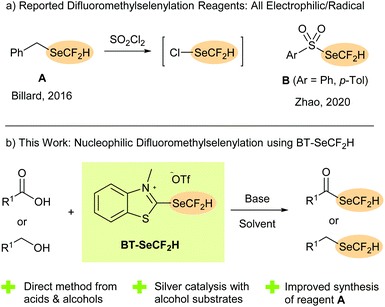
Incorporating fluorine atoms and larger fluorine-containing functional groups is a tried and tested method for modulating the physical characteristics, biological activity and bioavailability of organic compounds.1 While well-established moieties such as the CF3 group remain the most widely studied, the investigation of alternative fluorinated motifs that offer new possibilities for fine-tuning a molecule's properties has become a major area of research.2 Organoselenium derivatives are fundamental for many biological functions, with selenium itself being an essential human micronutrient. Multiple selenoethers and selenoesters have accordingly attracted attention as potential therapeutics including as anti-cancer, anti-microbial and anti-viral agents.3 Selenium derivatives have also found applications in materials science4 and as versatile synthetic intermediates and catalysts, especially in oxidation and radical chemistry.5Combining the beneficial effects of fluorine substitution with organoselenium chemistry is an attractive approach for developing new functional (bio)molecules and materials. In recent years, significant research interest has focused on fluoroalkylselenyl groups with the SeCF3 moiety in particular being the subject of several studies.6 The difluoromethylselenyl group (SeCF2H), on the other hand, has been less extensively investigated despite the well-known advantages partially fluorinated groups can offer over the corresponding perfluoro analogues (e.g. lipophilicity modulation, conformational effects, potential for hydrogen bonding).1,7 One reason for the lack of studies on the SeCF2H group is the scarcity of synthetic routes to access it. Traditionally, indirect methods involving either insertion of difluorocarbene into a selenol8 or formal nucleophilic difluoromethylation of a diselenide or cyanoselenide were employed.9 Direct difluoromethylselenylation methods, in which SeCF2H is installed as a whole group, do not require access to a selenium-containing precursor and allow SeCF2H to be more readily studied alongside other fluorinated or non-fluorinated groups in structure–activity relationship (SAR) investigations. To date, however, only two reagent classes have been developed for direct difluoromethylselenylation, with both serving as electrophilic or radical sources of the SeCF2H group. The selenoether BnSeCF2H (A, Scheme 1a), which is itself produced only in low yield (13–36%) from BnSeCN, typically requires in situ activation with SO2Cl2, with ClSeCF2H serving as the actual difluoromethylselenylation reagent.10 Sulfonyl derivatives B (Ar = Ph, p-Tol, Scheme 1a) react under milder conditions but are themselves synthesised from A.11 While nucleophilic approaches are commonly employed to install the SeCF3 group, to the best of our knowledge, no direct nucleophilic difluoromethylselenylation method has been reported and there are currently no sources of the −SeCF2H anion available.
In 2019, we introduced 2-fluoroalkylchalcogenyl-substituted benzothiazolium salts as new reagents for installing fluorine-containing groups onto organic molecules. These BT-reagents can be prepared from relatively inexpensive starting materials and serve as practical sources of fluoroalkylchalcogenyl anions in synthetically appealing deoxygenative functionalisation reactions of readily available aliphatic alcohols12 and carboxylic acids.13,14 In addition to perfluoroalkyl derivatives such as BT-SRF (RF = CnF2n–1) and BT-SeCF3, recent work showed that the partially fluorinated analogue BT-SCF2H could be successfully engaged in efficient deoxygenative difluoromethylthiolation reactions, providing (difluoromethyl)thioesters directly from carboxylic acids under mild conditions.13 Inspired by these results, we considered whether a (difluoromethyl)selenium analogue could be accessed and, if so, whether it would act as a source of hitherto unexplored −SeCF2H anions for nucleophilic transformations. Herein, we report the successful synthesis of BT-SeCF2H and its application as a reagent in unprecedented deoxygenative difluoromethylselenylation reactions (Scheme 1b). In addition to providing SeCF2H-containing selenoesters from diverse carboxylic acids, silver catalysis allowed for the efficient synthesis of (difluoromethyl)selenoethers, including the established electrophilic reagent BnSeCF2H (A), directly from unactivated alcohols.
Results and discussion
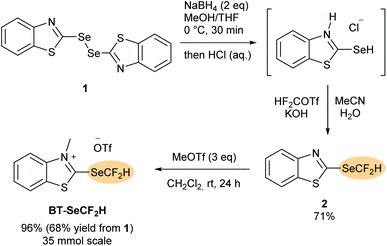
Synthesis of BT-SeCF2H
The synthesis of the new benzothiazolium salt BT-SeCF2H is shown in Scheme 2. As for the other BT-reagents,12–14 a two-stage approach was envisaged proceeding through a neutral non-methylated benzothiazole intermediate. In the first step, bis(benzothiazole)diselenide 115 was reduced to the corresponding selenol using NaBH4. Following precipitation as the benzothiazolium chloride adduct, subsequent treatment with difluorocarbene generated under basic conditions from HCF2OTf afforded the stable heteroarene 2, which could be isolated in 71% yield upon column chromatography. N-Methylation using methyl trifluoromethanesulfonate in CH2Cl2 at rt followed by precipitation with diethyl ether afforded BT-SeCF2H in 96% yield (overall yield of 68% from 1, 35 mmol scale). BT-SeCF2H was obtained as an off-white solid that required no further purification and is stable at least over several months when stored under air at room temperature.Deoxydifluoromethylselenylation of carboxylic acids
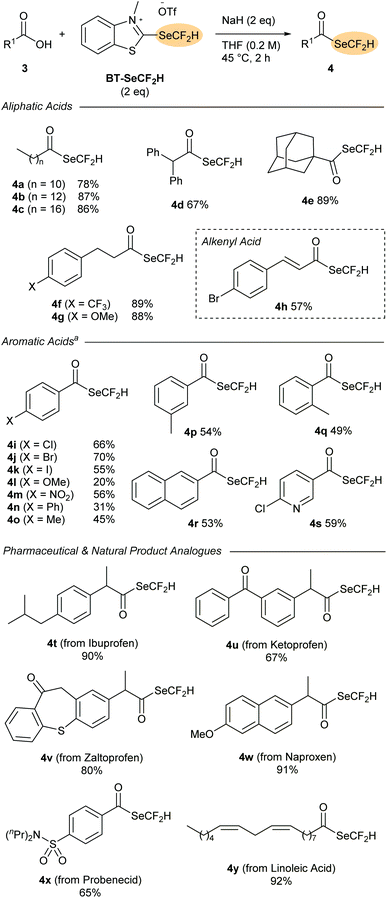
With BT-SeCF2H in hand, we sought to investigate its reactivity as a nucleophilic difluoromethylselenylating reagent. Inspired by the successful application of BT-SCF2H in deoxygenative substitution reactions of carboxylic acids,13 BT-SeCF2H (1.25 eq.) was first reacted with n-dodecanoic acid 3a and NaH (2 eq.) in THF. After 2 h at rt, we were delighted to observe clean formation of the (difluoromethyl)selenoester 4a in 45% NMR yield. Selenoesters have found multiple applications as pharmaceutical candidates and synthetic reagents, but studies on difluoromethyl derivatives are lacking.16 In 2020, Wang and co-workers reported the only methodology for preparing (difluoromethyl)selenoesters; a radical process from aldehydes employing BnSeCF2H (A) together with AIBN.10f The successful synthesis of (difluoromethyl)selenoesters using BT-SeCF2H not only offers a complementary route starting from readily available carboxylic acids, it also represents the first reported nucleophilic difluoromethylselenylation process. Mechanistically, 4a likely results from an initial attack of the carboxylate to the 2-position of BT-SeCF2H followed either by elimination of −SeCF2H and subsequent addition/elimination to a 2-carboxybenzothiazolium intermediate, or alternatively through a concerted rearrangement process.‡ Increasing the amount of BT-SeCF2H to 2 eq. and raising the reaction temperature to 45 °C improved the NMR yield to 81%, with 4a being isolated in 78% yield after column chromatography.The scope of the deoxygenative difluoromethylselenylation reaction was then tested with a range of carboxylic acid derivatives 3 (Scheme 3). A wide selection of aliphatic substrates could be successfully converted into the corresponding (difluoromethyl)selenoesters 4a–g in excellent yields (67–89%). Primary, secondary and even tertiary derivatives were all tolerated with 1-adamantanecarboxylic acid 3e providing selenoester 4e in 89% yield after column chromatography. Aromatic acids could also be successfully employed with these reactions being conducted at room temperature. A wide range of functional groups were tolerated with electron-neutral and comparatively electron-deficient moieties leading to the highest yields. The successful formation of the halogen-substituted products 4i–k is particularly noteworthy as these compounds could serve as SeCF2H-containing building blocks amenable to subsequent functionalisation through cross-coupling. As demonstrated by the series 4o–q, substituents at the ortho-, meta- and para-positions were tolerated with little difference in the product yields observed.
Finally, the applicability of the deoxydifluoromethylselenylation method to the synthesis of SeCF2H-containing pharmaceutical analogues was evaluated. A range of (difluoromethyl)selenoesters of common non-steroidal anti-inflammatory drugs (NSAIDs) could be prepared in excellent yields directly from the pharmaceutical compound (4t–w, 67–91%). The sulphonamide probenecid (3x), which is used to treat gout, could also be converted in 65% yield, while the naturally occurring fatty acid linoleic acid (3y) provided selenoester 4y in 92% yield.
Deoxydifluoromethylselenylation of alcohols
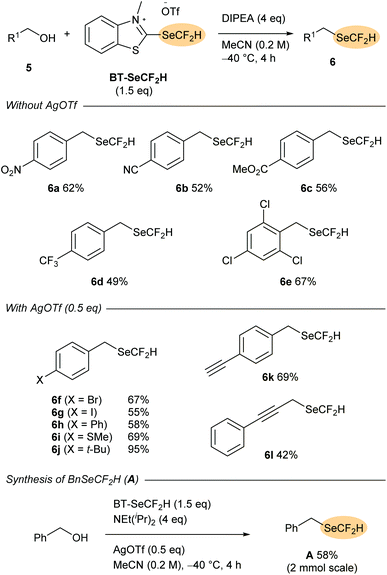
Having established the reactivity of BT-SeCF2H as a nucleophilic reagent for the difluoromethylselenylation of carboxylic acids, we next turned our attention to the synthesis of selenoethers directly from aliphatic alcohols. Although more widely studied than (difluoromethyl)selenoesters, synthetic routes to alkyl-SeCF2H compounds are largely limited to indirect methods that require pre-installation of a diselenide or cyanoselenide motif onto the substrate.9 To date, only a handful of direct difluoromethylselenylation reactions affording aliphatic products have been disclosed involving either nucleophilic attack onto in situ-activated BnSeCF2H (A)10c,e or, in a very recent report from Zhang and co-workers, radical group transfer from PhSO2SeCF2H (B).11cIn an initial test reaction, 4-nitrobenzyl alcohol 5a was reacted with BT-SeCF2H (1.25 eq.) and NEt(iPr)2 (2 eq.) in MeCN at rt. After 2 h, 1H and 19F NMR indicated the formation of the desired selenoether 6a in 42% yield. Increasing the amount of reagent and base and adding them in portions, as well as the optimisation of the temperature (−40 °C) and reaction time (4 h) allowed for an increase in the NMR yield of 6a to 65% with the pure product being isolated in 62% yield after column chromatography. At this stage, the generality of the method was tested with a selection of benzylic alcohols (Scheme 4). While a series of substrates bearing electron-withdrawing substituents such as –CN, –CF3 and –CO2Me provided the corresponding (difluoromethyl)selenoethers 6a–e in good yields, more electron-rich derivatives reacted only with low efficiency. Addition of these alcohols to the BT-reagent followed by elimination of −SeCF2H would lead to a comparatively less electrophilic 2-alkoxybenzothiazolium intermediate. Nucleophilic substitution at this species is likely less favoured, and decomposition of the −SeCF2H anion may outcompete product formation. With the aim of providing a stabilising counter-cation, which could increase the lifetime of −SeCF2H in the reaction medium, silver(I) salts were tested as catalytic additives. While 4-bromobenzyl-containing selenoether 6f was provided in only 31% yield under the standard conditions described above, addition of Ag2O (0.25 eq., 0.5 eq. of Ag+) led to an increase in NMR yield to 63%. Moreover, selenoether 6f was obtained in 81% NMR yield (67% isolated) when the reaction was conducted in the presence of AgOTf (0.5 eq.).
Under these silver catalysis conditions, good yields were obtained with a selection of electron-neutral and electron-rich benzyl alcohols (5g–k, up to 95% with 4-(tert-butyl)benzyl alcohol 5h), while the propargylic substrate 5l also reacted with moderate efficiency (42% yield of 6l). Notably, the method is also tolerant of terminal alkynes (6k), which are known to be activated by silver(I). Finally, direct deoxytrifluoromethylselenylation of benzyl alcohol was tested as a method for preparing BnSeCF2H (A). This electrophilic and radical difluoromethylselenylation reagent was introduced by Billard and co-workers in 201610a and has been previously synthesised from benzyl bromide in a two-step sequence involving nucleophilic difluoromethylation of BnSeCN.10a,e,11c Subjecting BnOH to the optimised conditions with BT-SeCF2H (1.5 eq.), AgOTf (0.5 eq.) and NEt(iPr)2 (4 eq.) resulted in smooth formation of the established reagent A, which could be isolated in 58% yield after column chromatography on a 2 mmol scale. This yield is notably higher than that obtained in the previously-reported difluoromethylation of BnSeCN (13–36%)10a,e,11c and suggests that direct deoxygenative difluoromethylselenylation could serve as a useful complementary approach to prepare reagent A and, by extension, its derivatives B.
Conclusions
In conclusion, BT-SeCF2H has been introduced as a practical reagent for hitherto unexplored nucleophilic difluoromethylselenylation reactions. Deoxygenative substitution of carboxylic acids provides (difluoromethyl)selenoesters, while silver catalysis allows for the efficient synthesis of benzylic and propargylic CF2H-substituted selenoethers, including the established electrophilic reagent BnSeCF2H (A), directly from unactivated alcohols. In opening up nucleophilic approaches, we believe this work will inspire new routes towards difluoromethylselenylated compounds and accelerate the study of the SeCF2H group in medicinal and materials chemistry.Conflicts of interest
M.N.H. and S.D. are co-inventors on a European and International Patent Application concerning the synthesis and use of benzothiazolium reagents for installing fluorine-containing functional groups (EP 3 677 576 A1; WO 2020141195 A1).Acknowledgements
This work is funded by the Dahlem Research School, the Studienstiftung des deutschen Volkes (scholarship to M.T.) and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project-ID 387284271 – SFB 1349 (gefördert durch die Deutsche Forschungsgemeinschaft (DFG) – Projektnummer 387284271 – SFB 1349). Financial support from the Fonds der Chemischen Industrie (FCI, Sachkostenzuschuss) is also gratefully acknowledged. We would like to acknowledge the assistance of the Core Facility BioSupraMol supported by the DFG.Notes and references
- Selected reviews: (a) K. Müller, C. Faeh and F. Diederich, Fluorine in Pharmaceuticals: Looking Beyond Intuition, Science, 2007, 317, 1881 CrossRef; (b) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Fluorine in medicinal chemistry, Chem. Soc. Rev., 2008, 37, 320 RSC; (c) R. Berger, G. Resnati, P. Metrangolo, E. Weber and J. Hulliger, Organic fluorine compounds: a great opportunity for enhanced materials properties, Chem. Soc. Rev., 2011, 40, 3496 RSC; (d) J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011), Chem. Rev., 2014, 114, 2432 CrossRef CAS PubMed; (e) T. Fujiwara and D. O'Hagan, Successful fluorine-containing herbicide agrochemicals, J. Fluorine Chem., 2014, 167, 16 CrossRef CAS; (f) A. Harsanyi and G. Sandford, Organofluorine chemistry: applications, sources and sustainability, Green Chem., 2015, 17, 2081 RSC; (g) M. G. Campbell and T. Ritter, Modern Carbon–Fluorine Bond Forming Reactions for Aryl Fluoride Synthesis, Chem. Rev., 2015, 115, 612 CrossRef CAS PubMed; (h) C. Ni and J. Hu, The unique fluorine effects in organic reactions: recent facts and insights into fluoroalkylations, Chem. Soc. Rev., 2016, 45, 5441 RSC; (i) R. Szpera, D. F. J. Moseley, L. B. Smith, A. J. Sterling and V. Gouverneur, The Fluorination of C−H Bonds: Developments and Perspectives, Angew. Chem., Int. Ed., 2019, 58, 14824 CrossRef CAS PubMed; (j) T. Koike and M. Akita, Recent progress in photochemical radical di- and mono-fluoromethylation, Org. Biomol. Chem., 2019, 17, 5413 RSC; (k) M. Inoue, Y. Sumii and N. Shibata, Contribution of Organofluorine Compounds to Pharmaceuticals, ACS Omega, 2020, 5, 10633 CrossRef CAS PubMed.
- D. Cahard and J.-A. Ma, Emerging Fluorinated Motifs: Synthesis, Properties and Applications, Wiley-VCH, Weinheim, 2020 Search PubMed.
- Selected reviews: (a) C. W. Nogueira, G. Zeni and J. B. T. Rocha, Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology, Chem. Rev., 2004, 104, 6255 CrossRef CAS; (b) M. P. Rayman, Selenium and human health, Lancet, 2012, 379, 1256 CrossRef CAS; (c) V. K. Jain and K. I. Priyadarsini, Organoselenium Compounds in Biology and Medicine: Synthesis, Biological and Therapeutic Treatments, Royal Society of Chemistry, Cambridge (UK), 2018 Search PubMed.
- Selected reviews: (a) M. A. Malik, M. Afzaal and P. O'Brien, Precursor Chemistry for Main Group Elements in Semiconducting Materials, Chem. Rev., 2010, 110, 4417 CrossRef CAS PubMed; (b) Q. Li, Y. Zhang, Z. Chen, X. Pan, Z. Zhang, J. Zhu and X. Zhu, Organoselenium chemistry-based polymer synthesis, Org. Chem. Front., 2020, 7, 2815 RSC.
- Selected reviews: (a) A. T. Diplock and F. D. Kurzer, Organic Selenium Compounds: Their Chemistry and Biology, Biochem. Soc. Trans., 1974, 2, 571 CrossRef; (b) T. Wirth, Organoselenium Chemistry in Stereoselective Reactions, Angew. Chem., Int. Ed., 2000, 39, 3740 CrossRef CAS; (c) D. M. Freudendahl, S. A. Shahzad and T. Wirth, Recent Advances in Organoselenium Chemistry, Eur. J. Org. Chem., 2009, 1649 CrossRef CAS; (d) D. Freudendahl, S. Santoro, S. Shahzad, C. Santi and T. Wirth, Green Chemistry with Selenium Reagents: Development of Efficient Catalytic Reactions, Angew. Chem., Int. Ed., 2009, 48, 8409 CrossRef CAS PubMed.
- Reviews: (a) A. Tlili, E. Ismalaj, Q. Glenadel, C. Ghiazza and T. Billard, Synthetic Approaches to Trifluoromethylselenolated Compounds, Chem. – Eur. J., 2018, 24, 3659 CrossRef CAS; (b) C. Ghiazza and A. Tlili, Copper-promoted/copper-catalyzed trifluoromethylselenolation reactions, Beilstein J. Org. Chem., 2020, 16, 305 CrossRef CAS.
- (a) J. A. Erickson and J. I. McLoughlin, Hydrogen Bond Donor Properties of the Difluoromethyl Group, J. Org. Chem., 1995, 60, 1626 CrossRef CAS; (b) Q. A. Huchet, B. Kuhn, B. Wagner, H. Fischer, M. Kansy, D. Zimmerli, E. M. Carreira and K. Müller, On the polarity of partially fluorinated methyl groups, J. Fluorine Chem., 2013, 152, 119 CrossRef CAS; (c) Y. Zafrani, D. Yeffet, G. Sod-Moriah, A. Berliner, D. Amir, D. Marciano, E. Gershonov and S. Saphier, Difluoromethyl Bioisostere: Examining the “Lipophilic Hydrogen Bond Donor” Concept, J. Med. Chem., 2017, 60, 797 CrossRef CAS PubMed.
- (a) H. Suzuki, M. Yoshinaga, K. Takaoka and Y. Hiroi, Simple Synthesis of Aryl Difluoromethyl Selenides and Tellurides, Synthesis, 1985, 497 CrossRef CAS; (b) V. P. Mehta and M. F. Greaney, S-, N-, and, Se-Difluoromethylation Using Sodium Chlorodifluoroacetate, Org. Lett., 2013, 15, 5036 CrossRef CAS; (c) N. B. Heine and A. Studer, Radical Difluoromethylation of Thiols with (Difluoromethyl)triphenylphosphonium Bromide, Org. Lett., 2017, 19, 4150 CrossRef CAS PubMed.
- (a) Y.-m. Lin, W.-b. Yi, W.-z. Shen and G.-p. Lu, A Route to α-Fluoroalkyl Sulfides from α-Fluorodiaroylmethanes, Org. Lett., 2016, 18, 592 CrossRef CAS; (b) T. Dong, J. Nie and C.-P. Zhang, A convenient, transition metal-free synthesis of difluoromethyl selenoethers from organic selenocyanates and TMSCF2H, Tetrahedron, 2018, 74, 5642 CrossRef CAS; (c) S. Jin, Z. Kuang and Q. Song, Precise Construction of SCF2H or SeCF2H Groups on Heteroarenes Generated in Situ from CF3-Containing 1,3-Enynes, Org. Lett., 2020, 22, 615 CrossRef CAS.
- (a) Q. Glenadel, E. Ismalaj and T. Billard, Benzyltrifluoromethyl (or Fluoroalkyl) Selenide: Reagent for Electrophilic Trifluoromethyl (or Fluoroalkyl) Selenolation, J. Org. Chem., 2016, 81, 8268 CrossRef CAS; (b) C. Ghiazza, T. Billard and A. Tlili, Trifluoromethyl- and Fluoroalkylselenolations of Alkynyl Copper(I) Compounds, Chem. – Eur. J., 2017, 23, 10013 CrossRef CAS PubMed; (c) C. Ghiazza, Q. Glenadel, A. Tlili and T. Billard, Trifluoromethylselenolation and Fluoroalkylselenolation of Alkenes by Electrophilic Addition, Eur. J. Org. Chem., 2017, 3812 CrossRef CAS; (d) Q. Glenadel, E. Ismalaj and T. Billard, A Metal-Free Route to Heterocyclic Trifluoromethyl- and Fluoroalkylselenolated Molecules, Org. Lett., 2018, 20, 56 CrossRef CAS PubMed; (e) C. Ghiazza, A. Tlili and T. Billard, Direct α-C–H Trifluoromethylselenolation of Carbonyl Compounds, Eur. J. Org. Chem., 2018, 3680 CrossRef CAS; (f) R.-L. Guo, X.-Q. Zhu, X.-L. Zhang and Y.-Q. Wang, Synthesis of difluoromethylselenoesters from aldehydes via a radical process, Chem. Commun., 2020, 56, 8976 RSC.
- (a) K. Lu, Q. Li, X. Xi, T. Zhou and X. Zhao, Metal-Free Difluoromethylselenolation of Arylamines Under Visible-Light Photocatalysis, J. Org. Chem., 2020, 85, 1224 CrossRef CAS; (b) K. Lu, X. Xi, T. Zhou, L. Lei, Q. Li and X. Zhao, Copper-catalysed direct difluoromethylselenolation of aryl boronic acids with Se-(difluoromethyl) 4-methylbenzenesulfonoselenoate, Tetrahedron Lett., 2021, 68, 152897 CrossRef CAS; (c) H. Zhang, F. Yu, C. Li, P. Tian, Y. Zhou and Z.-Y. Cao, Iron-Catalyzed, Site-Selective Difluoromethylthiolation (−SCF2H) and Difluoromethylselenation (−SeCF2H) of Unactivated C(sp3)–H Bonds in N-Fluoroamides, Org. Lett., 2021, 23, 4721 CrossRef CAS PubMed.
- (a) S. Dix, M. Jakob and M. N. Hopkinson, Deoxytrifluoromethylthiolation and Selenylation of Alcohols by Using Benzothiazolium Reagents, Chem. – Eur. J., 2019, 25, 7635–7639 CrossRef CAS; (b) A. Ariamajd, N. J. Gerwien, B. Schwabe, S. Dix and M. N. Hopkinson, Benzothiazolium salts as reagents for the deoxygenative perfluoroalkylthiolation of alcohols, Beilstein J. Org. Chem., 2021, 17, 83 CrossRef CAS PubMed.
- M. Tironi, L. M. Maas, A. Garg, S. Dix, J. P. Götze and M. N. Hopkinson, Deoxygenative Tri- and Difluoromethylthiolation of Carboxylic Acids with Benzothiazolium Reagents, Org. Lett., 2020, 22, 8925 CrossRef CAS PubMed.
- M. N. Hopkinson and S. Dix, Fluorine-containing compounds for use as nucleophilic reagents for transferring functional groups onto high value organic compounds, Eur. Pat. ApplEP19150201, 2019 Search PubMed; M. N. Hopkinson and S. Dix, Fluorine-containing compounds for use as nucleophilic reagents for transferring functional groups onto high value organic compounds, International Pat. Appl. PCT/EP2020/050031, 2020 Search PubMed.
- K. Shibata and O. Mitsunobu, Preparation of 1,4-Dienes from 2-(2-Hydroxyalkylseleno)benzothiazoles by the Reaction Involving Se→O Azaaromatic Ring Rearrangement, Bull. Chem. Soc. Jpn., 1992, 65, 3163 CrossRef CAS.
- Selected examples of selenoesters in medicine: (a) N. Díaz-Argelich, I. Encío, D. Plano, A. P. Fernandes, J. A. Palop and C. Sanmartín, Novel Methylselenoesters as Antiproliferative Agents, Molecules, 2017, 22, 1288 CrossRef PubMed; (b) A. Csonka, A. Kincses, M. Nové, Z. Vadas, C. Sanmartín, E. Dominguez-Álvarez and G. Spengler, Selenoesters and Selenoanhydrides as Novel Agents Against Resistant Breast Cancer, Anticancer Res., 2019, 39, 3777 CrossRef CAS; (c) M. L. De la Cruz-Claure, A. A. Cèspedes-Llave, M. T. Ulloa, M. Benito-Lama, E. Domínguez-Álvarez and A. Bastida, Inhibition–Disruption of Candida glabrata Biofilms: Symmetrical Selenoesters as Potential Anti-Biofilm Agents, Microorganisms, 2019, 7, 664 CrossRef CAS PubMed; (d) T. Mosolygó, A. Kincses, A. Csonka, Á. S. Tönki, K. Witek, C. Sanmartín, M. A. Marć, J. Handzlik, K. Kieć-Kononowicz, E. Domínguez-Álvarez and G. Spengler, Selenocompounds as Novel Antibacterial Agents and Bacterial Efflux Pump Inhibitors, Molecules, 2019, 24, 1487 CrossRef; (e) G. Spengler, A. Kincses, T. Mosolygó, M. A. Marć, M. Nové, M. Gajdács, C. Sanmartín, H. E. McNeil, J. M. A. Blair and E. Domínguez-Álvarez, Antiviral, Antimicrobial and Antibiofilm Activity of Selenoesters and Selenoanhydrides, Molecules, 2019, 24, 4264 CrossRef CAS. Selected examples of selenoesters as synthetic intermediates: (f) A. P. Kozikowski and A. Ames, Copper(I) promoted acylation reactions. A transition metal mediated version of the Friedel-Crafts reaction, J. Am. Chem. Soc., 1980, 102, 860 CrossRef CAS; (g) D. L. Boger and R. J. Mathvink, Tandem free-radical alkene addition reactions of acyl radicals, J. Am. Chem. Soc., 1990, 112, 4003 CrossRef CAS; (h) E. Wenkert and D. Chianelli, Nickel-catalysed decarbonylation of thioesters, J. Chem. Soc., Chem. Commun., 1991, 627 RSC; (i) S. Grélaud, V. Desvergnes and Y. Landais, Stereocontrolled (Me3Si)3Si H-Mediated Radical and Ionic Hydride Transfer in Synthesis of 2,3,5-Trisubstituted THF, Org. Lett., 2016, 18, 1542 CrossRef PubMed; (j) G. Pandey, S. K. Tiwari, B. Singh, K. Vanka and S. Jain, p-Selective (sp2)-C–H functionalization for an acylation/alkylation reaction using organic photoredox catalysis, Chem. Commun., 2017, 53, 12337 RSC; (k) X. Fan and Z. Gu, Palladium/Norbornene-Catalyzed Ortho-Acylation and Ipso-Selenation via C(O)–Se Bond Cleavage: Synthesis of α-Carbonyl Selane, Org. Lett., 2018, 20, 1187 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, reaction optimisation, and characterisation data. See DOI: 10.1039/d1qo01104a |
| ‡ A concerted mechanism proceeding through a 4-membered transition state was suggested by DFT studies on the related deoxydifluoromethylthiolation of carboxylic acids with BT-SCF2H (see ref. 13). |
| This journal is © the Partner Organisations 2021 |