A biomimetic synthesis-enabled stereochemical assignment of rhodotomentones A and B, two unusual caryophyllene-derived meroterpenoids from Rhodomyrtus tomentosa†
Abstract
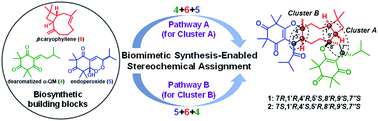
Rhodotomentones A and B (1 and 2), two unusual caryophyllene-derived meroterpenoids (CDMTs) featuring a rare 6/6/9/4/6/6 hexacyclic ring system, along with their biogenetically-related CDMTs 7 and 12–15, were isolated from Rhodomyrtus tomentosa. The planar structures of 1 and 2 were established by comprehensive spectroscopic data analyses. Based on their unique structural features, compounds 1 and 2 were hypothesized to be biosynthesized from three different building blocks. Inspired by two potentially biogenetic pathways, we completed the biosynthetic building block-based syntheses of 1 and 2, which enabled the unambiguous stereochemical assignment of 1 and 2. In addition, three CDMTs 10, 14, and 17 exhibited potent in vitro antiviral activities against respiratory syncytial virus (RSV).


 Please wait while we load your content...
Please wait while we load your content...