Clover leaf-shaped supramolecules assembled using a predesigned metallo-organic ligand†
Qixia
Bai‡
a,
Tun
Wu‡
a,
Zhe
Zhang
*ac,
Lianghuan
Xu
a,
Zhengbin
Tang
a,
Yuming
Guan
a,
Ting-Zheng
Xie
 a,
Mingzhao
Chen
a,
Peiyang
Su
a,
Heng
Wang
b,
Pingshan
Wang
a,
Mingzhao
Chen
a,
Peiyang
Su
a,
Heng
Wang
b,
Pingshan
Wang
 *a and
Xiaopeng
Li
*a and
Xiaopeng
Li
 *b
*b
aInstitute of Environmental Research at Greater Bay Area; Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education; Guangzhou Key Laboratory for Clean Energy and Materials; Guangzhou University, Guangzhou 510006, China. E-mail: chemwps@csu.edu.cn; zhezhang2018@gzhu.edu.cn
bCollege of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen, Guangdong 518055, China. E-mail: xiaopengli@szu.edu.cn
cGuangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou 510632, China
First published on 8th April 2021
Abstract
Inspired by the clover plant in nature, clover leaf-shaped supramolecular structures with three hexagons fused to create a triangular core were designed and self-assembled using a combination of Ru–Zn, Ru–Co, Ru–Mn or Ru–Ni metal ions. These results lay the foundation for further applications of heterometallic multinuclear metallo-supramolecules.
An important subfield of supramolecular chemistry, coordination-driven self-assembly has emerged in many synthetic systems that have dynamic characteristics over the past few decades.1,2 These metallo-supramolecular structures, that have increasing complexity and diversity, play an increasingly significant role in catalysis,3 sensing,4 drug delivery and release,5 gas storage,6 and smart materials7 due to their specific sizes and shapes. Of the diverse library of organic building blocks that exist, 2,2′:6′,2′′-terpyridine (tpy) has been extensively used as a tridentate motif because of its excellent complexation ability toward different metal ions.8 In addition to the common one-step self-assembly methodology that utilizes organic ligands and metal ions, the kinetically inert <tpy-Ru2+-tpy> connectivity allows for an alternative step-wise self-assembly approach for constructing supramolecular structures with increased complexity.9 Usually, a stable Ru(II)-organic ligand is synthesized and subsequently self-assembles with other metal ions with weak coordination, such as Zn(II), Cd(II) and Fe(II), to form heterometallic metallo-supramolecules.10 However, the lack of other suitable metal ions has created great obstacles for the diversity of tpy-based metallo-supramolecules.11 In most of these cases, Zn(II), Cd(II) and Fe(II) only serve as the connectors in the final supramolecular structures.12 The question this raises is whether we are able to use other metal ions to serve as connectors and to introduce potentially new functionality to the supramolecule on account of the redox properties, magnetic properties, and photo-activities of the ions.13 Recently, Ni(II), Co(II), Mn(II), and Cu(II) were used in a self-assembly process with tpy.14 However, this was still limited to the self-assembly of a single-type of metal ion with organic building blocks. Perhaps due to the challenges of the design and characterization of complex metal–organic building blocks, few cases have expanded the scope of this method to metal–organic ligands.15,16
Herein, we report the design and synthesis of four clover leaf-shaped bimetallic supramolecular structures (Scheme 1). The structures were obtained through the coordination of tetratopic metal–organic ligand L, which contains Ru(II), with 4 different transition metals, Zn(II), Co(II), Mn(II), and Ni(II). As designed, these complexes exhibit distinctive redox properties. The key metal–organic ligand L, which has four uncomplexed terpyridinyl units, was obtained via a 4-fold Suzuki coupling reaction of 4-(2,2′:6′,2′′-terpyridyl)-phenylboronic acid with a precursor, 5 (Scheme S1, ESI†). L and Zn(NTf2)2 were self-assembled in MeCN/MeOH at a stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The assembly was stirred at 60 °C for 8 h followed by addition of excess LiNTf2. The resulting solid was washed with water and MeOH. The reddish supramolecular metal complex Zn6L3 was obtained in a relatively high yield (98%). Subsequently, self-assembly with other metals was undertaken using some common metal salts, i.e., CoCl2·6H2O, MnClO4·6H2O and NiSO4·7H2O (Scheme S2, ESI†). As a result, a series of bimetallic clover leaf-shaped supramolecules with high symmetry were obtained. The structures were characterized using 1D and 2D NMR spectroscopy, electrospray ionization-mass spectrometry (ESI-MS), traveling wave ion mobility-mass spectrometry (TWIM-MS),17 gradient tandem-mass spectrometry (gMS2),18 transmission electron microscopy (TEM), and cyclic voltammetry (CV).
2. The assembly was stirred at 60 °C for 8 h followed by addition of excess LiNTf2. The resulting solid was washed with water and MeOH. The reddish supramolecular metal complex Zn6L3 was obtained in a relatively high yield (98%). Subsequently, self-assembly with other metals was undertaken using some common metal salts, i.e., CoCl2·6H2O, MnClO4·6H2O and NiSO4·7H2O (Scheme S2, ESI†). As a result, a series of bimetallic clover leaf-shaped supramolecules with high symmetry were obtained. The structures were characterized using 1D and 2D NMR spectroscopy, electrospray ionization-mass spectrometry (ESI-MS), traveling wave ion mobility-mass spectrometry (TWIM-MS),17 gradient tandem-mass spectrometry (gMS2),18 transmission electron microscopy (TEM), and cyclic voltammetry (CV).
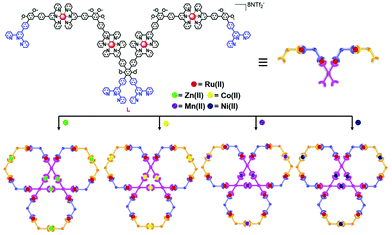 | ||
| Scheme 1 Self-assembly of clover leaf-shaped supramolecular structures obtained through the coordination of L with four different metal ions. | ||
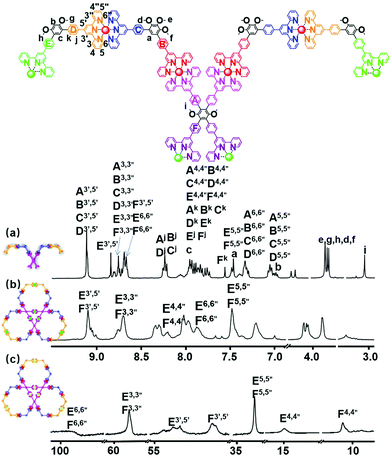
Fig. 1 shows the 1H NMR spectra of (a) ligand L, (b) Zn6L3 and (c) Co6L3. Three sets of distinctive signals appear at 9.11, 8.86 and 8.73 ppm, split in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, in the aromatic region of the spectrum of L. These are assigned to the three sets of tpyH3′,5′ protons of the Ru-based tpy moieties (A–D, E, and F tpy-phenyl peaks). In addition, the characteristic tpyH6,6′′ protons exhibited two sets of peaks, proving the formation of a highly symmetric structure (Fig. 1a). The other assignments were confirmed with the aid of 2D COSY and NOESY NMR spectroscopy (Fig. S16 and S17, ESI†). Shown in Fig. 1b, the signals of all the coordinated tpy moieties merged into broad peaks caused by their large planar structures. Compared with the signals of the free tpy groups of L, the proton signals attributed to E-tpyH3′,5′ and F-tpyH3′,5′ were shifted downfield, from 8.86 and 8.73 ppm to 9.12 ppm. Meanwhile, an expected upfield shift of the tpyH6,6′′ proton signals from 8.9 and 8.7 ppm to 7.82 ppm can be observed due to the electronic shielding effect that arises after coordination with the metals. The remaining signals of the Zn6L3 spectrum were confirmed using 2D COSY and NOESY NMR spectroscopy (Fig. S20 and S21, ESI†). In order to acquire more evidence of the structure, diffusion-ordered NMR spectroscopy (DOSY) was used to measure the size of Zn6L3. The DOSY spectrum (Fig. S22, ESI†) of Zn6L3 shows that the protons are found in a narrow band at log
1 ratio, in the aromatic region of the spectrum of L. These are assigned to the three sets of tpyH3′,5′ protons of the Ru-based tpy moieties (A–D, E, and F tpy-phenyl peaks). In addition, the characteristic tpyH6,6′′ protons exhibited two sets of peaks, proving the formation of a highly symmetric structure (Fig. 1a). The other assignments were confirmed with the aid of 2D COSY and NOESY NMR spectroscopy (Fig. S16 and S17, ESI†). Shown in Fig. 1b, the signals of all the coordinated tpy moieties merged into broad peaks caused by their large planar structures. Compared with the signals of the free tpy groups of L, the proton signals attributed to E-tpyH3′,5′ and F-tpyH3′,5′ were shifted downfield, from 8.86 and 8.73 ppm to 9.12 ppm. Meanwhile, an expected upfield shift of the tpyH6,6′′ proton signals from 8.9 and 8.7 ppm to 7.82 ppm can be observed due to the electronic shielding effect that arises after coordination with the metals. The remaining signals of the Zn6L3 spectrum were confirmed using 2D COSY and NOESY NMR spectroscopy (Fig. S20 and S21, ESI†). In order to acquire more evidence of the structure, diffusion-ordered NMR spectroscopy (DOSY) was used to measure the size of Zn6L3. The DOSY spectrum (Fig. S22, ESI†) of Zn6L3 shows that the protons are found in a narrow band at log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D = −9.88, which also demonstrates the formation of a discrete structure. The diffusion coefficient D was calculated to be 1.32 × 10−10 m2 s−1, from which the hydrodynamic radius, according to the Stokes–Einstein equation, is 2.42 nm for Zn6L3 (D = 4.84 nm). This result is consistent with the modelling data (4.67 nm). The paramagnetic nature of Co(II) is well-known, therefore, the Co(II) complexes were hard to characterize using 1H NMR. Nevertheless, we obtained the 1H NMR spectrum of Co6L3 (Fig. S23–25, ESI†) which spreads out over a wide range from 3 to 100 ppm (Fig. 1c). Although the 2D COSY and 2D NOESY spectra of the tpy protons could not be obtained because of fast relaxation, the 1H NMR signals from the tpy protons of Co6L3 could be assigned based on their characteristic chemical shifts and literature reports.14,19 Compared with Co(II), Mn(II) and Ni(II) exhibit stronger paramagnetic behaviour with shorter relaxation times, thus resulting in unsatisfactory 1H NMR spectra.20
D = −9.88, which also demonstrates the formation of a discrete structure. The diffusion coefficient D was calculated to be 1.32 × 10−10 m2 s−1, from which the hydrodynamic radius, according to the Stokes–Einstein equation, is 2.42 nm for Zn6L3 (D = 4.84 nm). This result is consistent with the modelling data (4.67 nm). The paramagnetic nature of Co(II) is well-known, therefore, the Co(II) complexes were hard to characterize using 1H NMR. Nevertheless, we obtained the 1H NMR spectrum of Co6L3 (Fig. S23–25, ESI†) which spreads out over a wide range from 3 to 100 ppm (Fig. 1c). Although the 2D COSY and 2D NOESY spectra of the tpy protons could not be obtained because of fast relaxation, the 1H NMR signals from the tpy protons of Co6L3 could be assigned based on their characteristic chemical shifts and literature reports.14,19 Compared with Co(II), Mn(II) and Ni(II) exhibit stronger paramagnetic behaviour with shorter relaxation times, thus resulting in unsatisfactory 1H NMR spectra.20
In addition, ESI-MS coupled with TWIM-MS was applied to validate the proposed structures. Fig. 2a shows a series of peaks with continuous charges from 11+ to 21+ for Zn6L3 due to the successive loss of the NTf2− counterion. After deconvolution, the obtained molecular weight of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 007 Da agreed well with the proposed molecular composition [(C234H210N36O12)3Ru12Zn6(NTF2−)36]. The experimental isotope pattern of each charged state is consistent with the simulated isotopic distribution (Fig. S6, ESI†). TWIM-MS showed a series of charged states with a narrow drift time distribution ranging from 11+ to 20+, excluding the formation of other isomers or conformers (Fig. 2b). Moreover, the molecular weights of Co6L3, Mn6L3 and Ni6L3 were also confirmed to correspond with their proposed molecular compositions (Fig. 2c, e and Fig. S4, ESI†). Similarly, the complexes with Co(II), Mn(II) and Ni(II) have comparable drift times in the same charge states (Fig. 2d, f and Fig. S5, ESI†), indicating that these complexes have similar shapes.
007 Da agreed well with the proposed molecular composition [(C234H210N36O12)3Ru12Zn6(NTF2−)36]. The experimental isotope pattern of each charged state is consistent with the simulated isotopic distribution (Fig. S6, ESI†). TWIM-MS showed a series of charged states with a narrow drift time distribution ranging from 11+ to 20+, excluding the formation of other isomers or conformers (Fig. 2b). Moreover, the molecular weights of Co6L3, Mn6L3 and Ni6L3 were also confirmed to correspond with their proposed molecular compositions (Fig. 2c, e and Fig. S4, ESI†). Similarly, the complexes with Co(II), Mn(II) and Ni(II) have comparable drift times in the same charge states (Fig. 2d, f and Fig. S5, ESI†), indicating that these complexes have similar shapes.
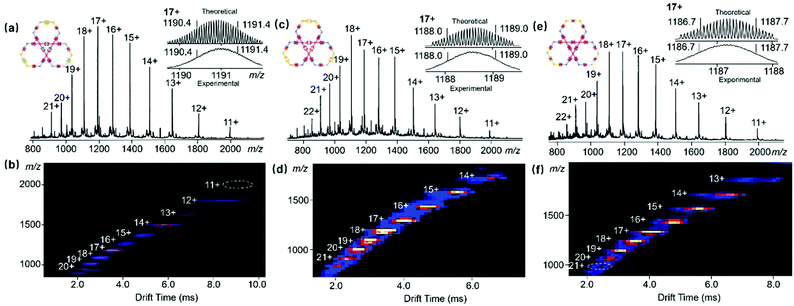 | ||
| Fig. 2 ESI-MS of (a) Zn6L3, (c) Co6L3 and (e) Mn6L3; TWIM-MS plots (m/z vs. drift time) of (b) Zn6L3, (d) Co6L3 and (f) Mn6L3. | ||
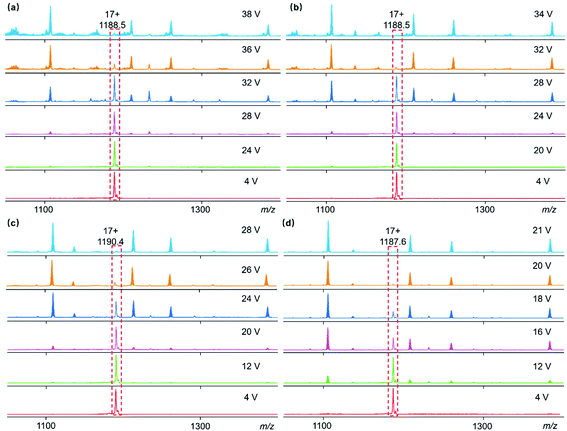
In order to examine the stability of the supramolecular complex, gMS2 experiments were performed on the 17+ ions at m/z 1190.4 via collision-induced dissociation with collision energies ranging from 4 to 28 V (Fig. 3c). There was no obvious fragmentation peak observed below 20 V and when the voltage reached 28 V the complex ions completely dissociated. The stability of Co6L3, Mn6L3 and Ni6L3 was examined under the same test conditions. The 17+ ions of Ni6L3 dissociated at 38 V, while Co6L3 and Mn6L3 became fragments at 34 V and 21 V, respectively. The stabilities of these supermolecules in the gas phase were estimated and were found to depend on the metal ions with a relative order of Ni > Co > Zn > Mn. This is similar to the relative order of stabilities observed for previously reported simple complexes.21,22
Furthermore, TEM also provided evidence for the formation of the clover-type bimetallic supramolecular structure. As shown in Fig. 4b, a reasonable measured diameter of 4.90 nm could be observed from the TEM image. This is similar to the size simulated from molecular modelling (4.67 nm) (Fig. 4b and Fig. S26–S28, ESI†). Finally, CV was used to characterize the electrical properties of the supramolecules, and a three-electrode working system consisting of a 3 mm glassy carbon electrode (WE), platinum wire electrode (CE) and Ag/AgCl electrode (RE) was used for testing. Due to the oxidation of the Ru(II)/Ru(III) and Ru(III)/Ru(IV) couples,23 ligand L has two oxidation peaks near 1.05 and 1.25 V (Fig. 5). In contrast, the Ru2+/3+ and Ru3+/4+ oxidation peaks of the supramolecular structure are slightly shifted compared with ligand L. Since Zn(II) is already in its highest oxidation state, only the oxidation peaks of Ru can be observed at 0.84 and 1.16 V.24 In Fig. 5b and c, the irreversible oxidation of Co(II) in Co6L3 can be seen peaking at −0.35 V, while the irreversible oxidation peak of Ni(II) is located at −0.48 V. The supramolecular structure of Mn6L3 gives rise to similar CV curves seen in ligand L.25,26 The photochemical properties of these complexes were also studied using UV-visible spectroscopy and low temperature fluorescence spectroscopy. The absorption spectra of the ligand and all complexes have a characteristic absorption peak near 495 nm, which can be attributed to the metal-to-ligand charge transfer transitions of the tpy-Ru-tpy unit (Fig. S29, ESI†).27 The emissions from L and the supramolecules were detected in CH3CN solution under 73 K (Fig. S30†). The emission spectra of Ni6L3, Mn6L3 and Co6L3 overlapped with a major peak at 653 nm while the major peak of Zn6L3 shows a slight shift to 648 nm.28,29
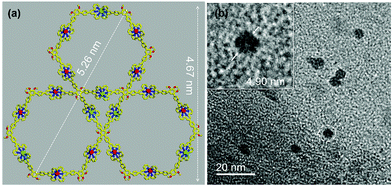 | ||
| Fig. 4 (a) Representative energy-minimized structure obtained from molecular modelling of Zn6L3, (b) TEM images of Zn6L3. | ||
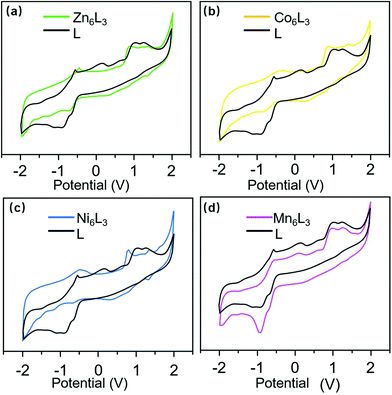 | ||
| Fig. 5 CV of L with (a) Zn6L3, (b) Co6L3, (c) Ni6L3, and (d) Mn6L3 (in a 0.1 M solution of Bu4NPF6 in CH3CN). | ||
In conclusion, four clover leaf-shaped metallo-supramolecular structures were successfully designed and synthesized. This report is the first example where Mn2+, Co2+, Ni2+ metal ions are used in a heterometallic multinuclear metallo-supramolecular system. The structures were characterized using 1D and 2D NMR, high-resolution ESI-MS, TWIM-MS, gMS2, TEM, CV, UV-vis and fluorescence spectroscopy. Moreover, we anticipate that these multinuclear metallo-supramolecules may serve as a model system for further study of the self-assembly behavior and physical properties of 2D materials.
Author contributions
All authors have given approval to the final version of the manuscript. Z.Z., X.L. and P.W. designed the experiments; T.W. and Q.B. completed the synthesis; T.W. and Z.T. carried out the NMR analysis; Q.B. and T.W. did the ESI-MS test and data curation; L.X., Y.G. and M.C. completed the TEM characterization; P.S. performed the low temperature fluorescence measurement; Q.B. and Z.Z. analyzed the experiment data; Q.B. and T.W. wrote the manuscript; Z.Z., X.L., H.W., T.X. and P.W. edited the manuscript. All the authors discussed the results and commented on and proofread the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (21971257 to P.W., 21806027 to P.S.), the Guangdong Natural Science Foundation (2019A1515011358 to Z.Z., 2018A030313479 to P.S.), the Science and Technology Research Project of Guangzhou (202002030257 to Z.Z.), the Open Fund of Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications (2020A07 to Z.Z.). The authors thank Dr Bokai Liao for the CV tests and the TEM tests from the Modern Analysis and Testing Center of Guangzhou University.Notes and references
- (a) R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Supramolecular coordination: self-assembly of finite two- and three-dimensional ensembles, Chem. Rev., 2011, 111, 6810–6918 CrossRef CAS PubMed; (b) M. D. Ward and P. R. Raithby, Functional behaviour from controlled self-assembly: challenges and prospects, Chem. Soc. Rev., 2013, 42, 1619–1636 RSC.
- (a) M. Han, D. M. Engelhard and G. H. Clever, Self-assembled coordination cages based on banana-shaped ligands, Chem. Soc. Rev., 2014, 43, 1848–1860 RSC; (b) G. R. Newkome and C. N. Moorefield, From 1 → 3 dendritic designs to fractal supramacromolecular constructs: understanding the pathway to the Sierpiński gasket, Chem. Soc. Rev., 2015, 44, 3954–3967 RSC; (c) H. Wang, Y. Li, N. Li, A. Filosa and X. Li, Increasing the size and complexity of discrete 2D metallosupramolecules, Nat. Rev. Mater., 2020, 6, 145–167 CrossRef.
- M. Schulze, V. Kunz, P. D. Frischmann and F. Wurthner, A supramolecular ruthenium macrocycle with high catalytic activity for water oxidation that mechanistically mimics photosystem II, Nat. Chem., 2016, 8, 576–583 CrossRef CAS PubMed.
- (a) L.-J. Chen, Y.-Y. Ren, N.-W. Wu, B. Sun, J.-Q. Ma, L. Zhang, H. Tan, M. Liu, X. Li and H.-B. Yang, Hierarchical self-assembly of discrete organoplatinum(II) metallacycles with polysaccharide via electrostatic interactions and their application for heparin detection, J. Am. Chem. Soc., 2015, 137, 11725–11735 CrossRef CAS PubMed; (b) Y. Liu, L. Perez, A. D. Gill, M. Mettry, L. Li, Y. Wang, R. J. Hooley and W. Zhong, Site-selective sensing of histone methylation enzyme activity via an arrayed supramolecular tandem assay, J. Am. Chem. Soc., 2017, 139, 10964–10967 CrossRef CAS PubMed.
- Y.-R. Zheng, K. Suntharalingam, T. C. Johnstone and S. J. Lippard, Encapsulation of Pt(IV) prodrugs within a Pt(II) cage for drug delivery, Chem. Sci., 2015, 6, 1189–1193 RSC.
- K. Schwamborn and R. M. Caprioli, Molecular imaging by mass spectrometry-looking beyond classical histology, Nat. Rev. Cancer, 2010, 10, 639–646 CrossRef CAS PubMed.
- (a) Z.-Y. Li, Y. Zhang, C.-W. Zhang, L.-J. Chen, C. Wang, H. Tan, Y. Yu, X. Li and H.-B. Yang, Cross-linked aupramolecular polymer gels constructed from discrete multi-pillar[5]arene metallacycles and their multiple stimuli-responsive behavior, J. Am. Chem. Soc., 2014, 136, 8577–8589 CrossRef CAS PubMed; (b) H. Wang, X. Qian, K. Wang, M. Su, W.-W. Haoyang, X. Jiang, R. Brzozowski, M. Wang, X. Gao, Y. Li, B. Xu, P. Eswara, X.-Q. Hao, W. Gong, J.-L. Hou, J. Cai and X. Li, Supramolecular Kandinsky circles with high antibacterial activity, Nat. Commun., 2018, 9, 1815–1824 CrossRef PubMed; (c) X. Yan, T. R. Cook, J. B. Pollock, P. Wei, Y. Zhang, Y. Yu, F. Huang and P. J. Stang, Responsive supramolecular polymer metallogel constructed by orthogonal coordination-driven self-assembly and host/guest interactions, J. Am. Chem. Soc., 2014, 136, 4460–4463 CrossRef CAS PubMed.
- (a) J.-H. Fu, Y.-H. Lee, Y.-J. He and Y.-T. Chan, Facile self-assembly of metallo-supramolecular ring-in-ring and spiderweb structures using multivalent terpyridine ligands, Angew. Chem., Int. Ed., 2015, 54, 6231–6235 CrossRef CAS PubMed; (b) T.-Z. Xie, S.-Y. Liao, K. Guo, X. Lu, X. Dong, M. Huang, C. N. Moorefield, S. Z. D. Cheng, X. Liu, C. Wesdemiotis and G. R. Newkome, Construction of a highly symmetric nanosphere via a one-pot reaction of a tristerpyridine ligand with Ru(II), J. Am. Chem. Soc., 2014, 136, 8165–8168 CrossRef CAS PubMed.
- Z. Jiang, Y. Li, M. Wang, B. Song, K. Wang, M. Sun, D. Liu, X. Li, J. Yuan, M. Chen, Y. Guo, X. Yang, T. Zhang, C. N. Moorefield, G. R. Newkome, B. Xu, X. Li and P. Wang, Self-assembly of a supramolecular hexagram and a supramolecular pentagram, Nat. Commun., 2017, 8, 15476–15485 CrossRef CAS PubMed.
- Z. Zhang, Y. Li, B. Song, Y. Zhang, X. Jiang, M. Wang, R. Tumbleson, C. Liu, P. Wang, X.-Q. Hao, T. Rojas, A. T. Ngo, J. L. Sessler, G. R. Newkome, S. W. Hla and X. Li, Intra- and intermolecular self-assembly of a 20 nm-wide supramolecular hexagonal grid, Nat. Chem., 2020, 12, 468–474 CrossRef CAS PubMed.
- T. Wu, J. Yuan, B. Song, Y.-S. Chen, M. Chen, X. Xue, Q. Liu, J. Wang, Y.-T. Chan and P. Wang, Stepwise self-assembly of a discrete molecular honeycomb using a multitopic metallo-organic ligand, Chem. Commun., 2017, 53, 6732–6735 RSC.
- S. Chakraborty and G. R. Newkome, Terpyridine-based metallosupramolecular constructs: tailored monomers to precise 2D-motifs and 3D-metallocages, Chem. Soc. Rev., 2018, 47, 3991–4016 RSC.
- X. Yang, D. Zhang, J. Li, W. Ji, N. Yang, S. Gu, Q. Wu, Q. Jiang, P. Shi and L. Li, A mitochondrion-targeting Mn(II)-terpyridine complex for two-photon photodynamic therapy, Chem. Commun., 2020, 56, 9032–9035 RSC.
- L. Wang, B. Song, S. Khalife, Y. Li, L.-J. Ming, S. Bai, Y. Xu, H. Yu, M. Wang, H. Wang and X. Li, Introducing seven transition metal ions into terpyridine-based supramolecules: self-assembly and dynamic ligand exchange study, J. Am. Chem. Soc., 2020, 142, 1811–1821 CrossRef CAS PubMed.
- T.-Z. Xie, X. Wu, K. J. Endres, Z. Guo, X. Lu, J. Li, E. Manandhar, J. M. Ludlow, C. N. Moorefield, M. J. Saunders, C. Wesdemiotis and G. R. Newkome, Supercharged, precise, megametallodendrimers via a single-step, quantitative, assembly process, J. Am. Chem. Soc., 2017, 139, 15652–15655 CrossRef CAS PubMed.
- M. Chen, J. Wang, S.-C. Wang, Z. Jiang, D. Liu, Q. Liu, H. Zhao, J. Yan, Y.-T. Chan and P. Wang, Truncated Sierpiński triangular assembly from a molecular mortise-tenon joint, J. Am. Chem. Soc., 2018, 140, 12168–12174 CrossRef CAS PubMed.
- S. Perera, X. Li, M. Soler, A. Schultz, C. Wesdemiotis, C. N. Moorefield and G. R. Newkome, Hexameric palladium(II) terpyridyl metallomacrocycles: assembly with 4,4′-bipyridine and characterization by TWIM mass spectrometry, Angew. Chem., Int. Ed., 2010, 49, 6539–6544 CrossRef CAS PubMed.
- X. Li, Y.-T. Chan, G. R. Newkome and C. Wesdemiotis, Gradient tandem mass spectrometry interfaced with Ion mobility separation for the characterization of supramolecular architectures, Anal. Chem., 2011, 83, 1284–1290 CrossRef CAS PubMed.
- E. C. Constable, K. Harris, C. E. Housecroft, M. Neuburger and J. A. Zampese, Turning {M(tpy)2}n+ embraces and CH⋯π interactions on and off in homoleptic cobalt(II) and cobalt(III) bis(2,2′: 6′,2′′-terpyridine) complexes, CrystEngComm, 2010, 12, 2949–2961 RSC.
- H. S. Chow, E. C. Constable, C. E. Housecroft, K. J. Kulicke and Y. Tao, When electron exchange is chemical exchange-assignment of 1H NMR spectra of paramagnetic cobalt(II)-2,2′: 6′,2′′-terpyridine complexes, Dalton Trans., 2005, 2, 236–237 RSC.
- M. Satterfield and J. S. Brodbelt, Relative binding energies of gas-phase pyridyl ligand/metal complexes by energy-variable collisionally activated dissociation in a quadrupole ion trap, Inorg. Chem., 2001, 40, 5393–5400 CrossRef CAS PubMed.
- M. A. Meier, B. G. Lohmeijer and U. S. Schubert, Relative binding strength of terpyridine model complexes under matrix-assisted laser desorption/ionization mass spectrometry conditions, J. Mass Spectrom., 2003, 38, 510–516 CrossRef CAS PubMed.
- M. Chen, D. Liu, J. Huang, Y. Li, M. Wang, K. Li, J. Wang, Z. Jiang, X. Li and P. Wang, Trefoiled propeller-shaped spiral terpyridyl metal-organic architectures, Inorg. Chem., 2019, 58, 11146–11154 CrossRef CAS PubMed.
- Z. Zhang, H. Wang, X. Wang, Y. Li, B. Song, O. Bolarinwa, R. A. Reese, T. Zhang, X.-Q. Wang, J. Cai, B. Xu, M. Wang, C. Liu, H.-B. Yang and X. Li, Supersnowflakes: stepwise self-assembly and dynamic exchange of rhombus star-shaped supramolecules, J. Am. Chem. Soc., 2017, 139, 8174–8185 CrossRef CAS PubMed.
- N. Elgrishi, M. B. Chambers, V. Artero and M. Fontecave, Terpyridine complexes of first row transition metals and electrochemical reduction of CO2 to CO, Phys. Chem. Chem. Phys., 2014, 16, 13635–13644 RSC.
- I. F. Mansoor, D. I. Wozniak, Y. Wu and M. C. Lipke, A delocalized cobaltoviologen with seven reversibly accessible redox states and highly tunable electrochromic behaviour, Chem. Commun., 2020, 56, 13864–13867 RSC.
- K. C. Robson, B. D. Koivisto, T. J. Gordon, T. Baumgartner and C. P. Berlinguette, Triphenylamine-modified ruthenium(II) terpyridine complexes: enhancement of light absorption by conjugated bridging motifs, Inorg. Chem., 2010, 49, 5335–5337 CrossRef CAS PubMed.
- X.-J. Yang, F. Drepper, B. Wu, W.-H. Sun, W. Haehnel and C. Janiak, From model compounds to protein binding: syntheses, characterizations and fluorescence studies of [RuII(bipy)(terpy)L]2+ complexes (bipy = 2,2′-bipyridine; terpy = 2,2′: 6′,2′′-terpyridine; L = imidazole, pyrazole and derivatives, cytochrome c), Dalton Trans., 2005, 2, 256–267 RSC.
- M. Galletta, S. Campagna, M. Quesada, G. Ulrich and R. Ziessel, The elusive phosphorescence of pyrromethene-BF2 dyes revealed in new multicomponent species containing Ru(II)-terpyridine subunits, Chem. Commun., 2005, 33, 4222–4224 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Full experimental details, the 1H NMR, 13C NMR, COSY, NOESY of the new compounds, ESI-MS spectra of related compounds. See DOI: 10.1039/d1qo00336d |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2021 |