Efficient stereoselective synthesis of chiral 3,3′-dimethyl-(2,2′-bipyridine)-diol ligand and applications in FeII-catalysis†
Abstract
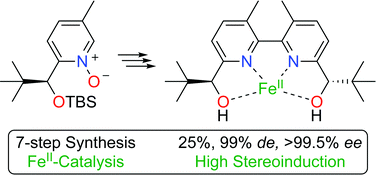
A seven step synthesis of a chiral 2,2′-bipyridinediol ligand with 3,3′-dimethyl substituents was achieved starting from commercially available materials. The O2-mediated oxidative homocoupling reaction of a chiral pyridine N-oxide was demonstrated to be the key step to prepare the S,S enantiomer of the title ligand with excellent stereoselectivities, i.e., 99% de and >99.5% ee. An unusual heptacoordination of FeII when complexed with the chiral 2,2′-bipyridinediol ligand was highlighted from single crystal diffraction analysis. Steric strain due to the 3,3′-dimethyl groups was revealed from the structural analysis of the obtained FeII complex. Asymmetric induction using this chiral 3,3′-dimethyl-(2,2′-bipyridine)-diol ligand was studied in the Mukaiyama aldol and thia-Michael reactions. An increase of chiral induction in the latter one was achieved using the FeII catalyst made from newly synthesized ligand vs. Bolm's ligand.


 Please wait while we load your content...
Please wait while we load your content...