Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stereoselective synthesis and applications of spirocyclic oxindoles
Alexander J.
Boddy
 and
James A.
Bull
and
James A.
Bull
 *
*
Department of Chemistry, Imperial College London, Molecular Sciences Research Hub, White City Campus, Wood Lane, London W12 0BZ, UK. E-mail: j.bull@imperial.ac.uk
First published on 6th January 2021
Abstract
The development of novel synthetic strategies to form new chemical entities in a stereoselective manner is an ongoing significant objective in organic and medicinal chemistry. This review analyses the development of new stereoselective approaches to spirocyclic oxindoles with spiro-3- to 8-membered rings. It highlights the importance of these structures for applications in medicinal chemistry, as intermediates or final products in total synthesis and as model compounds for the development of enantioselective catalytic methodologies.
Introduction
The application of spirocyclic structures in drug discovery has seen a dramatic increase in attention in recent years, alongside major developments in their synthetic chemistry.1 Defined as a bicycle connected by a single fully-substituted carbon atom, which is not connected by an adjacent atom, spirocycles are inherently highly 3-dimensional structures. The shared tetrahedral sp3-carbon atom positions the planes of the 2 rings orthogonally, despite the torsional strain this may impose on the substituents of the rings.2 Spirocyclic compounds can improve certain physicochemical properties such as lipophilicity, aqueous solubility and metabolic stability, in comparison to the respective monocyclic structure.3 Furthermore, they access relatively underexplored chemical space and novel intellectual property (IP) space. Saturated spirocycles provide a dense, rigid scaffold, with the potential to append more substituents, and so occupy an increased number of defined vectors compared to flat aromatic compounds.4 All of these factors have contributed to a greater uptake in medicinal chemistry and have demanded significant advances in organic synthesis to provide spirocycles in a controlled and stereoselective manner.5This review focuses specifically on spirocyclic oxindoles (spirooxindoles or spiroindolones). These are a widespread motif within modern organic synthesis, drug discovery and natural product chemistry. Stereoselective synthetic methods towards this privileged class of spirocycles have seen enormous development in recent years. This review aims to combine the analysis of recent synthetic strategies with an overview of the importance of these scaffolds for medicinal chemistry and in natural product synthesis. Since 1950 there have been 6896 publications containing spirooxindoles, 3283 of these publications have appeared since 2012.15 Oxindoles are often used as rigid scaffolds for testing new asymmetric synthetic methodology and due to the demand of discovery chemistry for controlled and modular syntheses, and with the plethora of publications in this area, we will focus on stereoselective processes. This review examines spirocyclic oxindoles containing a spiro-(3 to 8)-membered ring, in turn, analysing carbocyclic then monoheteroatom nitrogen-containing and oxygen-containing spirocycles. The review will cover recent developments from 2013, following major work by Singh and Desta,6 until April 2020. Specific bioactivity and applications of each ring system will be discussed at the start of the relevant section and provide a reference work for the preparation of different spirocyclic patterns. Within each section, the discussion is split by the reaction type employed to construct the spirocycle.
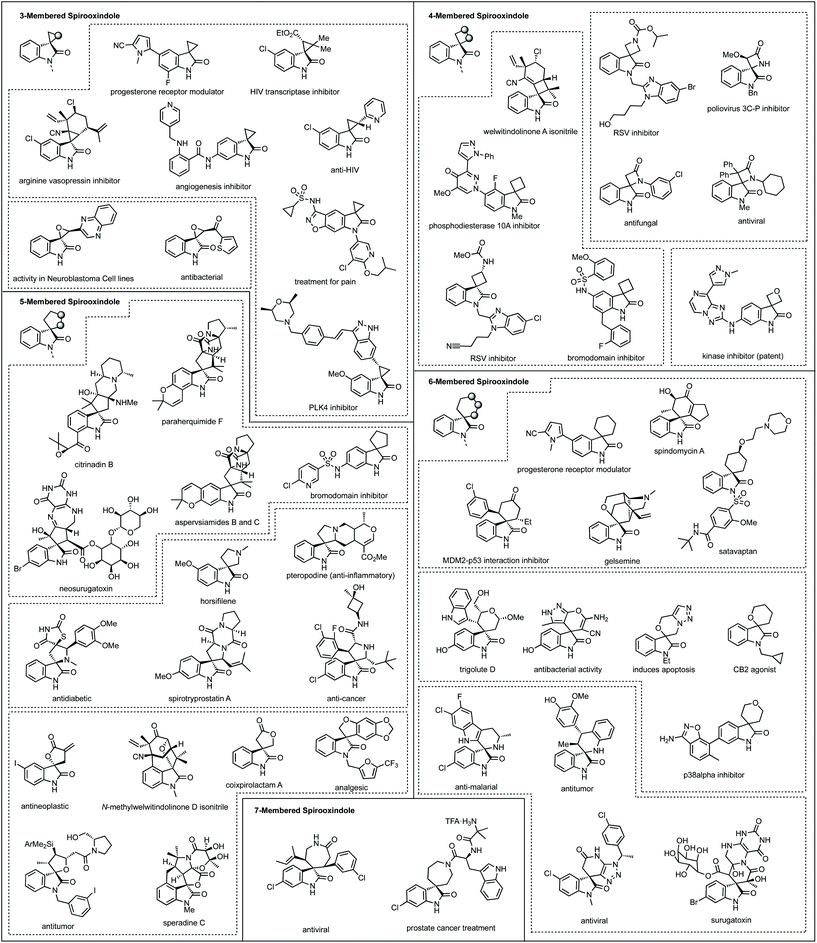
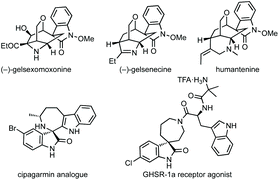
Fig. 1 shows a representative set of each ring system, which will be covered in this review, as they feature in medicinal or natural products.7 Notable biological activity is indicated, including use as anti-cancer agents,8 and anti-viral agents.9 Some ring types are not represented in these bioactive compounds, i.e. aziridines, likely due to their instability relative to larger ring sizes, but will nonetheless be featured in the review.
Given the importance of this structural class, spirocyclic oxindoles have been featured in other reviews discussing their synthesis,10 including asymmetric synthesis,11 use of isatin starting materials12 or the synthesis of target product scaffolds.13 General reviews on spirocyclic compounds (i.e. spiroindolenes) also often contain spirocyclic oxindoles without specifically focusing on these.14 We expect the analysis presented in this review of the structural types, synthesis and applications to lead to further studies, and aid in the identification of future opportunities to expand the applications of this fascinating class of compounds.
Frequency analysis of spirooxindoles
To quantify the importance of spiroindolones in the medicinal chemistry and organic synthesis literature, we analysed publications which feature spiroindolones containing up to one heteroatom in the spirocycle between 1970–2020 (Fig. 2).15 The number of publications in which these structures feature has grown significantly over the last 50 years and has consistently reached numbers above 400 per year since 2013. Even accounting for the generalised increase in publications, this represents an extensive level of attention. The number of publications on 5- and 6-membered rings (silver and gold in Fig. 2) dominates the contribution to this total. However, in the last decade the relative contribution of 3-, 4- and 7-membered spirocycles (dark/light blues and orange) has increased.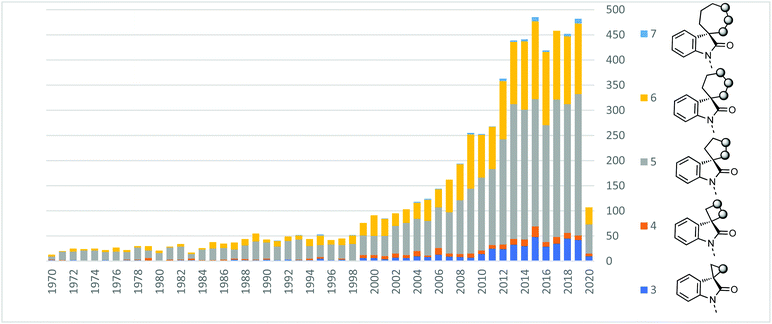 | ||
| Fig. 2 Incidence of 3-to 7-membered spirooxindoles in the literature up to April 2020. No. of heteroatoms in ring ≤1.15 | ||
We also analysed the frequency of the different types of rings which feature in this review (Fig. 3). Considering 3-membered spirocycles, we can see that cyclopropane rings are much more common than their aziridine or epoxide analogues. Similarly, cyclobutanes far outnumber azetidines or oxetanes. Notably the number of oxetanes is surprisingly low, especially, when compared to azetidines. The position of the heteroatom has a significant influence on the frequency of a certain ring. This may generally correlate with ease of synthesis or lower complexity, i.e. 3,4′-spirotetrahydropyran oxindoles far outnumber the 3,2′- or 3,3′-analogues. 7-Membered rings are generally underrepresented when bridged examples are discounted (these will generally have been counted in the numbers for other ring sizes as these are a less significant contribution). There is only one example of a non-bridged mono-heteroatom containing 8-membered spirooxindole in the literature (synthesised as an analogue of cipargamin).16
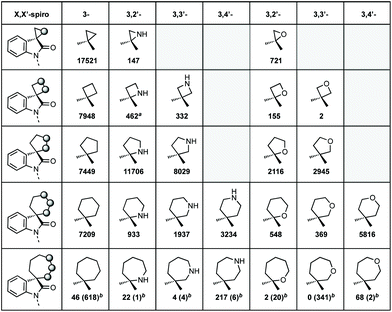 | ||
Fig. 3 The spirocyclic rings covered in this review and the number of hits for each heteroatom position.15a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Beta-lactams or derivatives thereof. b Beta-lactams or derivatives thereof. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Numbers in brackets indicates bridged examples. Numbers in brackets indicates bridged examples. | ||
Although there will be a clear correlation between the number of publications for each ring size, we have tried to discuss each ring type on an equal basis, though inevitably the 5-membered nitrogen section is largest.
Three-membered rings
Spirocyclopropyl oxindoles
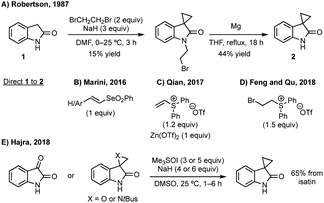
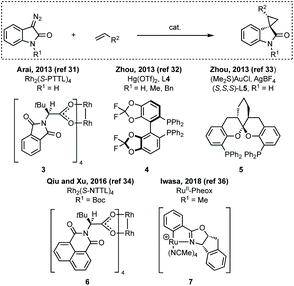
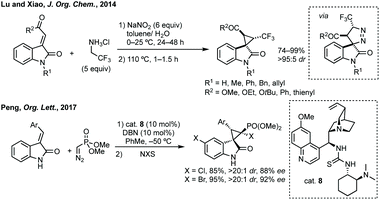
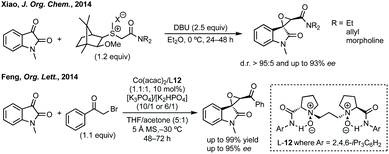
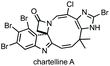
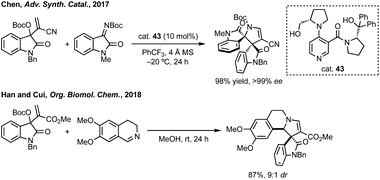
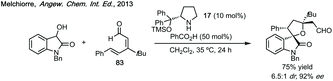
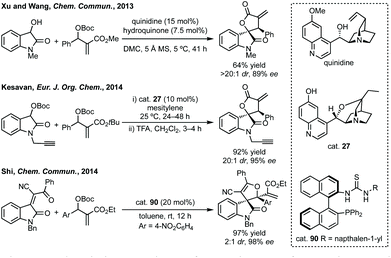
Three-membered ring containing spiroindolones feature in pharmaceutical compounds as well as being used as reactive intermediates, i.e. in ring opening reactions.17 These ring opening reactions can often be coupled with ring closing reactions to form spirocycles of larger ring size. There has recently been an excellent review on the catalytic enantioselective synthesis of polysubstituted spirocyclopropyl oxindoles by Cao and Zhou,18 as well as a review of transition metal-free strategies by Ashfeld.19In 2014, Lu and Xiao showed a [3 + 2]-cycloaddition between 3-ylideneoxindoles and in situ generated 2,2,2-trifluorodiazoethane could afford a pyrazoline which upon heating under reflux in toluene would ring contract to afford 3,3′-cyclopropyl spirooxindoles.38 Using chemistry first developed by Carreira,39 sodium nitrite was used to oxidise 2,2,2-trifluoroethylamine·HCl to generate 2,2,2-trifluorodiazoethane which can undergo a [3 + 2]-cycloaddition with the electron deficient alkene followed by heating to liberate N2 and form the cyclopropane with high yield and dr (Scheme 3). Using a similar cycloaddition and ring contraction strategy, Babu demonstrated the synthesis of aryl substituted 3,3′-cyclopropyl spirooxindoles while Han and Chen have reported the synthesis of difluoromethyl substituted spirocyclic cyclopropanes.40,41 A significant advance in this methodology accesses enantioenriched spirocyclic cyclopropanes through a 1,3-dipolar cycloaddition between dimethyl (diazomethyl)phosphonate and 3-ylideneoxindoles followed by ring contraction mediated by NCS or NBS (this also caused chlorination/bromination by SEAr).42 Peng used thiourea catalyst 8 derived from a cinchona alkaloid to induce enantioselectivity in the pyrazoline formation and this ee was retained in the 5- to 3-membered ring contraction (Scheme 3).
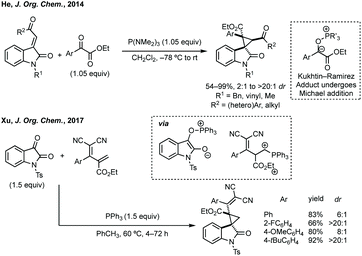 | ||
| Scheme 4 P(NMe2)3-mediated reductive cyclopropanation via Kukhtin–Ramirez (K–R) adduct and K–R adduct reaction with diene activated by PPh3. | ||
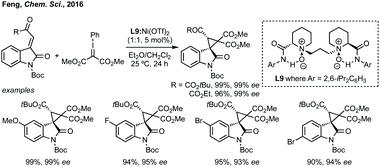
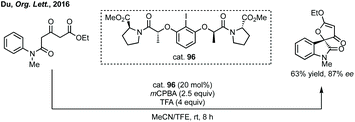
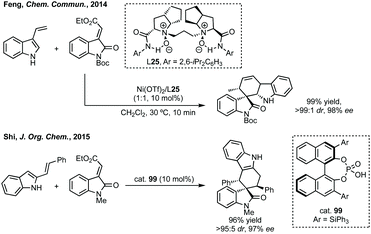
Du developed a Michael addition/alkylation cascade reaction between 3-chlorooxindoles and arylidenepyrazolones, alkenyl thiazolones (also developed by Sheng and Feng) or, more recently, 2,3-dioxopyrrolidines.46 In a quite distinct method, a Ni-catalysed enantioselective cyclopropanation developed by Feng utilised phenyliodonium ylides to generate a free carbene which can react with 3-ylideneoxindoles to generate 3,3′-cyclopropyl spirooxindoles in high yield, dr and ee (Scheme 5).47 More recently, Feng used a related system for the Mg catalysed reaction of 3-ylidene oxindoles and sulfonium ylides.48
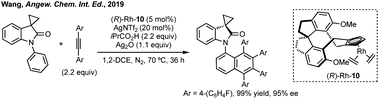
Wang reported an enantioselective Satoh–Miura type reaction using a RhIII catalyst 10 to perform a dual C–H activation forming an axially chiral spirocycle in high enantioselectivity (Scheme 7).50
Spiroaziridinyl oxindoles
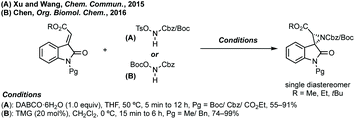
Spiroaziridines don't commonly feature in natural products or medicines, but are utilised in synthesis, and protected versions could be envisaged to be of use in biology.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr vs. 6.6
1 dr vs. 6.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
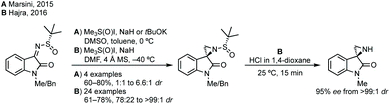
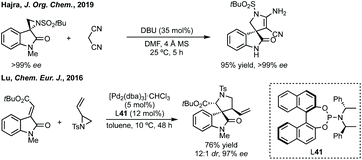
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr).55 Both reports use trimethylsulfoxonium iodide with either NaH or tBuOK and Hajra found that using DMF as solvent at lower temperature gave much higher diastereoselectivity. Hajra demonstrated one example of deprotection of the sulfinimide converting a protected aziridine with >99
1 dr).55 Both reports use trimethylsulfoxonium iodide with either NaH or tBuOK and Hajra found that using DMF as solvent at lower temperature gave much higher diastereoselectivity. Hajra demonstrated one example of deprotection of the sulfinimide converting a protected aziridine with >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr to the free aziridine with 95% ee, which was subsequently shown to be unstable.55
1 dr to the free aziridine with 95% ee, which was subsequently shown to be unstable.55
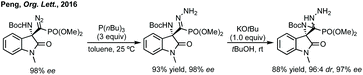
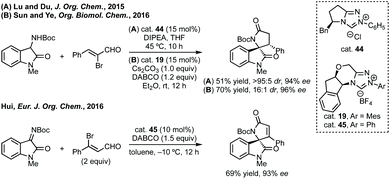
Peng developed an asymmetric Mannich reaction of α-diazophosphonates as nucleophiles with isatin N-Boc ketimines catalysed by an asymmetric phosphoric acid (Scheme 11).56 The product diazo functionality could be reduced using tributylphosphine to afford the chiral hydrazone which could be cyclised to afford the enantiopure aziridine, with undefined stereochemistry at the hydrazine/phosphonate chiral centre.
Spiroepoxy oxindoles
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr.65
1 dr.65
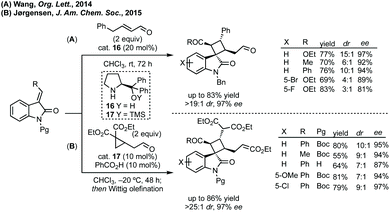
The first report of an enantioselective epoxidation to form a spiro-epoxyoxindole was by Metzner and Briere in 2007, though only one example with 30% ee was given.66 In 2011, Gasperi developed a moderately stereoselective epoxidation of 3-ylideneoxindoles using tert-butyl hydroperoxide with a prolinol catalyst.67 More recently, Gasperi reported a full study of this work and disclosed a highly enantioselective epoxidation reaction of this type, when the oxindole protecting group was Boc, though the diastereoselectivity was poor.68 In 2014, Xiao reported the use of camphor-derived sulfonium salts in an asymmetric epoxidation of isatins (Scheme 13).69 Substitution on the oxindole did not significantly affect the high enantioselectivity, though changing the R group on the sulfonium salt did reduce the enantioselectivity slightly. Feng described an enantioselective Darzens reaction to synthesise spiro-epoxyoxindoles using L-12 as a hydrogen bonding ligand to induce enantioselectivity in an aldol reaction which is followed by cyclisation to afford the three-membered ring (Scheme 13).70 Lower enantioselectivities were observed when the aryl group of the acyl bromides or the fused oxindole ring were substituted (ee <85%).
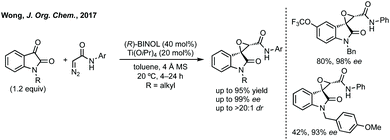
Improved enantioselectivity was achieved by Wong in 2017 in an asymmetric Darzens reaction using diazoacetamides (Scheme 14).71 High yields and enantioselectivities (up to 99% ee) were observed using a titanium/BINOL complex and this reaction had a broad scope without reduction in enantioselectivity.
Four-membered rings
Spirocyclobutyl oxindoles
Advances in stereoselective spirocyclobutane oxindoles have been mainly limited to achievements in C–H activation chemistry and [2 + 2]-cycloadditions.
Baudoin formed 3-cyclobutyl N-methyl-oxindole through C–H activation when trying to develop an arylation/electrocyclic cascade reaction.82,83 Xu could form the same unsubstituted cyclobutane, as well as 5- and 6-membered analogues, in an intramolecular 1,5-HAT using aryl iodides by visible light photoredox catalysis.84 Gouverneur recently developed a silyl radical-mediated hydrosulfamoylation using sulfonyl chlorides and could effect a cascade spirocyclisation (Giese-type addition followed by aryl C–H transfer) from cyclobutene 15, albeit with only poor diastereoselectivity (Scheme 16).85
 | ||
| Scheme 17 Advances in organocatalytic [2 + 2]-cycloaddition of 3-ylideneoxindoles with α,β-unsaturated aldehydes, either directly or in situ generated. | ||
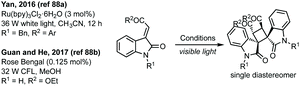
Yan in 2016 and then Guan and He in 2017 have independently published a photocatalysed [2 + 2]-cycloaddition of 3-ylideneoxindoles to form a bispirooxindole cyclobutane as a single diastereomer (Scheme 18).88 Out of a possible 8 diastereomers, one diastereomer was formed in the cycloaddition reaction.
Spiroazetidinyl oxindoles
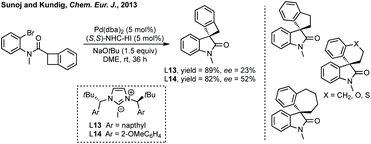
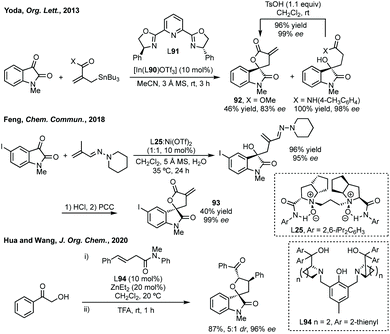
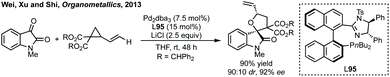
There are very few examples of spirocyclic azetidinyl oxindoles. Indeed, an analysis of all N-containing 4-membered 3,2′-spiro oxindole structures shows that all of them are beta-lactams or derivatives. Whereas for the corresponding 3,3′-spirocycles only one out of 332 is a β-lactam or derivative thereof (yet these are typically symmetrical and easily installed via traditional methods and do not feature heavily in this section). β-Lactams dominate the nitrogen containing bioactive compounds. This may reflect the lack of synthetic methods towards the unsubstituted spiroazetidinyl oxindoles.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 er (Scheme 21).102
17 er (Scheme 21).102
Spirooxetanyl oxindoles
Five-membered rings
Spirocylopentyl oxindoles
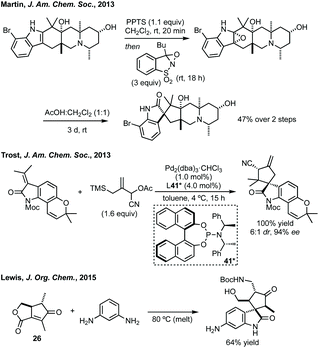
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3 followed by stereoselective collapse of the epoxide results in spirocyclopentane formation. Sarpong, Simpkins, Sun and Li have reported total syntheses of numerous natural products using a similar spirocyclisation strategy employing various epoxidising reagents.113 A recent study of the biosynthetic spirocyclisation of the paraherquimides (related natural products) by Sherman and coworkers showed that this semi-pinacol rearrangement was the biosynthetic pathway to these spirooxindoles.114 Wood synthesised (+)-Citrinadin B forming the spirocyclopentane in a Pd-catalysed enyne cyclisation, initially developed by Trost.115 Trost has developed an asymmetric [3 + 2] Pd-trimethylenemethane (TMM) cycloaddition to form the spirocyclopentane core of Marcfortine B and C (Scheme 25).116 Lewis developed an efficient complexity generating spirocyclisation heating phenylenediamine and 26 to form the spirocyclopentane, by ring opening of the ester and ortho-alkylation by a Friedel–Crafts reaction, in 64% yield and 3.7
C3 followed by stereoselective collapse of the epoxide results in spirocyclopentane formation. Sarpong, Simpkins, Sun and Li have reported total syntheses of numerous natural products using a similar spirocyclisation strategy employing various epoxidising reagents.113 A recent study of the biosynthetic spirocyclisation of the paraherquimides (related natural products) by Sherman and coworkers showed that this semi-pinacol rearrangement was the biosynthetic pathway to these spirooxindoles.114 Wood synthesised (+)-Citrinadin B forming the spirocyclopentane in a Pd-catalysed enyne cyclisation, initially developed by Trost.115 Trost has developed an asymmetric [3 + 2] Pd-trimethylenemethane (TMM) cycloaddition to form the spirocyclopentane core of Marcfortine B and C (Scheme 25).116 Lewis developed an efficient complexity generating spirocyclisation heating phenylenediamine and 26 to form the spirocyclopentane, by ring opening of the ester and ortho-alkylation by a Friedel–Crafts reaction, in 64% yield and 3.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr as a precursor to surugatoxin aglycone (Scheme 25, for structure of natural product see Fig. 5).117 Zhang and Jia recently described the total synthesis of similisines A and B (Fig. 5), enantiomeric trisindole structures containing a spirocyclopentane oxindole core, through a key acid-mediated Friedel–Crafts cyclisation, though this was low yielding and non-stereoselective.118
1 dr as a precursor to surugatoxin aglycone (Scheme 25, for structure of natural product see Fig. 5).117 Zhang and Jia recently described the total synthesis of similisines A and B (Fig. 5), enantiomeric trisindole structures containing a spirocyclopentane oxindole core, through a key acid-mediated Friedel–Crafts cyclisation, though this was low yielding and non-stereoselective.118
 | ||
| Scheme 26 Selected isatin derived MBH carbonates in a cycloaddition with 3-methyleneoxindoles and β,γ-unsaturated α-keto esters. | ||
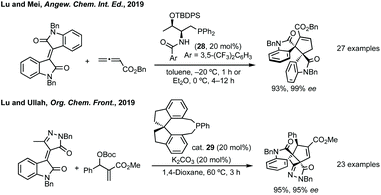
Further advances in this field have been made using asymmetric phosphorus catalysis to activate allenes, MBH carbonates or alkynones to form spirooxindoles.124,125 In Lu and Mei's 2019 report threonine derived cat. 28 was found to give the highest yield and enantioselectivity in Et2O for the [3 + 2] annulation of isoindigos and allenes (Scheme 27).126 Lu and Mei additionally showed unsymmetrical isoindigos in this process with high regiocontrol, as well as the formal syntheses of a number of complex natural products. Lu, with Ullah, then reported the annulation of pyrazoloneyldiene oxindoles with MBH carbonates using asymmetric phosphorus catalyst, SITCP 29 (Scheme 27).127 Both routes exploit the regioselective addition of the activated electrophile (MBH carbonate or allene) to the more electrophilic alkene carbon. Related reactions have been developed using isocyanides to activate similar electrophiles including allenes.128
Domino Michael addition/aldol (or alternative cyclisation)
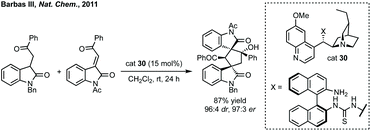
In 2011, Barbas III designed a bifunctional thiourea catalyst 30 for the domino Michael addition/aldol reaction to form bispirooxindoles from 3-substituted oxindoles and 3-methylene oxindoles (Scheme 28).129 Since, this Michael addition/cyclisation strategy based upon hydrogen bonding catalysis has been employed to access spirocyclopentane oxindoles on a large number of occasions.130 Notable examples include Kanger's use of 3-ylidene oxindoles undergoing asymmetric thiourea catalysed Michael addition alpha to the nitro group of a γ-nitroketone and spontaneous stereoselective aldol formation (determined by stereochemistry of the first step) to give the five-membered ring (for a related reaction see Scheme 75).131 Johnston and Cordova used a prolinol aminocatalyst to promote a Michael addition between an alkyne substituted oxindole and an α,β-unsaturated aldehyde followed by cyclisation to form an enantioenriched spirocyclopentane with moderate dr.132 Shi has utilised asymmetric phosphoric acid catalysts to employ various vinyl indoles to react with 3-ylidene oxindoles, formed in situ from 3-indolylmethanol, in a Michael/alkylation cascade.133![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
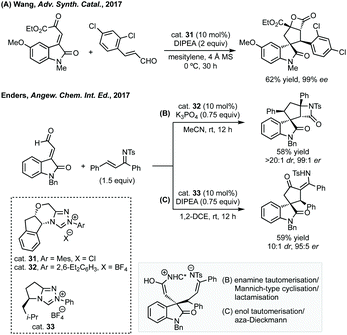
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr was achieved using cat. 31 and DIPEA for the spirooxindole products (Scheme 29A). Subsequently, Enders published a related study where fused β-lactam spirooxindoles could be formed (Scheme 29B).136 Enders also showed that using a different NHC catalyst (cat. 33), base and solvent, a different spirocyclopentane scaffold could be formed in good yield and high dr and er (Scheme 29C). This switchable reactivity occurs from the same intermediate formed by Michael addition. This intermediate can then undergo either (B) intramolecular Mannich reaction then lactamisation or (C) aza-Dieckmann type cyclisation and tautomerisation.
1 dr was achieved using cat. 31 and DIPEA for the spirooxindole products (Scheme 29A). Subsequently, Enders published a related study where fused β-lactam spirooxindoles could be formed (Scheme 29B).136 Enders also showed that using a different NHC catalyst (cat. 33), base and solvent, a different spirocyclopentane scaffold could be formed in good yield and high dr and er (Scheme 29C). This switchable reactivity occurs from the same intermediate formed by Michael addition. This intermediate can then undergo either (B) intramolecular Mannich reaction then lactamisation or (C) aza-Dieckmann type cyclisation and tautomerisation.
 | ||
| Scheme 30 Phase transfer catalysed enantioselective double Michael addition chemistry to spirocyclopentanes. | ||
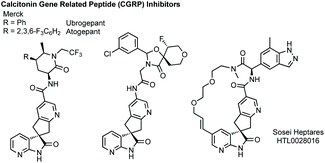
Spirocyclopentanyl oxindoles feature in a number of lead Calcitonin Gene-Related Peptide (CGRP) medicines developed by Merck, and more recently Sosei Heptares, for treatment of migraine (Fig. 6).139
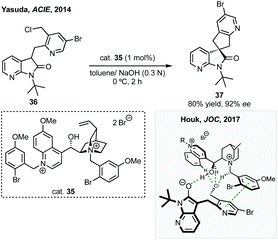
Towards an efficient asymmetric synthesis of the spirocyclopentane core of these compounds Merck developed an enantioselective phase transfer catalysed spirocyclisation.140 Using a doubly quaternised cinchona alkaloid derived phase transfer catalyst 35 up to 96% ee was achieved for the transformation of substrates such as 36 to 37 in quantitative yield which could conceivably be elaborated via the halogenated pyridine (Scheme 31). Merck subsequently collaborated with Houk to model how the novel phase transfer catalysts promote the reaction and induce enantioselectivity (Scheme 31).141 They proposed three key electrostatic interactions: (1) hydrogen bonding between the hydroxyl of the catalyst and the oxindole enolate; (2) a chloride–CH interaction activating the leaving group; (3) a π–π interaction between the pyridine of the formed cyclopentane and the quinicludine benzyl group. Merck also recently published on the monitoring of the reaction kinetics of these inherently complex, dual-phased reaction mixtures in an automated fashion.142 These studies remain a significant advance in asymmetric phase transfer catalysis, as well as in the synthesis of enantioenriched spirooxindoles.
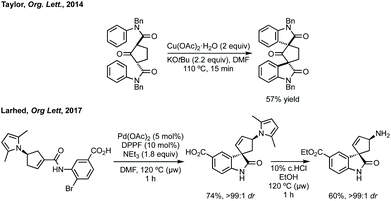
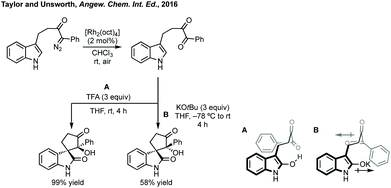
Taylor et al. reported a Cu(II)-mediated double C–H/Ar–H coupling of bis-anilides to form bispirooxindoles (Scheme 33).150 This strategy was notable for the trans-diastereoselectivity observed and the flexibility in increasing the size of the central ring. Larhed and co-workers, in collaboration with AstraZeneca, have built on their previous work on the Heck–Mizoroki reaction to generate functionalised cyclopentenes,151 to develop an intramolecular variant. Exploiting the selectivity of the Heck–Mizoroki reaction to afford spirocyclopentenes with high diastereocontrol (Scheme 33).152 García-López and others have reported C–H activation and carbene insertion procedures to afford spirocyclopentanes.153
Related to these C–H activation approaches is the stereoselective oxidation of spirocyclopentane oxindole C–H bonds using Ru or Mn catalysis.154 Initially developed by Bach in 2014, selective oxidation of one of the enantiotopic carbons of the cyclopentane gave cyclopentanones in high er.
Spiropyrrolidinyl oxindoles
[3 + 2]-Cycloaddition
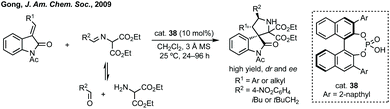
The utility of this approach by Gong has been demonstrated by the multitude of reports in this area since. These advances include the combination of an isatin derived dipole reacting with an external alkene dipolarophile,176 or other dipolaraphiles177 such as alkynes178 or allenes.179 Often these dipoles are derived from aminooxindoles180 or they could be malonitrile dipolarophiles,181 azomethine imines182 or pyridinium ylides.183 There are also many applications of this methodology for the synthesis of bispirooxindoles.184 Advances have also been made with related systems using copper catalysis.185 This azomethine ylide cycloaddition has been used by Hoffman-La Roche to synthesise MDM2 antagonist MI-888 (Fig. 7, ref. 157), including >100 g scale synthesis of the final enantiopure product by chiral resolution.186
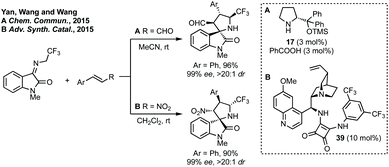
For the construction of 3,2′-spiropyrrolidinyl oxindoles, isatin derived ketimines can be used.187N-2,2,2-Trifluoroethylisatin ketimines are very popular as a starting material because of the resultant inclusion of a CF3 group in the final product. In 2015, Yan, K. Wang and R. Wang demonstrated the first enantioselective [3 + 2]-cycloaddition of N-2,2,2-trifluorethylisatin ketimines using prolinol cat. 17 (Scheme 37A).188 The same authors subsequently reported a similar cycloaddition catalysed by a cinchona alkaloid derived squaramide catalyst (Scheme 37B).189 These reports were followed by many diastereoselective190 and enantioselective191 cycloadditions using N-2,2,2-trifluoroethylisatin ketimines as 1,3-dipole starting materials.
 | ||
| Scheme 37 Selected reports on the use of N-2,2,2-trifluoroethylisatin ketimines in [3 + 2]-cycloadditions. | ||
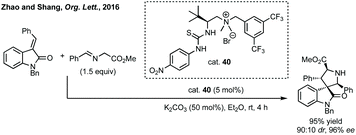
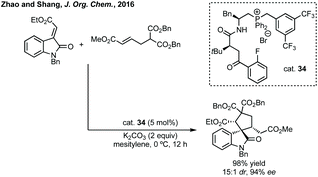
Zhao and Shang developed an asymmetric phase transfer catalysed [3 + 2]-cycloaddition using a thiourea containing ammonium salt 40 with K2CO3 as base to form polysubstituted 3,3′-spiropyrrolidinyl oxindoles in up to 99% ee (Scheme 38).192
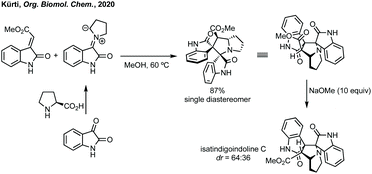
Finally, Kürti demonstrated the utility of [3 + 2]-cycloaddition chemistry. Kürti demonstrated the total synthesis of natural isatindigoindoline C in short sequence from isatin through a diastereoselective [3 + 2]-cycloaddition followed by base mediated epimerisation (Scheme 39).193 The natural stereochemistry of isatindigoindoline C was thus confirmed as anti by comparison of the 1H NMR spectra.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
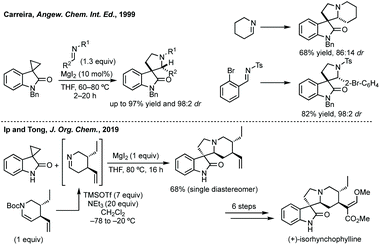
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (Scheme 40).194 Carreira and others have used this ring expansion/cycloaddition strategy on multiple occasions to affect racemic and stereoselective syntheses of natural products,195 as well as being adapted.196 Recently, Ip and Tong employed Carreira's method as the key step in the first enantioselective total synthesis of Rhynchophylline and Isoryhnchophylline using a cyclic imine (Scheme 40).197
2) (Scheme 40).194 Carreira and others have used this ring expansion/cycloaddition strategy on multiple occasions to affect racemic and stereoselective syntheses of natural products,195 as well as being adapted.196 Recently, Ip and Tong employed Carreira's method as the key step in the first enantioselective total synthesis of Rhynchophylline and Isoryhnchophylline using a cyclic imine (Scheme 40).197
 | ||
| Scheme 40 Carreira's seminal ring expansion strategy and Ip and Tong's application of this methodology in total synthesis. | ||
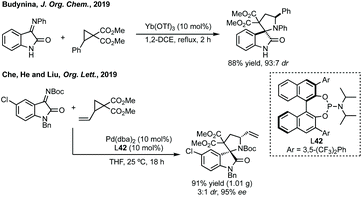
Budynina has performed a similar ring expansion in a sequential azide anion ring opening followed by a Staudinger/Wittig/Mannich reaction.198 Whereas Hajra has ring expanded 3-spiroaziridinyl oxindoles using malonitrile (Scheme 41).199 This type of ring expansion chemistry has also been carried out in an inverse fashion, i.e. Lu reacted a 3-ylidene oxindole with a vinyl aziridine (Scheme 41).200 In a related aziridine ring expansion, Hajra used Cu(OTf)2 as catalyst to ring expand an aziridine reacting with a 3-substituted isatin to form a 3,2′-spiropyrrolidine.201
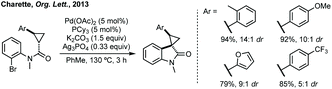
Budynina ring expanded a cyclopropane with an isatin derived ketimine (Scheme 42).202 Chu, He and Liu have recently reported an enantioselective cycloaddition of vinyl cyclopropanes with isatin derived imines using ligand 42, to form 3,2′-spiro-derivatives (Scheme 42).203
Domino conjugate addition/cyclisation
Conjugate addition/cyclisation is a common tactic employed to access spiropyrrolidinyl oxindole scaffolds stereoselectively, and highlights the continuum between concerted [3 + 2]-annulation chemistry and stepwise sequences. Stepwise but simultaneous addition/cyclisation sequences will be dealt with first followed by discrete additions and sequential asynchronous cyclisations. Domino Michael addition/cyclisation reactions have been separated according to the isatin reactants: (A) 3-isothiocyanato oxindoles or (B) oxindoles with a nucleophilic C3 substituent reacting with olefins and (C) 3-ylidene oxindoles.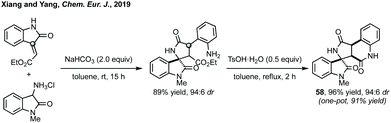 | ||
| Scheme 46 Selected example of the combination of strategy B and C, using a nucleophilic C3 oxindole substituent and 3-ylidene oxindole. | ||
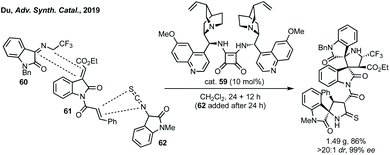
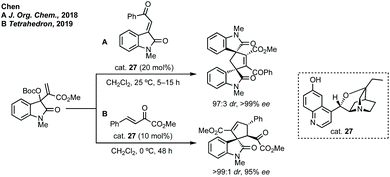
A remarkable extension of strategies A and C with dipolar cycloaddition has been developed by Du where compounds containing a spiropyrrolidine oxindole and bispirooxindole were formed by a dual Michael/Mannich and Michael/cyclisation sequence (Scheme 47).229 Using dimeric squaramide cat. 59 the reaction between N-2,2,2-trifluoroethylisatin ketimine 60 and 3-methyleneoxindole 61 could be promoted, followed by the reaction between 3-isothiocyanato oxindole 62 and the pendant α,β-unsaturated amide on 61. The bispirooxindole-spirooxindole compounds with seven stereocentres were afforded in high yield, dr and ee, including on gram scale.
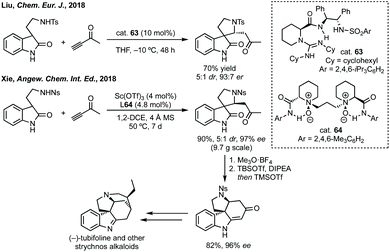
Before this, Sasai had developed the same reaction using a chiral phosphine catalyst but with maximum 84% ee.231 More recently, Wu and Zhang used a chiral bisphosphine catalyst for the same reaction.232 A clear demonstration of the utility of these methods was given by Xie who showed the total synthesis of some strychnos alkaloids (Scheme 48). A related reaction has been developed by Miesch involving a copper catalysed hydroamination process.233 In a related strategy, Peng and Shao reported an asymmetric propargylation followed by iodocyclisation to construct polycyclic spirooxindoles in one-pot or as a discrete asymmetric coupling step followed by cyclisation.234
Discrete coupling strategies
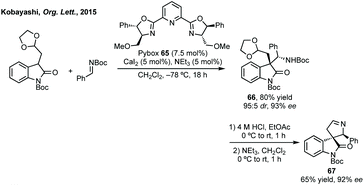
In this section strategies where a discrete coupling followed by cyclisation will be discussed. A common strategy towards spiropyrrolidine oxindoles is an asymmetric Mannich reaction using ketimines followed by cyclisation. In 2012, Lu and then Li and Wang reported significant advances in enantioselective Michael addition and allylic alkylation of nitroalkanes using cinchona alkaloid derived catalysts.235 Reductive cyclisation of the nitro group in the product then afforded spirolactams in high ee. In 2015, Kobayashi developed a calcium/Pybox asymmetric Mannich reaction, which could be cyclised upon deprotection and basic cyclisation (Scheme 49).236,237 Using CaI2 with Pybox ligand 65 in CH2Cl2 at −78 °C afforded the Mannich product in high dr (trans product favoured) and excellent enantioselectivity. From acetal product 66, treatment with HCl followed by NEt3 afforded 3,3′-spiropyrrolinyl oxindole 67 in 65% yield and 92% ee.In 2016, Ooi used triazolium phase transfer catalyst 68 to effect the C–H amination of a hydroxylamine derivative in high ee for 5- and 6-membered saturated nitrogen heterocycles (Scheme 50).238 More recently, Du and Chen developed an asymmetric allylic alkylation from 3-phenyloxindoles using phase transfer catalyst 69 and Pd(OAc)2 with Na2CO3 as base (Scheme 50).239 This remarkable reaction afforded good yields of the 3,2′-spiropyrrolidine oxindole products in high ee. The products could be readily derivatised to numerous spirocycles including spirocyclohexanes, piperidines and pyrrolidines. Luo and Zhu developed a Heck/carbonylative cyclisation sequence to 3,3′-spiropyrrolidone oxindoles from non-isatin derived starting materials (Scheme 50).240 They employed chiral bidentate phosphine ligand L70 with Pd2(dba)3, K2CO3 and PivOH in toluene and a CO atmosphere to affect the Heck/carbonylation cascade. Notably, the methodology was limited to aryl protected lactams but high yields and enantioselectivities were observed when using the readily removable PMB group. The authors showed the application of this methodology to the synthesis of a CRTH2 receptor antagonist241 in 6 steps in 35% overall yield and 98% ee (Scheme 50).
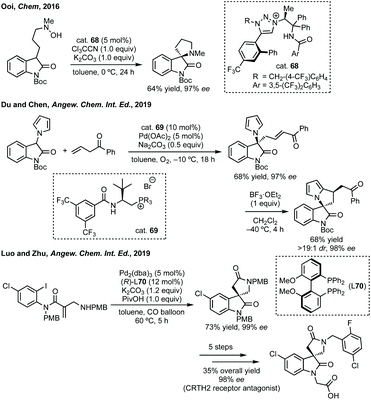 | ||
| Scheme 50 Selected examples of phase transfer catalysed or metal-catalysed cross-coupling strategies to spiropyrrolidines. PMB = para-methoxybenzyl. | ||
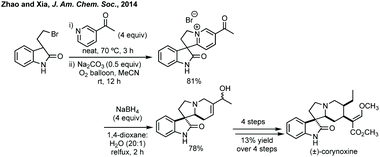
In a clearly distinct strategy Zhao and Xia developed a cross-dehydrogenative coupling of pyridines with 3-substituted oxindoles.242 The pyridinium salts afforded could be reduced diastereoselectively with NaBH4 in order to access racemic corynoxine in a rapid fashion (Scheme 51). Although the pyridine scope was limited to electron withdrawing groups at C3, the reaction notably worked on unprotected oxindoles.
Addition to isatin derived ketimines is a common route to spiropyrrolidines. Liu reported a one-pot Mannich/hydroamination approach using isatin ketimines.243 Zhou used a triple catalysis cascade reaction to generate an isatin derived ketimine in situ which could then undergo Brønsted base catalysed 6π-electrocyclisation.244 Hajra developed an enantioselective tanden aza-Henry reaction-cyclisation of isatin-derived ketimines and nitroalkane mesylates to 3,2-spiropyrrolidine oxindoles (Scheme 52).245 These conditions were also applicable to piperidine derivatives with a chain extended nitro mesylate substrate. Xu reported a Rh-catalysed arylation of these ketimines, when using o-tolylboroxine, treatment of the product with NBS and Boc deprotection allowed cyclisation to product 72 (Scheme 52). More recently, Zhu and Zhang reported an enantioselective para-C–H functionalisation of N-monosubstituted anilines with isatin derived ketimines using cat. 73.246 The enantioenriched 3-aminoooxindoles were readily cyclised to spiropyrrolidines in good yield and high ee (Scheme 52).
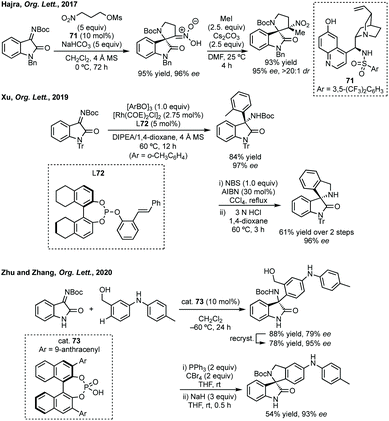 | ||
| Scheme 52 Synthesis of 3,2′-spiropyrrolidine oxindoles following enantioselective additions to isatin derived ketimines. | ||
Spirotetrahydrofuranyl oxindoles253
Cycloaddition
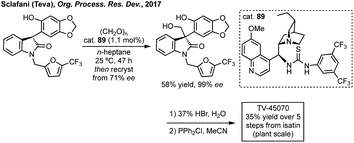
In 2016, Bisai reported an enantioselective aldol reaction of dimeric oxindoles which resulted in ring opening of one of the oxindoles, and in doing so developed a highly enantioselective thiourea catalysed aldol reaction with formaldehyde.281 The first process scale synthesis of TV-45070 (Fig. 9) employed a phase-transfer catalysed asymmetric alkylation using a Lygo phase transfer catalyst.282 Due to the requirement for multiple protecting groups in the first process scale synthesis of TV-45070, a new route was developed using a thiourea catalysed aldol reaction similar to the one developed by Bisai (Scheme 59). Only moderate enantioselectivity was observed using cat. 89 (up to 73% ee), but this could be improved by recrystallisation, followed by further two steps to afford the final API.
Yin has developed a Pd-catalysed cascade reaction involving dearomatisation of furans to form the THF core of the spirocycle.295 Other metal-mediated approaches include the use of Cu-,296 Ti-,297 Ru-298 or Ni-catalysed299 spirocyclisations. Trost has also applied his development of Pd-allyl complexes previously discussed in the spiropyrrolidine section to the synthesis of spiroTHFs.300 Similar to Moody's use of diazo compounds to synthesise spiropyrrolidines, OH insertion/cyclisation could be used to synthesise spiroTHFs301 and there have been many other approaches using Rh- or Cu-catalysed decomposition of diazo compounds.302
Hajra and Kumar have independently developed Lewis acid-mediated ring expansion of spiroepoxides with allylsilanes to afford spiroTHF oxindoles with moderate to good dr (Scheme 63).305 In 2016, Hajra had used spiroepoxy oxindoles in a regioselective Friedel–Crafts alkylation, the alcohol product could then undergo an Appel reaction and spontaneous cyclisation through the phenol to afford 2H-spirobenzofuran oxindoles.306
Six-membered rings
Spirocylohexanyl oxindoles
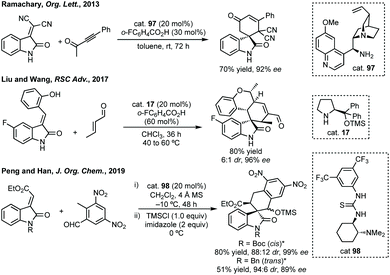 | ||
| Scheme 65 Selected [4 + 2] annulations. * Indicates stereochemical relationship between OTMS and ester group on cyclohexane ring. | ||
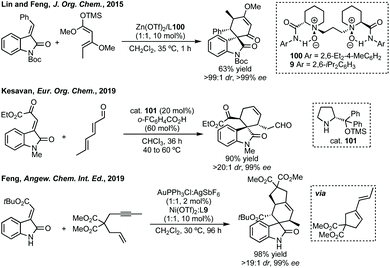
In 2011, Melchiorre and Barbas III reported asymmetric Diels–Alder reactions between 3-vinyl indoles and electron poor olefins using iminium ion catalysis and hydrogen bonding catalysis.327 These works laid the foundations for a body of work which provide tetrahydrocarbazoles fused with spirooxindoles.328 Notably, in 2014, Feng reported the asymmetric Diels–Alder reaction between 3-vinyl indoles and 3-methylene oxindoles using Ni catalysis (Scheme 66).329 Also, in 2015 Shi developed a similar reaction using 2-vinyl indoles using chiral phosphoric acid catalysis (Scheme 66).330 Oxygenated analogues of these tetrahydrocarbazoles, which could be produced by an Oxa-Pictet–Spengler reaction can undergo a Claisen rearrangement to spirocyclohexanes (which as we have seen can also form spiroTHF products).331
Antilla described the use of chiral Mg-phosphate catalysis for an asymmetric Diels–Alder reaction.332 For a related Diels–Alder reaction, Lin and Feng used Zn(OTf)2 complexed with L100 in up to 99% ee (Scheme 67).333,334 In 2019, Kesavan described an asymmetric Diels–Alder reaction between 2,4-dienals and 3-methylene oxindoles catalysed by prolinol cat. 101 (Scheme 67).335 Feng has recently reported Au-catalysed cycloisomerisation followed by Ni-catalysed Diels–Alder cycloaddition to enantioenriched spirocyclohexanes (Scheme 67).336 Feng and Dong have also disclosed a Dy(OTf)3-mediated ring-opening/[4 + 2]-cycloaddition of cyclobutenones and 3-methylene oxindoles.337
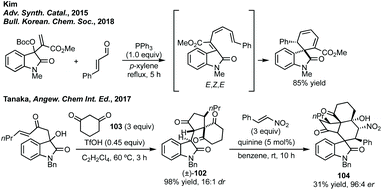
In terms of other cascade rearrangements, Kim has developed a diastereoselective 6π-electrocyclisation from MBH precursors.338 Kim further developed this to a one-pot PPh3 mediated coupling of MBH carbonates and enals where favourable disrotatory ring closure from the E,Z,E-isomer proceeds to the major diastereomer (Scheme 68).339 In a distinct complexity-generating reaction, Tanaka could form racemic intermediate 102 in a [4 + 1] annulation of 3-methylene oxindole and diketone 103 by treatment with TfOH (Scheme 68).340 In a Michael–Henry cascade reaction 102 could react with an electron-poor olefin (such as a nitroalkene) and form polycyclic spirocyclohexane oxindole containing product 104 with excellent enantioselectivity. The yields for these products were low due to only one enantiomer of 102 reacting, therefore, the reaction could also serve to furnish highly enantioenriched 102 in a kinetic resolution.
Spiropiperidinyl oxindoles
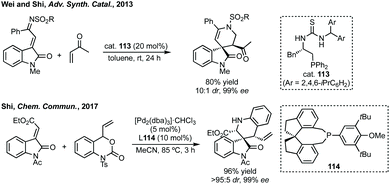
Similar to other ring sizes we have considered, there have been a number of efforts made towards the synthesis of rings with more than one heteroatom, here we only consider the synthesis of piperidines or δ-lactam scaffolds.356 Wei and Shi developed a [4 + 2]-cycloaddition of vinyl ketones and α,β-unsaturated imines derived from isatins catalysed by a bifunctional asymmetric phosphorus-thiourea catalyst (Scheme 72, R = 2,4,6-triisopropyl phenyl).357 In metal-mediated approaches, gold catalysed spirocyclisation of in situ generated indoles and isatins was reported by Subba Reddy.358 Related to Liu and Feng's reaction (ref. 355), Kumar developed an enantioselective aza-Diels Alder reaction catalysed by Dy(OTf)3 and a ligand similar to 112 where Ar = 2,6-iPr2C6H4.359 Feng, Li and Xiao have independently developed 1,5-hydride transfer reactions to spiropiperidines.360 More recently, Shi developed a Pd-catalysed decarboxylative [4 + 2] cycloaddition strategy using vinyl benzoxazinanones and 3-methylene oxindoles (Scheme 72).361
Spirotetrahydropyranyl oxindoles
In other approaches, Subba Reddy has developed a BF3·OEt2 mediated Prins cascade cyclisation between aldehydes and butanamides to furnish spiroTHP oxindoles in high yield and good dr.408 Hu used Rh-carbenes generated from 3-diazooxindoles to undergo C–H insertion/aldol condensation to afford spiroTHP oxindoles in high yield and diastereoselectivity.409 Another example of C–H activation has been shown by Messaoudi with glycosides.410 The intramolecular Co-catalysed Pauson–Khand cyclisation of 1,7-enynes generated 3,2′-spiroTHPs with high diastereoselectivity.411
Seven-membered rings
Applications
Natural products containing seven-membered spirocyclic oxindoles are predominantly bridged carbocyclic examples of gelsemium alkaloids.412 There are not many examples of these rings in medicinal chemistry, though nitrogen containing examples do feature in some patents.413 A spiroazepane oxindole was synthesised as a cipargamin analogue with antimalarial activity,16 and an example with activity against a prostate cancer target (Fig. 1).414 While natural products tend to favour bridged carbocyclic seven-membered rings (Fig. 11) and there are some methodologies to synthesise bridged seven-membered rings, this section will focus on non-bridged examples.415 Additionally, we have grouped the methodologies in terms of similarity rather than into C/N/O-containing rings due to the small number of publications, the vast majority of which are aimed at spiroazepane synthesis.Natural product synthesis
Total synthesis efforts centre around the synthesis of gelsemium alkaloids. Carreira has described elegant approaches to gelsemoxonine (see Fig. 11 for structure).416 Ferreira constructed the oxindole of gelsenicine with an oxidative cyclisation of a Weinreb amide with an aromatic ring.417 Fukuyama and Ma have independently described divergent syntheses to many gelsedine type alkaloids.418 Takayama recently described an asymmetric synthesis of (−)-14-hydroxygelsenicine and six other gelsemium alkaloids.419 The spirooxindole was constructed in a diastereoselective Heck cyclisation (Scheme 80).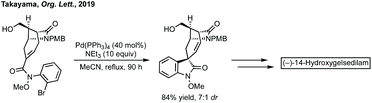 | ||
| Scheme 80 Pd-Catalysed Heck reaction as key step in the total synthesis of (−)-14-hydroxygelsedilam. | ||
NHC catalysis
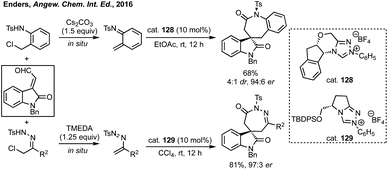
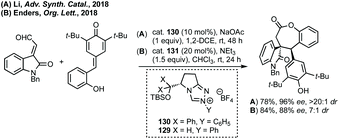
In 2016, Enders pioneered the use of NHCs for the [3 + 4]-cycloaddition of isatin derived enals with aza-o-quinone methides or azoalkenes to form spiro-benzazepinones or spiro-diazepinones (Scheme 81).420 Using cat. 128 with Cs2CO3 to form the aza-o-quinone methide in situ from the N-(o-chloromethyl)aryl amide in EtOAc gave high enantioselectivity and atroposelectivity. Switching starting material in order to make diazepinones was highly stereoselective using cat. 129.Li developed an NHC catalysed enantioselective synthesis of spirobenzoxepinones in a [4 + 3] cycloaddition of isatin derived enals and quinone methides (Scheme 82).421 High enantioselectivity (up to >99% ee and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) was achieved using triazolium cat. 130 in combination with NaOAc in 1,2-dichloroethane. Enders published a similar reaction a month after Li's study, which employed cat. 131 to achieve up to 95
1 dr) was achieved using triazolium cat. 130 in combination with NaOAc in 1,2-dichloroethane. Enders published a similar reaction a month after Li's study, which employed cat. 131 to achieve up to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er and good dr.422 This was followed by work by Yan Li and Ye on a related [4 + 3]-cycloaddition of isatin derived enals and aurone-derived azadienes.423 Using a similar NHC catalyst interesting spiroazepinones were formed with high enantioselectivity and diastereoselectivity. The compounds exhibited moderate cytotoxicity against cancer cell lines.
5 er and good dr.422 This was followed by work by Yan Li and Ye on a related [4 + 3]-cycloaddition of isatin derived enals and aurone-derived azadienes.423 Using a similar NHC catalyst interesting spiroazepinones were formed with high enantioselectivity and diastereoselectivity. The compounds exhibited moderate cytotoxicity against cancer cell lines.
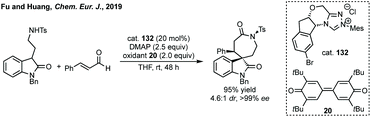
More recent advances include Fu and Huang's NHC catalysed [4 + 3]-annulation of α,β-unsaturated aldehydes and amine substituted oxindoles (oxotryptamines, Scheme 83).424 Song and Gong's previously discussed excellent Cu/NHC dual catalysis could be used with ethynyl benzoxazinanones to construct chiral azepines.404
MBH carbonates
Chen and coworkers reported a [4 + 3] cycloaddition of a bromo-substituted MBH derivative and an aza-o-quinone methide precursor (Scheme 84).425 Chen used tri-(4-fluoro)phenyl phosphine as a Lewis base to form an allylic ylide from the MBH precursor and Cs2CO3 as base to generate the aza-o-quinone methide. Xu published a related reaction at a similar time,426 using tributyl phosphine as Lewis base catalysis with a related MBH precursor and a Boc protected aza-o-quinone methide precursor to generate seven-membered spirocycles in good yields, including on gram-scale. In a similar fashion, Du recently employed ortho-quinone methides in combination with an isatin derived MBH precursor to affect a [4 + 3]-cycloaddition.427 Using DABCO as Lewis base catalyst in MeCN, high diastereoselectivity with electron-rich ortho-quinone methides was observed (Scheme 84). | ||
| Scheme 84 Selected [4 + 3]-cycloadditions to 7-membered spirocycles using MBH carbonates or MBH precursors. | ||
Du and Chen have collaboratively reported an asymmetric Ir catalysed [4 + 3]-cycloaddition between an MBH carbonate and π-allyl precursor (Scheme 85).428 The π-allyl precursor includes a vinylogous leaving group i.e. vinyl-OBoc, ethylene oxazinanones or vinyl aziridines (for six-membered rings) which forms an asymmetric Ir-allyl complex to react with the DABCO activated MBH carbonate.
Other approaches to spiroazepanes include a desymmetrising Cu-catalysed C–N bond formation in high ee (Scheme 86).429 Budynina has used azide anion ring opening of spirocyclopropyl oxindoles (ref. 198) in a Staudinger, domino Michael/aza-Wittig and reduction sequence.430 Yang developed a Pd-NHC catalysed allylic alkylation, the products of which could readily elaborated to a spiroazepinone.431
Eight-membered rings
As with seven-membered spiro-oxindoles there are very few examples of syntheses capable of accessing the eight-membered analogues. Shi and Zhao independently published the Pd-catalysed [5 + 3]-cycloaddition of N-2,2,2-trifluoromethylisatin ketimines and vinylethylene carbonates (Scheme 87).432 Shi employed (Pd2dba3)·CHCl3 and Xantphos as a ligand for the decarboxylative allylic substitution and a racemic phosphoric acid catalyst to effect the cyclisation. Meanwhile, Zhao used Pd(PPh3)4 in combination with PPh3 as ligand and pyridine as base for the cyclisation. Both procedures afford the anti-product as the major product in high yields and dr. Shi demonstrated a preliminary result for the enantioselective allylation using tBu-RuPhos as ligand affording the eight-membered spirocycle in 63% ee. Furthermore, Shi showed that epoxidation of the endogenous double bond with mCPBA would lead to spontaneous epoxide ring opening to form spiropyrrolidine 134 (Scheme 87A).Summary and conclusions
We have reviewed the developments in state-of-the-art stereoselective spiroindolone synthesis between 2013 and 2020. The progress of synthetic methodology for each ring size (3- to 8-) has been discussed. The importance of these advances should not be understated, with reference to the significant potential of many of these structures in medicinal chemistry, which we have highlighted with numerous examples. The trends we have observed within this review can be summarised as follows:1. Spirooxindoles represent a very important class of structures within medicinal chemistry, featuring in approved medicines with a large variety of biological activity, as well as acting as the structural core in a significant number of natural products (egFig. 1). Where possible we have highlighted how the synthetic methodologies discussed in this review have influenced the process scale synthesis of these pharmaceuticals, for example in Scheme 59.
2. Spirooxindoles serve as a benchmark in asymmetric synthesis. Many of the methods reviewed here have made substantial advances in asymmetric catalysis. This may be due to the fused backbone of the indolone providing a flat and rigid platform for construction of 3D spirocycles. These advances are doubly valuable because they fulfill the object of advancing asymmetric methodology while making biologically relevant scaffolds for screening against biologically relevant targets.
3. The advances that have been observed in spirooxindole synthesis generally reflect the advance of organic synthesis since 2013. While there has been a significant rise in the number of publications on this topic, there are a growing number of excellent and innovative reports targeting these scaffolds (Fig. 2). This review has covered advances in stereoselective NHC catalysis, chiral acid catalysis, aminocatalysis, metal catalysis including cross-coupling, hydrogen bonding catalysis and phase transfer catalysis. We have also highlighted total syntheses of natural products containing these core structures.
There will likely be sustained interest in these scaffolds because of the trends observed in recent years coupled with the success of many of the pharmaceutical agents. We envisage that future developments may be targeted to the following objectives:
1. More general methods to access multiple ring sizes. Currently, there are few methodologies that can access multiple ring sizes with simple changes i.e. to starting material structure. For example, ring expansion strategies making use of small rings are useful to access more than one ring size. Ideal methods could also access more than one heteroatom pattern on the spirocyclic ring and be able to control ring substitution.
2. A wider variety of synthetic targets through asymmetric synthesis. Unsubstituted rings can often be synthesised as racemates using traditional methods. Yet, asymmetric synthesis of unsubstituted rings is a challenge and though there are examples within this review there is still a requirement to access the unsubstituted scaffold.
3. Small and larger ring spirocycles. As could be seen from the analysis of the publication numbers (Fig. 2) and reflected in the number of strategies discussed in this review, there is a plethora of methods for 5- and 6-membered rings. Future endeavours in this area should seek to synthesise small or larger rings, eg azetidines and oxetanes are increasingly useful for medicinal chemistry and have not received the same level of attention. Furthermore, 7- and 8-membered rings have not received significant attention due to the difficulty in accessing these structures and we envisage that synthetic advances seen in this review will likely be applied to larger ring synthesis. 8-Membered ring spirocycles, as the first member of the medium rings, may require significant new method development. Higher medium ring homologues were not within the scope of this review, but could pose interesting targets for medicinal chemistry. New syntheses of these smaller and larger ring spirocycles will lead to improved access to this valuable and underexplored chemical space.
We hope that this review will serve as a reference for medicinal and synthetic chemists aiming to synthesise this type of ring structure and inspire future advances in the synthesis of spirooxindoles.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge The Royal Society for funding [University Research Fellowship, UF140161 (to J. A. B)], and AstraZeneca and EPSRC for iCASE studentship funding.Notes and references
- Y. Zheng, C. M. Tice and S. B. Singh, The use of spirocyclic scaffolds in drug discovery, Bioorg. Med. Chem. Lett., 2014, 24, 3673 CrossRef CAS.
- Y. J. Zheng and C. M. Tice, The utilization of spirocyclic scaffolds in novel drug discovery, Expert Opin. Drug Discovery, 2016, 11, 831 CrossRef.
- S. L. Degorce, M. S. Bodnarchuk and J. S. Scott, Lowering Lipophilicity by Adding Carbon: AzaSpiroHeptanes, a logD Lowering Twist, ACS Med. Chem. Lett., 2019, 10, 1198 CrossRef CAS.
- (a) G. Müller, T. Berkenbosch, J. C. J. Benningshof, D. Stumpfe and J. Bajorath, Charting Biologically Relevant Spirocyclic Compound Space, Chem. – Eur. J., 2017, 23, 703 CrossRef. For benefits of saturated heterocycles vs. aromatic cycles see: (b) T. J. Ritchie and S. J. F. Macdonald, The impact of aromatic ring count on compound developability - are too many aromatic rings a liability in drug design?, Drug Discovery Today, 2009, 14, 1011 CrossRef CAS; (c) T. J. Ritchie, S. J. F. MacDonald, R. J. Young and S. D. Pickett, The impact of aromatic ring count on compound developability: further insights by examining carbo- and hetero-aromatic and -aliphatic ring types, Drug Discovery Today, 2011, 16, 164 CrossRef CAS.
- E. M. Carreira and T. C. Fessard, Four-Membered Ring-Containing Spirocycles: Synthetic Strategies and Opportunities, Chem. Rev., 2014, 114, 8257 CrossRef CAS.
- G. S. Singh and Z. Y. Desta, Isatins As Privileged Molecules in Design and Synthesis of Spiro-Fused Cyclic Frameworks, Chem. Rev., 2012, 112, 6104 CrossRef CAS.
- T. L. Pavlovska, R. G. Redkin, V. V. Lipson and D. V. Atamanuk, Molecular diversity of spirooxindoles. Synthesis and biological activity, Mol. Diversity, 2016, 20, 299 CrossRef CAS.
- (a) B. Yu, D. Q. Yu and H. M. Liu, Spirooxindoles: Promising scaffolds for anticancer agents, Eur. J. Med. Chem., 2015, 97, 673 CrossRef CAS; (b) B. Yu, Y.-C. Zheng, X.-J. Shi, P.-P. Qi and H.-M. Liu, Natural Product-Derived Spirooxindole Fragments Serve as Privileged Substructures for Discovery of New Anticancer agents, Anticancer Agents Med. Chem., 2016, 16, 1315 CrossRef CAS.
- N. Ye, H. Chen, E. A. Wold, P. Y. Shi and J. Zhou, Therapeutic Potential of Spirooxindoles. as Antiviral Agents, ACS Infect. Dis., 2016, 2, 382 CrossRef CAS.
- (a) M. M. M. Santos, Recent advances in the synthesis of biologically active spirooxindoles, Tetrahedron, 2014, 70, 9735 CrossRef CAS; (b) E. Sansinenea, E. F. Martínez and A. Ortiz, Organocatalytic Synthesis of Chiral Spirooxindoles with Quaternary Stereogenic Centers, Eur. J. Org. Chem., 2020, 5101 CAS.
- (a) B. M. Trost and M. K. Brennan, Asymmetric Syntheses of. Oxindole and Indole Spirocyclic Alkaloid Natural Products, Synthesis, 2009, 3003 CrossRef CAS; (b) F. Zhou, Y. L. Liu and J. Zhou, Catalytic Asymmetric Synthesis of Oxindoles Bearing a Tetrasubstituted Stereocenter at the C-3 Position, Adv. Synth. Catal., 2010, 352, 1381 CrossRef CAS; (c) L. Hong and R. Wang, Recent Advances in Asymmetric Organocatalytic Construction of 3,3′-Spirocyclic Oxindoles, Adv. Synth. Catal., 2013, 355, 1023 CrossRef CAS; (d) D. Cheng, Y. Ishihara, B. Tan and C. F. Barbas, Organocatalytic Asymmetric Assembly Reactions: Synthesis of Spirooxindoles via Organocascade Strategies, ACS Catal., 2014, 4, 743 CrossRef CAS; (e) R. Narayan, M. Potowski, Z. J. Jia, A. P. Antonchick and H. Waldmann, Catalytic Enantioselective 1,3-Dipolar Cycloadditions of Azomethine Ylides for Biology-Oriented Synthesis, Acc. Chem. Res., 2014, 47, 1296 CrossRef CAS; (f) R. Dalpozzo, Recent Catalytic Asymmetric Syntheses of 3,3-Disubstituted Indolin-2-ones and 2,2-Disubstituted Indolin-3-ones, Adv. Synth. Catal., 2017, 359, 1772 CrossRef CAS; (g) G. J. Mei and F. Shi, Catalytic asymmetric synthesis of spirooxindoles: recent developments, Chem. Commun., 2018, 54, 6607 RSC.
- (a) M. Xia and R.-Z. Ma, Recent Progress on Routes to Spirooxindole Systems Derived from Isatin, J. Heterocycl. Chem., 2014, 51, 539 CrossRef CAS; (b) Y. Liu, H. Wang and J. Wan, Recent Advances in Diversity Oriented Synthesis through Isatin-based Multicomponent Reactions, Asian J. Org. Chem., 2013, 2, 374 CrossRef CAS; (c) G. Mohammadi Ziarani, R. Moradi and N. Lashgari, Asymmetric synthesis of chiral oxindoles using isatin as starting material, Tetrahedron, 2018, 74, 1323 CrossRef CAS.
- (a) S. Peddibhotla, 3-Substituted-3-hydroxy-2-oxindole, an Emerging New Scaffold for Drug Discovery with Potential Anti-Cancer and other Biological Activities, Curr. Bioact. Compd., 2009, 5, 20 CrossRef CAS; (b) J. Kaur, B. P. Kaur and S. S. Chimni, Recent advances in the catalytic synthesis of 3-aminooxindoles: an update, Org. Biomol. Chem., 2020, 18, 4692 RSC.
- (a) A. K. Franz, N. V. Hanhan and N. R. Ball-Jones, Asymmetric Catalysis for the Synthesis of Spirocyclic Compounds, ACS Catal., 2013, 3, 540 CrossRef CAS; (b) A. Ding, M. Meazza, H. Guo, J. W. Yang and R. Rios, New development in the enantioselective synthesis of spiro compounds, Chem. Soc. Rev., 2018, 47, 5946 RSC; (c) J. Bariwal, L. G. Voskressensky and E. V. Van Der Eycken, Recent advances in spirocyclization of indole derivatives, Chem. Soc. Rev., 2018, 47, 3831 RSC.
- Analysis carried out on SciFinder on 06/04/2020 and 07/04/2020. A general substructure search was carried out with each ring size and heteroatom position iteratively with the following restrictions placed: (1) exclude metal-containing results; (2) exclude isotope-containing results; (3) only include if hit has references; (4) only include if hit is a single component; (5) limit references to biological or preparative sources.
- B. K. S. Yeung, B. Zou, M. Rottmann, S. B. Lakshminarayana, S. H. Ang, S. Y. Leong, J. Tan, J. Wong, S. Keller-Maerki and C. Fischli, et al., Spirotetrahydro β-Carbolines (Spiroindolones): A New Class of Potent and Orally Efficacious Compounds for the Treatment of Malaria, J. Med. Chem., 2010, 53, 5155 CrossRef CAS.
- A. A. Akaev, M. Y. Melnikov and E. M. Budynina, Chameleon-Like Activating Nature of the Spirooxindole Group in Donor-Acceptor Cyclopropanes, Org. Lett., 2019, 21, 9795 CrossRef CAS.
- Z. Y. Cao and J. Zhou, Catalytic asymmetric synthesis of polysubstituted spirocyclopropyl oxindoles: organocatalysis versus transition metal catalysis, Org. Chem. Front., 2015, 2, 849 RSC.
- E. P. Bacher and B. L. Ashfeld, Transition metal-free strategies for the stereoselective construction of spirocyclopropyl oxindoles, Tetrahedron, 2020, 76, 130692 CrossRef CAS.
- B. Sampson, Y. Liu, N. K. Patel, M. Feher, B. Forrest, S.-W. Li, L. Edwards, R. Laufer, Y. Lang and F. Ban, et al., The Discovery of Polo-Like Kinase 4 Inhibitors: Design and Optimization of Spiro[cyclopropane-1,3′[3H]indol]-2′(1′H)-ones as Orally Bioavailable Antitumor Agents, J. Med. Chem., 2015, 58, 130 CrossRef.
- S. Chen, H. He, B. Lagu, H. Qin, C. Wu and Y. Xiao, Tricyclic Sulfonamide Derivatives, PCT Int. ApplWO2015102929A1, 2015 Search PubMed.
- D. Brunner, J. Malberg, B. G. Shankar, S. Kolczewski, A. Limberg, E. Prinssen, C. Riemer and T. Stoll, 2-Oxo-2,3-dihydro-indoles for the Treatment of CNS Disorders, PCT Int. ApplWO2014040969A1, 2014 Search PubMed.
- (a) T. Jiang, K. L. Kuhen, K. Wolff, H. Yin, K. Bieza, J. Caldwell, B. Bursulaya, T. Y. H. Wu and Y. He, Design, synthesis and biological evaluations of novel oxindoles as HIV-1 non-nucleoside reverse transcriptase inhibitors. Part I, Bioorg. Med. Chem. Lett., 2006, 16, 2105 CrossRef CAS; (b) T. Jiang, K. L. Kuhen, K. Wolff, H. Yin, K. Bieza, J. Caldwell, B. Bursulaya, T. Tuntland, K. Zhang and D. Karanewsky, et al., Design, synthesis and biological evaluations of novel oxindoles as HIV-1 non-nucleoside reverse transcriptase inhibitors. Part 2, Bioorg. Med. Chem. Lett., 2006, 16, 2109 CrossRef CAS; (c) B. Yu, Z. Yu, P. P. Qi, D. Q. Yu and H. M. Liu, Discovery of orally active anticancer candidate CFI-400945 derived from biologically promising spirooxindoles: Success and challenges, Eur. J. Med. Chem., 2015, 95, 35 CrossRef CAS; (d) S. Cockerill, C. Pilkington, J. Lumley, R. Angell and N. Mathews, Pharmaceutical Compounds, PCT Int. ApplWO2013068769A1, 2013 Search PubMed.
- (a) F. C. Stevens, W. E. Bloomquist, A. G. Borel, M. L. Cohen, C. A. Droste, M. L. Heiman, A. Kriauciunas, D. J. Sall, F. C. Tinsley and C. D. Jesudason, Potent oxindole based human β3 adrenergic receptor agonists, Bioorg. Med. Chem. Lett., 2007, 17, 6270 CrossRef CAS; (b) A. Nardi, F. Jakob, I. Konetzki, T. Craan, C. Hesslinger and R. Doodeman, Novel Substituted Pyrimidine Compounds, PCT Int. ApplWO2016008593A1, 2016 Search PubMed Also see ref. 25.
- D. W. Robertson, J. H. Krushinski, G. D. Pollock, H. Wilson, R. F. Kauffman and J. S. Hayes, Dihydropyridazinone cardiotonics: synthesis and inotropic activity of 5′-(1,4,5,6-tetrahydro-6-oxo-3-pyridazinyl)-spiro[cycloalkane-1,3′-(3H)indol-2′(1′)H-ones, J. Med. Chem., 1987, 30, 824 CrossRef CAS.
- M. Palomba, L. Rossi, L. Sancineto, E. Tramontano, A. Corona, L. Bagnoli, C. Santi, C. Pannecouque, O. Tabarrini and F. Marini, A new vinyl selenone-based domino approach to spirocyclopropyl oxindoles endowed. with anti-HIV RT activity, Org. Biomol. Chem., 2016, 14, 2015 RSC.
- M. Zhou, K. En, Y. Hu, Y. Xu, H. C. Shen and X. Qian, Zinc triflate-mediated cyclopropanation of oxindoles with vinyldiphenyl sulfonium triflate: a mild reaction with broad functional group compatibility, RSC Adv., 2017, 7, 3741 RSC.
- H. Qin, Y. Miao, K. Zhang, J. Xu, H. Sun, W. Liu, F. Feng and W. Qu, A convenient cyclopropanation process of oxindoles via bromoethylsulfonium salt, Tetrahedron, 2018, 74, 6809 CrossRef CAS.
- S. Hajra, S. Roy and S. A. Saleh, Domino Corey-Chaykovsky Reaction for One-Pot Access to Spirocyclopropyl Oxindoles, Org. Lett., 2018, 20, 4540 CrossRef CAS.
- (a) S. A. Bonderoff and A. Padwa, Rh(II)-Catalyzed Reactions of Differentially Substituted Bis(diazo) functionalities, Org. Lett., 2013, 15, 4114 CrossRef CAS; (b) S. Muthusamy and R. Ramkumar, Solvent- and transition metal-free synthesis of spiro[cyclopropane-1,3-oxindoles] from cyclic diazoamides, Tetrahedron Lett., 2014, 55, 6389 CrossRef CAS; (c) G. Karthik, T. Rajasekaran, B. Sridhar and B. V. S. Reddy, Catalyst and solvent-free cyclopropanation of electron-deficient olefins with cyclic diazoamides for the synthesis of spiro[cyclopropane-1,3′-indolin]-2′-one derivatives, Tetrahedron Lett., 2014, 55, 7064 CrossRef CAS; (d) S. Muthusamy and R. Ramkumar, Highly Diastereoselective Synthesis of Spirocyclopropane-oxindoles Using InCl3 as a Catalyst in Water, Synlett, 2015, 26, 2156 CrossRef CAS.
- A. Awata and T. Arai, Catalytic Asymmetric Cyclopropanation with Diazooxindole, Synlett, 2013, 24, 29 CAS.
- Z. Y. Cao, F. Zhou, Y. H. Yu and J. Zhou, A Highly Diastereo- and Enantioselective Hg(II)-Catalyzed Cyclopropanation of Diazooxindoles and Alkenes, Org. Lett., 2013, 15, 42 CrossRef CAS.
- Z.-Y. Cao, X. Wang, C. Tan, X.-L. Zhao, J. Zhou and K. Ding, Highly Stereoselective Olefin Cyclopropanation of Diazooxindoles Catalyzed by a C2-Symmetric Spiroketal Bisphosphine/Au(I) Complex, J. Am. Chem. Soc., 2013, 135, 8197 CrossRef CAS.
- Y. Chi, L. Qiu and X. Xu, Highly enantioselective synthesis of spirocyclopropyloxindoles via a Rh(II)-catalyzed asymmetric cyclopropanation reaction, Org. Biomol. Chem., 2016, 14, 10357 RSC.
- Z. Y. Cao, W. Wang, K. Liao, X. Wang, J. Zhou and J. Ma, Catalytic enantioselective synthesis of cyclopropanes featuring vicinal all-carbon quaternary stereocenters with a CH2F group; study of the influence of C-F⋯H-N interactions on reactivity, Org. Chem. Front., 2018, 5, 2960 RSC.
- M. Tone, Y. Nakagawa, S. Chanthamath, I. Fujisawa, N. Nakayama, H. Goto, K. Shibatomi and S. Iwasa, Highly stereoselective spirocyclopropanation of various diazooxindoles with olefins catalyzed using Ru(II)-complex, RSC Adv., 2018, 8, 39865 RSC.
- J. L. Meloche and B. L. Ashfeld, A Rhodium(II)-Catalyzed Formal [4 + 1]-Cycloaddition toward Spirooxindole Pyrrolone Construction Employing Vinyl Isocyanates as 1,4-Dipoles, Angew. Chem., Int. Ed., 2017, 56, 6604 CrossRef CAS.
- T.-R. Li, S.-W. Duan, W. Ding, Y.-Y. Liu, J.-R. Chen, L.-Q. Lu and W.-J. Xiao, Synthesis of CF3-Containing 3,3′-Cyclopropyl Spirooxindoles by Sequential [3 + 2] Cycloaddition/Ring Contraction of Ylideneoxindoles with 2,2,2-Trifluorodiazoethane, J. Org. Chem., 2014, 79, 2296 CrossRef CAS.
- B. Morandi and E. M. Carreira, Iron-Catalyzed Cyclopropanation with Trifluoroethylamine Hydrochloride and Olefins in Aqueous Media: In Situ Generation of Trifluoromethyl Diazomethane, Angew. Chem., Int. Ed., 2010, 49, 938 CrossRef CAS.
- G. Ramu, N. Hari Krishna, G. Pawar, K. N. Visweswara Sastry, J. B. Nanubolu and B. Nagendra Babu, Solvent-Controlled, Tunable Domino Reaction of 3-Ylideneoxindoles with in Situ-Generated α-Aryldiazomethanes: A Facile Access to 3-Spirocyclopropyl-2-oxindole and Pyrazoloquinazolinone, ACS Omega, 2018, 3, 12349 CrossRef CAS.
- W. Y. Han, J. Zhao, J. S. Wang, B. D. Cui, N. W. Wan and Y. Z. Chen, Syntheses of CF2H-containing spirocyclopropyloxindoles from in situ generated CF2HCHN2 and 3-ylideneoxindoles, Tetrahedron, 2017, 73, 5806 CrossRef CAS.
- N. Huang, L. Zou and Y. Peng, Enantioselective 1,3-Dipolar Cycloaddition of Methyleneindolinones with α-Diazomethylphosphonate to Access Chiral Spiro-phosphonylpyrazoline-oxindoles Catalyzed by Tertiary Amine Thiourea and 1,5-Diazabicyclo[4.3.0]non-5-ene, Org. Lett., 2017, 19, 5806 CrossRef CAS.
- (a) R. Zhou, C. Yang, Y. Liu, R. Li and Z. He, Diastereoselective Synthesis of Functionalized Spirocyclopropyl Oxindoles via P(NMe2)3-Mediated Reductive Cyclopropanation, J. Org. Chem., 2014, 79, 10709 CrossRef CAS. For a further study see: (b) E. E. Wilson, K. X. Rodriguez and B. L. Ashfeld, Stereochemical implications in the synthesis of 3,3′-spirocyclopropyl oxindoles from β-aryl/alkyl-substituted alkylidene oxindoles, Tetrahedron, 2015, 71, 5765 CrossRef CAS.
- D. Yin, H. Liu, C. D. Lu and Y. J. Xu, Dialkyl Phosphite-Initiated Cyclopropanation of α,β-Unsaturated Ketones Using α-Ketoesters or Isatin Derivatives, J. Org. Chem., 2017, 82, 3252 CrossRef CAS.
- L. Zhang, H. Lu, G.-Q. Xu, Z.-Y. Wang and P.-F. Xu, PPh3 Mediated Reductive Annulation Reaction between Isatins and Electron Deficient Dienes to Construct Spirooxindole Compounds, J. Org. Chem., 2017, 82, 5782 CrossRef CAS.
- (a) J. H. Li, T. F. Feng and D. M. Du, Construction of Spirocyclopropane-Linked Heterocycles Containing Both Pyrazolones and Oxindoles through Michael/Alkylation Cascade Reactions, J. Org. Chem., 2015, 80, 11369 CrossRef CAS; (b) Y. X. Song and D. M. Du, Asymmetric synthesis of spirooxindole-fused spirothiazolones via squaramide-catalysed reaction of 3-chlorooxindoles with 5-alkenyl thiazolones, Org. Biomol. Chem., 2019, 17, 5375 RSC; (c) S. Wang, Z. Guo, Y. Wu, W. Liu, X. Liu, S. Zhang and C. Sheng, Organocatalytic asymmetric synthesis of highly functionalized spiro-thiazolone-cyclopropane-oxindoles bearing two vicinal spiro quaternary centers, Org. Chem. Front., 2019, 6, 1442 RSC; (d) Y. Zhu, T. Lu, A. Geng, H. Cui and L. Zhang, Ultrafast and Diastereoselective Synthesis of 3-Spirocyclopropyl-2-oxindoles Bearing Three Continuous All-Carbon Quaternary Centers, Heterocycles, 2019, 98, 711 CrossRef; (e) J. B. Wen and D. M. Du, Squaramide-catalysed asymmetric cascade reactions of 2,3-dioxopyrrolidines with 3-chlorooxindoles, Org. Biomol. Chem., 2020, 18, 1647 RSC.
- J. Guo, Y. Liu, X. Li, X. Liu, L. Lin and X. Feng, Nickel(II)-catalyzed enantioselective cyclopropanation of 3-alkenyl-oxindoles with phenyliodonium ylide via free carbene, Chem. Sci., 2016, 7, 2717 RSC.
- (a) L. Wang, W. Cao, H. Mei, L. Hu and X. Feng, Catalytic Asymmetric Synthesis of Chiral Spiro-cyclopropyl Oxindoles from 3-Alkenyl-oxindoles and Sulfoxonium Ylides, Adv. Synth. Catal., 2018, 360, 4089 CrossRef CAS; (b) For a related metal-free diastereoselective transformation (when using a chiral sulfur ylide in combination with an asymmetric hydrogen bonding catalyst achieved a maximum 39% ee see: J. W. Kang, X. Li, F. Y. Chen, Y. Luo, S. C. Zhang, B. Kang, C. Peng, X. Tian and B. Han, Protecting group-directed annulations of tetra-substituted oxindole olefins and sulfur ylides: regio- and chemoselective synthesis of cyclopropane- and dihydrofuran-fused spirooxindoles, RSC Adv., 2019, 9, 12255 RSC.
- C. L. Ladd, D. Sustac Roman and A. B. Charette, Silver-Promoted, Palladium-Catalyzed Direct Arylation of Cyclopropanes: Facile Access to Spiro 3,3′-Cyclopropyl Oxindoles, Org. Lett., 2013, 15, 1350 CrossRef CAS.
- H. Li, X. Yan, J. Zhang, W. Guo, J. Jiang and J. Wang, Enantioselective Synthesis of C-N Axially Chiral N-Aryloxindoles by Asymmetric Rhodium-Catalyzed Dual C-H Activation, Angew. Chem., Int. Ed., 2019, 58, 6732 CrossRef CAS.
- J. Li, T. Du, G. Zhang and Y. Peng, 3-Bromooxindoles as nucleophiles in asymmetric organocatalytic Mannich reactions with N-Ts-imines, Chem. Commun., 2013, 49, 1330 RSC.
- (a) M. A. Loreto, A. Migliorini, P. A. Tardella and A. Gambacorta, Novel Spiroheterocycles by Aziridination of α-methylene-γ- and -δ-lactams, Eur. J. Org. Chem., 2007, 2365 CrossRef CAS; (b) I. Ammetto, T. Gasperi, M. A. Lorerto, A. Migliorini, F. Palmarelli and P. A. Tardella, Synthesis of Functionalized Spiroaziridine-oxindoles from 3-Ylideneoxindoles: An Easy Route to 3-(Aminoalkyl)oxindoles, Eur. J. Org. Chem., 2009, 6189 CrossRef CAS; (c) T. Gasperi, M. A. Loreto, A. Migliorini and C. Ventura, Synthesis of Aziridine- and Oxirane-2-phosphonates Spiro-Fused with Oxindoles, Eur. J. Org. Chem., 2011, 385 CrossRef CAS.
- (a) Q. L. Wang, T. Cai, J. Zhou, F. Tian, X. Y. Xu and L. X. Wang, An unprecedented base-promoted domino reaction of methyleneindolinones and N-tosyloxycarbamates for the construction of bispirooxindoles and spiroaziridine oxindoles, Chem. Commun., 2015, 51, 10726 RSC; (b) Y. Y. Liu, S. W. Duan, R. Zhang, Y. H. Liu, J. R. Chen and W. J. Xiao, Base-catalyzed controllable reaction of 3-ylideneoxindoles with O-Boc hydroxycarbamates for the synthesis of amidoacrylates and spiroaziridine oxindoles, Org. Biomol. Chem., 2016, 14, 5224 RSC.
- M. A. Marsini, J. T. Reeves, J. N. Desrosiers, M. A. Herbage, J. Savoie, Z. Li, K. R. Fandrick, C. A. Sader, B. McKibben and D. A. Gao, et al., Diastereoselective Synthesis of α-Quaternary Aziridine-2-carboxylates via Aza-Corey-Chaykovsky Aziridination of N-tert-Butanesulfinyl Ketimino Esters, Org. Lett., 2015, 17, 5614 CrossRef CAS.
- (a) S. Hajra, S. M. Aziz, B. Jana, P. Mahish and D. Das, Synthesis of Chiral Spiro-Aziridine Oxindoles via Aza-Corey-Chaykovsky reaction of Isatin Derived N-tert-Butanesulfinyl Ketimines, Org. Lett., 2016, 18, 532 CrossRef CAS; (b) S. Hajra and A. Biswas, Catalyst-Free Stereocontrolled Formal [3 + 2]-Cycloaddition of CO2 for the Synthesis of Enantiopure Spiro[Indoline-3,5′-Oxazolidine]-2,2′-Diones under Aqueous and Ambient Conditions, Org. Lett., 2020, 22, 4990 CrossRef CAS.
- J. Chen, X. Wen, Y. Wang, F. Du, L. Cai and Y. Peng, Asymmetric Mannich Reaction of Isatin-Based Ketimines with α-Diazomethylphosphonates Catalyzed by Chiral Silver Phosphate, Org. Lett., 2016, 18, 4336 CrossRef CAS.
- J. Q. Zhao, D. F. Yue, X. M. Zhang, X. Y. Xu and W. C. Yuan, The organocatalytic asymmetric Neber reaction for the enantioselective synthesis of spirooxindole 2H-azirines, Org. Biomol. Chem., 2016, 14, 10946 RSC.
- (a) K. Sharma, L. K. Sharma and R. Jain, Facile Synthesis of Spiro[3h-Indole-3,2′oxirane]-3′-(2-Oxo-2-(Thiophene-2-Yl-(2(1H)Ones and their Antibacterial Activity, Heterocycl. Lett., 2015, 5, 383 CAS; (b) M. Montana, F. Correard, O. Khoumeri, M. A. Esteve, T. Terme and P. Vanelle, Synthesis of New Quinoxalines Containing an Oxirane Ring by the TDAE Strategy and in Vitro Evaluation in Neuroblastoma Cell Lines, Molecules, 2014, 19, 14987 CrossRef.
- S. Monticelli, L. Castoldi, S. Touqeer, M. Miele, E. Urban and V. Pace, Recent Advances in the Synthesis and Reactivity of Spiro-Epoxyoxindoles, Chem. Heterocycl. Compd., 2018, 54, 389 CrossRef CAS.
- M. M. Lou, H. Wang, L. Song, H. Y. Liu, Z. Q. Li, X. S. Guo, F. G. Zhang and B. Wang, The Epoxidation of Carbonyl Compounds with a Benzyne-Triggered Sulfur Ylide, J. Org. Chem., 2016, 81, 5915 CrossRef CAS.
- V. Pace, L. Castoldi, A. D. Mamuye, T. Langer and W. Holzer, Chemoselective Addition of Halomethyllithiums to Functionalised Isatins: A Straightforward Access to Spiro-Epoxyoxindoles, Adv. Synth. Catal., 2016, 358, 172 CrossRef CAS.
- (a) D. Basavaiah, S. S. Badsara and B. C. Sahu, Baylis-Hillman Bromides as a Source of 1,3-Dipoles: Sterically Directed Synthesis of Oxindole-Fused Spirooxirane and Spirodihydrofuran Frameworks, Chem. – Eur. J., 2013, 19, 2961 CrossRef CAS; (b) Q. Fu and C. G. Yan, Facile synthesis of functionalized spiro[indoline-3,2′-oxiran]-2-ones by Darzens reaction, Beilstein J. Org. Chem., 2013, 9, 918 CrossRef CAS.
- B. Cheng, B. Zu, Y. Li, C. Tao, C. Zhang, R. Wang, Y. Li and H. Zhai, Synthesis of CF3-containing spiro-epoxyoxindoles via the Corey-Chaykovsky reaction of N-alkyl isatins with Ph2S+CH2CF3OTF−, Org. Biomol. Chem., 2018, 16, 3564 RSC.
- K. Luo, X. Yu, P. Chen, K. He, J. Lin and Y. Jin, Visible-light-induced aerobic epoxidation in cyclic ether: Synthesis of spiroepoxyoxindole derivatives, Tetrahedron Lett., 2020, 61, 151578 CrossRef CAS.
- S. Crotti, N. Di Iorio, C. Artusi, A. Mazzanti, P. Righi and G. Bencivenni, Direct Access to Alkylideneoxindoles via Axially Enantioselective Knoevenagel Condensation, Org. Lett., 2019, 21, 3013 CrossRef CAS.
- V. Schulz, M. Davoust, M. Lemarié, J. F. Lohier, J. S. O. De Santos, P. Metzner and J. F. Brière, Straightforward Stereoselective Synthesis of Spiro-epoxyoxindoles, Org. Lett., 2007, 9, 1745 CrossRef CAS.
- C. Palumbo, G. Mazzeo, A. Mazziotta, A. Gambacorta, M. A. Loreto, A. Migliorini, S. Superchi, D. Tofani and T. Gasperi, Noncovalent Organocatalysis: A Powerful Tool for the Nucleophilic. Epoxidation of α-Ylideneoxindoles, Org. Lett., 2011, 13, 6248 CrossRef CAS.
- M. Miceli, A. Mazziotta, C. Palumbo, E. Roma, E. Tosi, G. Longhi, S. Abbate, P. Lupattelli, G. Mazzeo and T. Gasperi, Asymmetric Synthesis of Spirooxindoles via Nucleophilic Epoxidation Promoted by Bifunctional Organocatalysis, Molecules, 2018, 23, 438 CrossRef.
- A. Boucherif, Q.-Q. Yang, Q. Wang, J.-R. Chen, L.-Q. Lu and W.-J. Xiao, Enantio- and Diastereoselective Synthesis of Spiro-epoxyoxindoles, J. Org. Chem., 2014, 79, 3924 CrossRef CAS.
- Y. Kuang, Y. Lu, Y. Tang, X. Liu, L. Lin and X. Feng, Asymmetic Synthesis of Spiro-epoxyoxindoles by the Catalytic Darzens Reaction of Isatins with Phenacyl Bromides, Org. Lett., 2014, 16, 4244 CrossRef CAS.
- G.-L. Chai, J.-W. Han and H. N. C. Wong, Asymmetric Darzens Reaction of Isatins with Diazoacetamides Catalyzed by Chiral BINOL-Titanium Complex, J. Org. Chem., 2017, 82, 12647 CrossRef CAS.
- (a) G. Zhu, G. Bao, Y. Li, W. Sun, J. Li, L. Hong and R. Wang, Efficient Catalytic Kinetic Resolution of Spiro-epoxyoxindoles with Concomitant Asymmetric Friedel-Crafts Alkylation of Indoles, Angew. Chem., Int. Ed., 2017, 56, 5332 CrossRef CAS; (b) G. Zhu, Y. Li, G. Bao, W. Sun, L. Huang, L. Hong and R. Wang, Catalytic Kinetic Resolution of Spiro-Epoxyoxindoles with 1-Napthols: Switchable Asymmetric Tandem Dearomatization/Oxa-Michael Reaction and Friedel-Crafts Alkylation of 1-Napthols at the C4 Position, ACS Catal., 2018, 8, 1810 CrossRef CAS.
- R. Zhou, H. Zhang, J. Liu, R. Liu, W. C. Gao, Y. Qiao and R. Li, Chemoselective P(NMe2)3-Mediated Reductive Epoxidation between Two Different Carbonyl Electrophiles: Synthesis of Highly Functionalized Unsymmetrical Epoxides, J. Org. Chem., 2018, 83, 8272 CrossRef CAS.
- M. Yoshikawa, H. Kamisaki, J. Kunitomo, H. Oki, H. Kokubo, A. Suzuki, T. Ikemoto, K. Nakashima, N. Kamiguchi and A. Harada, et al., Design and synthesis of a novel 2-oxindole scaffold as a highly potent and brain-penetrant phosphodiesterase 10A inhibitor, Bioorg. Med. Chem., 2015, 23, 7138 CrossRef CAS.
- R. K. Ujjinamatada, S. Samajdar, C. Abbineni, S. Mukeherjee, T. Linnanen and G. Wohlfahrt, Spiro[cyclobutane-1,3′-indolin]-2′-one Derivatives as Bromodomain Inhibitors, PCT Int. ApplWO2016203112A1, 2016 Search PubMed.
- A. Fensome, R. Bender, J. Cohen, M. A. Collins, V. A. Mackner, L. L. Miller, J. W. Ullrich, R. Winneker, J. Wrobel and P. Zhang, et al., New progesterone receptor antagonists: 3,3-disubstituted-5-aryloxindoles, Bioorg. Med. Chem. Lett., 2002, 12, 3487 CrossRef CAS.
- A. Tahri, S. M. H. Vendeville, T. H. M. Jonckers, P. J.-M. B. Raboisson, L. Hu, S. D. Demin and L. P. Cooy-Mans, RSV Antiviral Compounds, PCT Int. ApplWO2014060411A1, 2014 Search PubMed.
- (a) P. S. Baran and J. M. Richter, Enantioselective Total Syntheses of Welwitindolinone A and Fischerindoles I and G, J. Am. Chem. Soc., 2005, 127, 15394 CrossRef CAS; (b) P. S. Baran, T. J. Maimone and J. M. Richter, Total synthesis of marine natural products without using protecting groups, Nature, 2007, 446, 404 CrossRef CAS; (c) J. M. Richter, Y. Ishihara, T. Masuda, B. W. Whitefield, T. Llamas, A. Pohjakallio and P. S. Baran, Enantiospecific Total Synthesis of the Hapalindoles, Fischerindoles, and Welwitindolinones via a Redox Economic Approach, J. Am. Chem. Soc., 2008, 130, 17938 CrossRef CAS.
- (a) J. M. Ready, S. E. Reisman, M. Hirata, M. M. Weiss, K. Tamaki, T. V. Ovaska and J. L. Wood, A Mild and Efficient Synthesis of Oxindoles: Progress Towards the Synthesis of Welwitindolinone A Isonitrile, Angew. Chem., Int. Ed., 2004, 43, 1270 CAS; (b) S. E. Reisman, J. M. Ready, A. Hasuoka, C. J. Smith and J. L. Wood, Total Synthesis of (±)-Welwitindolinone A Isonitrile, J. Am. Chem. Soc., 2006, 128, 1448 CrossRef CAS; (c) S. E. Reisman, J. M. Ready, M. M. Weiss, A. Hasuoka, M. Hirata, K. Tamaki, T. V. Ovaska, C. J. Smith and J. L. Wood, Evolution of a Synthetic Strategy: Total Synthesis of (±)-Welwitindolinone A Isonitrile, J. Am. Chem. Soc., 2008, 130, 2087 CrossRef CAS.
- L. E. Overman and D. J. Poon, Asymmetric Heck Reactions via Neutral Intermediates: Enhanced Enantioselectivity with Halide Additives Gives Mechanistic Insights, Angew. Chem., Int. Ed. Engl., 1997, 36, 518 CrossRef CAS.
- D. Katayev, Y. X. Jia, A. K. Sharma, D. Banerjee, C. Besnard, R. B. Sunoj and E. P. Kündig, Synthesis of 3,3-Disubstituted Oxindoles by Palladium-Catalyzed Asymmetric Intramolecular α-Arylation of Amides: Reaction Development and Mechanistic Studies, Chem. – Eur. J., 2013, 19, 11916 CrossRef CAS.
- R. Rocaboy, D. Dailler, F. Zellweger, M. Neuburger, C. Salomé, E. Clot and O. Baudoin, Domino Pd0-Catalyzed C(sp3)-H Arylation/Electrocyclic Reactions via Benzazetidine Intermediates, Angew. Chem., Int. Ed., 2018, 57, 12131 CrossRef CAS.
- Though non-stereoselective, Zhang and co-workers discovered an unexpected cascade reaction upon treatment of a spirocyclopropyl oxetane with boron trifluoride diethyl etherate to form a spirocyclobutyl oxindole via a carbocationic intermediate: D. D. Wu, C. M. Huang, Y. H. Wu, H. K. Fun, J. H. Xu and Y. Zhang, Acid-mediated transformation of spirocyclopropyl oxetanes: a facile approach to spirocyclopropyl butenolides and γ-butyrolactones, RSC Adv., 2013, 3, 7529 RSC.
- J. Q. Chen, Y. L. Wei, G. Q. Xu, Y. M. Liang and P. F. Xu, Intramolecular 1,5-H transfer reaction of aryl iodides through visible-light photoredox catalysis: a concise method for the synthesis of natural product scaffolds, Chem. Commun., 2016, 52, 6455 RSC.
- S. M. Hell, C. F. Meyer, G. Laudadio, A. Misale, M. C. Willis, T. Noël, A. A. Trabanco and V. Gouverneur, Silyl Radical-Mediated Activation of Sulfamoyl Chlorides Enables Direct Access to Aliphatic Sulfonamides from Alkenes, J. Am. Chem. Soc., 2020, 142, 720 CrossRef CAS.
- L. W. Qi, Y. Yang, Y. Y. Gui, Y. Zhang, F. Chen, F. Tian, L. Peng and L. X. Wang, Asymmetric Synthesis of 3,3′-Spirooxindoles Fused with Cyclobutanes through Organocatalytic Formal [2 + 2] Cycloadditionns under H-Bond-Directing Dienamine Activation, Org. Lett., 2014, 16, 6436 CrossRef CAS.
- K. S. Halskov, F. Kniep, V. H. Lauridsen, E. H. Iversen, B. S. Donslund and K. A. Jørgensen, Organocatalytic Enamine-Activation of Cyclopropanes for Highly Stereoselective Formation of Cyclobutanes, J. Am. Chem. Soc., 2015, 137, 1685 CrossRef CAS.
- (a) Y. H. Jiang, R. Y. Yang, J. Sun and C. G. Yan, Diastereoselective synthesis of dispiro[indoline-3,1′-cyclobutane-2′,3′′-indolines] via visible light catalyzed cyclodimerization of 3-phenacylideneoxindoles, Heterocycl. Commun., 2016, 22, 151 CAS; (b) L. L. Wu, G. H. Yang, Z. Guan and Y. H. He, Metal-free visible-light-promoted intermolecular [2 + 2]-cycloaddition of 3-ylideneoxindoles, Tetrahedron, 2017, 73, 1854 CrossRef CAS.
- (a) J. W. Skiles and D. McNeil, Spiro indolinone beta-lactams, inhibitors of poliovirus and rhinovirus 3C-proteinases, Tetrahedron Lett., 1990, 31, 7277 CrossRef CAS; (b) W. Shi, Z. Jiang, H. He, F. Xiao, F. Lin, Y. Sun, L. Hou, L. Shen, L. Han and M. Zeng, et al., Discovery of 3,3′-Spiro[Azetidine]-2-oxo-indoline Derivatives as Fusion Inhibitors for Treatment of RSV Infection, ACS Med. Chem. Lett., 2018, 9, 94 CrossRef CAS.
- G. S. Singh and P. Luntha, Synthesis and antimicrobial activity of new 1-alkyl/cyclohexyl-3,3-diaryl-1′-methylspiro[azetidine-2,3′-indoline]-2′,4-diones, Eur. J. Med. Chem., 2009, 44, 2265 CrossRef CAS.
- R. Kumar, S. Giri and N. Nizamuddin, Synthesis of some 1′-(substituted phenyl)spiro[indole-3,4′-azetidine]-2(3H),2′-diones as potential fungicides, J. Agric. Food Chem., 1989, 37, 1094 CrossRef CAS.
- R. Jain, K. Sharma and D. Kumar, Green Synthesis of 1-(1,2,4-Triazol-4-yl)spiro[azetidine-2,3′-(3H)indole]-2′4′(1′H)-diones as Potential Insecticide Agents, J. Heterocycl. Chem., 2013, 50, 315 CrossRef CAS.
- C. Sun, X. Lin and S. M. Weinreb, Explorations on the Total Synthesis of the Unusual Marine Alkaloid Chartelline A, J. Org. Chem., 2006, 71, 3159 CrossRef CAS.
- Ref. 92 and (a) H. Deokar, J. Chaskar and A. Chaskar, Synthesis, Screening And Antimicrobial Activity Of Spiro Indolothiazolidinone, Oxazolidinone And Azetidine Derivatives Of Benzenesulfonyl Chloride, Pharm. Chem. J., 2012, 46, 429 CrossRef CAS; (b) B. V. Subba Reddy, G. Karthik, T. Rajasekaran, A. Antony and B. Sridhar, Rh2(OAc)4 atalyzed substrate selective [4 + 2]/[2 + 2] cycloaddition of acylketenes: a highly chemo- and regioselective synthesis of spiro(oxindolyl)oxazinones and β-lactams, Tetrahedron Lett., 2012, 53, 2396 CrossRef CAS.
- H. M. Zhang, Z. H. Gao and S. Ye, Bifunctional N-Heterocyclic Carbene-Catayzed Highly Enantioselective Synthesis of Spirocyclic Oxindolo-β-lactams, Org. Lett., 2014, 16, 3079 CrossRef CAS.
- (a) J. Xu, S. Yuan, J. Peng, M. Miao, Z. Chen and H. Ren, Enantioselective [2 + 2] annulation of simple aldehydes with isatin-derived ketimines via oxidative N-heterocyclic carbene catalysis, Chem. Commun., 2017, 53, 3430 RSC. For further insights see: (b) X. Li, R. Duan, Y. Wang, L. B. Qu, Z. Li and D. Wei, Insights into N-Heterocyclic Carbene-Catalyzed Oxidative α-C(sp3)-H Activation of Aliphatic Aldehydes and Cascade [2 + 2] Cycloaddition with Ketimines, J. Org. Chem., 2019, 84, 6117 CrossRef CAS.
- J. H. Jin, J. Zhao, W. L. Yang and W. P. Deng, Asymmetric Synthesis of Spirooxindole β-lactams via Isothiourea-catalyzed Mannich/lactamization Reaction of Aryl Acetic Acids with Isatin-derived Ketimines, Adv. Synth. Catal., 2019, 361, 1592 CrossRef CAS.
- S. Periyaraja, P. Shanmugam and A. B. Mandal, A Copper-Catalyzed One-Pot, Three-Component Diastereoselective Synthesis of 3-Spiroazetidinimine-2-oxindoles and Their Synthetic Transformation into Fluorescent Conjugated Indolones, Eur. J. Org. Chem., 2014, 954 CrossRef CAS.
- Throughout this review the stereochemical relationship is, where applicable, defined between the aromatic group of the oxindole and the substituent on the ring.
- S. Guo, P. Dong, Y. Chen, X. Feng and X. Liu, Chiral Guanidine/Copper Catalyzed Asymmetric Azide-Alkyne Cycloaddition/[2 + 2] Cascade Reaction, Angew. Chem., Int. Ed., 2018, 57, 16852 CrossRef CAS.
- G. Rainoldi, M. Faltracco, L. Lo Presti, A. Silvani and G. Lesma, Highly diastereoselective entry into chiral spirooxindole-based 4-methyleneazetidines via formal [2 + 2] annulation reaction, Chem. Commun., 2016, 52, 11575 RSC.
- G. Rainoldi, M. Faltracco, C. Spatti, A. Silvani and G. Lesma, Organocatalytic Access to Enantioenriched Spirooxindole-Based 4-Methyleneazetidines, Molecules, 2017, 22, 2016 CrossRef.
- (a) L. Su and M. H. Xu, Asymmetric Reformatsky-Type Reaction of Isatin-Derived N-Sulfinyl Ketimines: Efficient and Practical Synthesis of Enantiopure Chiral 2-Oxoindolinyl-β3,3-Amino Esters, Synthesis, 2016, 48, 2595 CrossRef CAS; (b) In 2017 Hajra synthesised the same intermediates with high dr: S. Hajra, S. S. Bhosale and A. Hazra, An asymmetric acetate-Mannich reaction of chiral isatin derived ketimines and its applications, Org. Biomol. Chem., 2017, 15, 9217 RSC.
- Q. Tan, X. Wang, Y. Xiong, Z. Zhao, L. Li, P. Tang and M. Zhang, Chiral Amino Alcohol Accelerated and Stereocontrolled Allylboration of Iminoisatins: Highly Efficient Construction of Adjacent Quaternary Stereogenic Centers, Angew. Chem., Int. Ed., 2017, 56, 4829 CrossRef CAS.
- J. S. Yu, H. Noda and M. Shibasaki, Exploiting β-Amino Acid Enolates in Direct Catalytic Diastereo- and Enantioselective C-C Bond-Forming Reactions, Chem. – Eur. J., 2018, 24, 15796 CrossRef CAS.
- C. Deutsch, D. Kuhn, T. Ross and L. Burgdorf, 8-Substituted 2-Amino-[1,2,4] Triazolo [1,5-A] Pyrazines as SYK Tryrosine Kinase Inhibitors and GCN2 Serin Kinase Inhibitors, PCT Int. ApplWO2013124026A1, 2013 Search PubMed.
- D. D. Wu, M. T. He, Q. Di Liu, W. Wang, J. Zhou, L. Wang, H. K. Fun, J. H. Xu and Y. Zhang, Photoinduced reactions of bicycloalkylidenes with isatin and isoquinolinetrione, Org. Biomol. Chem., 2012, 10, 3626 RSC.
- M. Palomba, E. Scarcella, L. Sancineto, L. Bagnoli, C. Santi and F. Marini, Synthesis of Spirooxindole Oxetanes Through a Domino Reaction of 3-Hydroxyoxindoles and Phenyl Vinyl Selenone, Eur. J. Org. Chem., 2019, 5396 CrossRef CAS.
- P. R. Gentry, T. M. Bridges, A. Lamsal, P. N. Vinson, E. Smith, P. Chase, P. S. Hodder, J. L. Engers, C. M. Niswender and J. Scott Daniels, et al., Discovery of ML326: The first sub-micromolar, selective M5 PAM, Bioorg. Med. Chem. Lett., 2013, 23, 2996 CrossRef CAS.
- X. Hao, X. Liu, W. Li, F. Tan, Y. Chu, X. Zhao, L. Lin and X. Feng, Chiral Lewis Acid Catalyzed Asymmetric Cycloadditions of Disubstituted Ketenes for the Synthesis of β-Lactones and δ-Lactones, Org. Lett., 2014, 16, 134 CrossRef CAS.
- E. Hayashi, M. Isogai, Y. Kagawa, N. Takayanagi and S. Yamada, Neosurugatoxin, a Specific Antagonist of Nicotinic Acetylcholine Receptors, J. Neurochem., 1984, 42, 1491 CrossRef CAS.
- Z. Bian, C. C. Marvin and S. F. Martin, Enantioselective Total Synthesis of (-)-Citrinadin A and Revision of Its Stereochemical Structure, J. Am. Chem. Soc., 2013, 135, 10886 CrossRef CAS.
- (a) D. A. Mundal and R. Sarpong, Synthetic Studies toward the Citrinadin A and B Core Architecture, Org. Lett., 2013, 15, 4952 CrossRef CAS; (b) E. V. Mercado-Marin, P. Garcia-Reynaga, S. Romminger, E. F. Pimenta, D. K. Romney, M. W. Lodewyk, D. E. Williams, R. J. Andersen, S. J. Miller and D. J. Tantillo, et al., Total synthesis and isolation of citrinalin and cylopiamine congeners, Nature, 2014, 509, 318 CrossRef CAS; (c) N. S. Simpkins, I. Pavlakos, M. D. Weller and L. Male, The cascade radical cyclisation approach to prenylated alkaloids: synthesis of stephacidin A and notoamide B, Org. Biomol. Chem., 2013, 11, 4957 RSC; (d) B. Zhang, W. Zheng, X. Wang, D. Sun and C. Li, Total Synthesis of Notoamides F, I, and R and Sclerotiamide, Angew. Chem., Int. Ed., 2016, 55, 10435 CrossRef CAS.
- A. E. Fraley, K. Caddell Haatveit, Y. Ye, S. P. Kelly, S. A. Newmister, F. Yu, R. M. Williams, J. L. Smith, K. N. Houk and D. H. Sherman, Molecular Basis for Spirocycle Formation in the Paraherquamide Biosynthetic Pathway, J. Am. Chem. Soc., 2020, 142, 2244 CrossRef CAS.
- K. Kong, J. A. Enquist, M. E. McCallum, G. M. Smith, T. Matsumaru, E. Menhaji-Klotz and J. L. Wood, An Enantioselective Total Synthesis and Stereochemical Revision of (+)-Citrinadin B, J. Am. Chem. Soc., 2013, 135, 10890 CrossRef CAS.
- B. M. Trost, D. A. Bringley, T. Zhang and N. Cramer, Rapid Access to Spirocyclic Oxindole Alkaloids: Application of the Asymmetric Palladium-Catalyzed [3 + 2] Trimethylenemethane Cycloaddition, J. Am. Chem. Soc., 2013, 135, 16720 CrossRef CAS.
- M. E. Hinze, J. L. Daughtry and C. A. Lewis, Access to the Surugatoxin Alkaloids: Chemo-, Regio-, and Stereoselective Oxindole Annulation, J. Org. Chem., 2015, 80, 11258 CrossRef CAS.
- L. Shi, L. Li, J. Wang, B. Huang, K. Zeng, H. Jin, Q. Zhang and Y. Jia, Total synthesis of natural spiro-trisindole enantiomers similisines A, B and their stereoisomers, Tetrahedron Lett., 2017, 58, 1934 CrossRef CAS.
- For a review see: Z. Chen, Z. Chen, W. Du and Y. Chen, Transformations of Modified Morita-Baylis-Hillman Adducts from Isatins Catalyzed by Lewis Bases, Chem. Rec., 2020, 20, 541 CrossRef CAS.
- For selected significant prior art in this area see: (a) B. M. Trost, N. Cramer and S. M. Silverman, Enantioselective Construction of Spirocyclic Oxindolic Cyclopentanes by Palladium-Catalyzed Trimethylenemethane-[3 + 2]-Cycloaddition, J. Am. Chem. Soc., 2007, 129, 12396 CrossRef CAS; (b) J. Peng, X. Huang, L. Jiang, H. L. Cui and Y. C. Chen, Tertiary Amine-Catalyzed Chemoselective and Asymmetric [3 + 2] Annulation of Morita-Baylis-Hillman Carbonates of Isatins with Propargyl Sulfones, Org. Lett., 2011, 13, 4584 CrossRef CAS; (c) B. Tan, N. R. Candeias and C. F. Barbas, Core-Structure-Motivated Design of a Phosphine-Catalyzed [3 + 2] Cycloaddition Reaction: Enantioselective Syntheses of Spirocyclopenteneoxindoles, J. Am. Chem. Soc., 2011, 133, 4672 CrossRef CAS.
- Y. Chen, B. D. Cui, Y. Wang, W. Y. Han, N. W. Wan, M. Bai, W. C. Yuan and Y. Z. Chen, Asymmetric [3 + 2] Cycloaddition Reaction of Isatin-Derived MBH Carbonates with 3-Methyleneoxindoles: Enantioselective Synthesis of 3,3′-Cyclopentenyldispirooxindoles Incorporating Two Adjacent Quaternary Spirostereocenters, J. Org. Chem., 2018, 83, 10465 CrossRef CAS.
- Y. Chen, B. D. Cui, M. Bai, W. Y. Han, N. W. Wan and Y. Z. Chen, Synthesis of chiral spiro-cyclopentene/cyclopentadiene-oxindoles through an asymmetric [3 + 2] cycloaddition of isatin-derived MBH carbonates and β,γ-unsaturated α-keto esters, Tetrahedron, 2019, 75, 2971 CrossRef CAS.
- For related work see: (a) J. Peng, G. Y. Ran, W. Du and Y. C. Chen, Tertiary-Amine-Catalyzed Asymmetric [3 + 2] Annulations. of Morita-Baylis-Hillman Carbonates of Isatins with Nitroolefins to Construct Spirooxindoles, Synthesis, 2015, 47, 2538 CrossRef CAS; (b) G. Y. Ran, P. Wang, W. Du and Y. C. Chen, α-Regioselective [3 + 2] annulations with Morita-Baylis-Hillman carbonates of isatins and 2-nitro-1,3-enynes, Org. Chem. Front., 2016, 3, 861 RSC; (c) G. Zhan, M.-L. Shi, Q. He, W.-J. Lin, Q. Ouyang, W. Du and Y.-C. Chen, Catalyst-Controlled Switch in Chemo- and Diastereoselectivities: Annulations of Morita-Baylis-Hillman Carbonates from Isatins, Angew. Chem., Int. Ed., 2016, 55, 2147 CrossRef CAS; (d) Z. H. Wang, C. W. Lei, X. Y. Zhang, Y. You, J. Q. Zhao and W. C. Yuan, Asymmetric domino 1,6-addition/annulation reaction of 3-cyano-4-alkenyl-2H-chromen-2-ones with isatin-derived MBH cyclopentenylspirooxindoles bearing 2H-chromen-2-ones, Org. Chem. Front., 2019, 6, 3342 RSC; (e) J. Q. Zhao, L. Yang, X. J. Zhou, Y. You, Z. H. Wang, M. Q. Zhou, X. M. Zhang, X. Y. Xu and W. C. Yuan, Organocatalyzed Dearomative Cycloaddition of 2-Nitrobenzofurans and Isatin-Derived Morita-Baylis-Hillman Carbonates: Highly Stereoselective Construction of Cyclopenta[b]benzofuran Scaffolds, Org. Lett., 2019, 21, 660 CrossRef CAS.
- F. Zhong, X. Han, Y. Wang and Y. Lu, Highly Enantioselective [3 + 2] Annulation of Morita-Baylis-Hillman Adducts Mediated by L-Threonine-Derived Phosphines: Synthesis of 3-Spirocyclopentene-2-oxindoles having Two Contiguous Quaternary Centers, Angew. Chem., Int. Ed., 2011, 50, 7837 CrossRef CAS.
- Work by Miao and co-workers on phosphorus promoted [3 + 2] cycloaddition of alkynes and 3-methylene oxindoles with moderate enantioselectivity achieved: (a) J. Zhang, C. Cheng, D. Wang and Z. Miao, Regio- and. Diastereoselective Construction of Spirocyclopenteneoxindoles through Phosphine-Catalyzed [3 + 2] Annulation of Methyleneindolinone with Alkynoate Derivatives, J. Org. Chem., 2017, 82, 10121 CrossRef CAS. For related non-stereoselective activation by phosphorus catalysts see: (b) L. Zhang, H. Lu, G.-Q. Xu, Z.-Y. Wang and P.-F. Xu, PPh3 Mediated Reductive Annulation Reaction between Isatins and Electron Deficient Dienes to Construct Spirooxindole Compounds, J. Org. Chem., 2017, 82, 5782 CrossRef CAS. Also see: (c) D. B. Ramachary, C. Venkaiah and P. M. Krishna, Stereoselective Synthesis of Five-Membered Spirooxindoles through Tomita Zipper Cyclization, Org. Lett., 2013, 15, 4714 CrossRef CAS; (d) C. Gomez, M. Gicquel, J. C. Carry, L. Schio, P. Retailleau, A. Voituriez and A. Marinetti, Phosphine-Catalyzed Synthesis of 3,3-Spirocyclopenteneoxindoles from g-Substituted Allenoates: Systematic Studies and Targeted Applications, J. Org. Chem., 2013, 78, 1488 CrossRef CAS.
- W. L. Chan, X. Tang, F. Zhang, G. Quek, G. J. Mei and Y. Lu, Phosphine-Catalyzed (3 + 2) Annulation of Isoindigos with Allenes: Enantioselective Formation of Two Vicinal Quaternary Stereogenic Centers, Angew. Chem., Int. Ed., 2019, 58, 6260 CrossRef CAS.
- J. Zhang, W.-L. Chan, L. Chen, N. Ullah and Y. Lu, Creation of bispiro[pyrazoline-3,3′-oxindoles] via a phosphine-catalyzed enantioselective [3 + 2] annulation of the Morita-Baylis-Hillman carbonates with pyrazoloneyldiene, Org. Chem. Front., 2019, 6, 2210 RSC.
- S. Su, C. Li, X. Jia and J. Li, Isocyanide-Based Multicomponent Reactions: Concise Synthesis of Spirocyclic Oxindoles with Molecular Complexity by Using a [1,5]-Hydrogen Shift as the Key Step, Chem. – Eur. J., 2014, 20, 5905 CrossRef CAS.
- B. Tan, N. R. Candeias and C. F. Barbas, Construction of bispirooxindoles containing three quaternary stereocentres in a cascade using a single multifunctional organocatalyst, Nat. Chem., 2011, 3, 473 CrossRef CAS.
- (a) X. Li, Y. M. Li, F. Z. Peng, S. T. Wu, Z. Q. Li, Z. W. Sun, H. Bin Zhang and Z. H. Shao, Highly Enantioselective One-Pot Synthesis of Spirocyclopentaneoxindoles Containing the Oxime Group by Organocatalyzed Michael Addition/ISOC/Fragmentation Sequence, Org. Lett., 2011, 13, 6160 CrossRef CAS; (b) K. Albertshofer, K. E. Anderson and C. F. Barbas, Assembly of Spirooxindole Derivatives via Organocatalytic Iminium-Enamine Cascade Reactions, Org. Lett., 2012, 14, 5968 CrossRef CAS; (c) W. Sun, G. Zhu, C. Wu, L. Hong and R. Wang, An Organocatalytic Cascade Strategy for the Enantioselective Construction of Spirocyclopentane Bioxindoles Containing Three Contiguous Stereocenters and Two Spiro Quaternary Centers, Chem. – Eur. J., 2012, 18, 6737 CrossRef CAS; (d) W. Sun, G. Zhu, C. Wu, L. Hong and R. Wang, ‘Organo-Metal’ Synergistic Catalysis: The 1 + 1 > 2 Effect for the Construction of Spirocyclopentane oxindoles, Chem. – Eur. J., 2012, 18, 13959 CrossRef CAS; (e) J. Sun, Y. J. Xie and C. G. Yan, Construction of Dispirocyclopentanebisoxindoles via Self-Domino Michael-Aldol Reactions of 3-Phenacylideneoxindoles, J. Org. Chem., 2013, 78, 8354 CrossRef CAS; (f) W. Sun, L. Hong, G. Zhu, Z. Wang, X. Wei, J. Ni and R. Wang, An Organocatalytic Michael-Michael Cascade. for the Enantioselective Construction of Spirocyclopentane Bioxindoles: Control of Four Contiguous Stereocenters, Org. Lett., 2014, 16, 544 CrossRef CAS; (g) L. Z. Ding, T. S. Zhong, H. Wu and Y. M. Wang, Highly Enantioselective Construction of Spirocyclopentaneoxindoles Containing Four Consecutive Stereocenters. through an Organocatalytic Iminium-Enamine Cascade Reaction, Eur. J. Org. Chem., 2014, 5139 CrossRef CAS; (h) J. Zhou, Q. L. Wang, L. Peng, F. Tian, X. Y. Xu and L. X. Wang, An organocatalytic domino Michael-alkylation reaction: highly enantioselective construction of spiro-cyclopentaneoxindoles and tetronic acid scaffolds, Chem. Commun., 2014, 50, 14601 RSC; (i) K. Suman and S. Thennarasu, Base catalysed domino and self-domino Michael-Aldol reactions: one-pot synthesis of dispirocyclopentaneoxindoles containing multiple chiral stereocenters, RSC Adv., 2015, 5, 23291 RSC; (j) B. L. Zhao and D. M. Du, Organocatalytic cascade Michael/Michael reaction for the asymmetric synthesis of spirooxindoles containing five contiguous stereocenters, Chem. Commun., 2016, 52, 6162 RSC; (k) Y. Du, J. Li, K. Chen, C. Wu, Y. Zhou and H. Liu, Construction of highly enantioenriched spirocyclopetaneoxindoles containing four consecutive stereocenters via thiourea-catalyzed asymmetric Michael-Henry cascade reactions, Beilstein J. Org. Chem., 2017, 13, 1342 CrossRef CAS; (l) R.-Y. Yang, J. Sun, G. Jin and C.-G. Yan, Synthesis of functionalized dispiro[indoline-3,1′-cyclopentane-3′,3′′-indolines] via cyclodimerization of 3-phenacylideneoxindolines with benzoylhydrazides and arylhydrazines, Mol. Diversity, 2018, 22, 21 CrossRef CAS; (m) B. L. Zhao, Y. Lin and D. M. Du, Enantioselective Construction of Bispirooxindoles via Squaramide-Catalysed Cascade Michael/Cyclization Reaction, Adv. Synth. Catal., 2019, 361, 3387 CrossRef CAS; (n) Y. Lin, B.-L. Zhao and D.-M. Du, Bifunctional Squaramide-Catalyzed Asymmetric [3 + 2] Cyclization of 2-(1-Methyl-2-oxoindolin-3-yl)malonitriles with Unsaturated Pyrazolones To Construct Spirooxindole-Fused Spiropyrazolones, J. Org. Chem., 2019, 84, 10209 CrossRef CAS; (o) S. Xu, X. Liu, X. Zuo, G. Zhou, Y. Gong, X. Liu and Y. Zhou, Oxindole-chromones C3 Synthons Directed Stereocontrolled Construction of Five Contiguous Stereocenters on Spiro[tetrahydrocyclopenta[b]chromanone-oxindole]s, Adv. Synth. Catal., 2019, 361, 5328 CrossRef CAS; (p) S. S. Vagh, P. Karanam, C. Liao, T. Lin, Y. Liou, A. Edukondalu, Y. Chen and W. Lin, Enantioselective Construction of Spirooxindole-Fused Cyclopenta[c]chromen-4-ones Bearing Five Contiguous Stereocenters via a Stepwise (3 + 2) Cycloaddition, Adv. Synth. Catal., 2020, 362, 1679 CrossRef CAS.
- (a) A. Noole, K. Ilmarinen, I. Järving, M. Lopp and T. Kanger, Asymmetric Synthesis of Congested Spiro-cyclopentaneoxindoles via an Organocatalytic Cascade Reaction, J. Org. Chem., 2013, 78, 8117 CrossRef CAS. Also see for 5- and 6-membered rings: (b) M. Monari, E. Montroni, A. Nitti, M. Lombardo, C. Trombini and A. Quintavalla, Highly Stereoselective [4 + 2] and [3 + 2] Spiroannulations of 2-(2-Oxoindolin-3-ylidene)acetic Esters Catalyzed by Bifunctional Thioureas, Chem. – Eur. J., 2015, 21, 11038 CrossRef CAS.
- L. Deiana, Y. Jiang, C. Palo-Nieto, S. Afewerki, C. A. Incerti-Pradillos, O. Verho, C. W. Tai, E. V. Johnston and A. Cõrdova, Combined Heterogeneous Metal/Chiral Amine: Multiple Relay Catalysis for Versatile Eco-Friendly Synthesis, Angew. Chem., Int. Ed., 2014, 53, 3447 CrossRef CAS.
- (a) F. Shi, H. H. Zhang, X. X. Sun, J. Liang, T. Fan and S. J. Tu, Organocatalytic Asymmetric Cascade Reactions of 7-Vinylindoles: Diastereo- and Enantioselective Synthesis of C7-Functionalized Indoles, Chem. – Eur. J., 2015, 21, 3465 CrossRef CAS; (b) T. Fan, H. H. Zhang, C. Li, Y. Shen and F. Shi, The Application of N-Protected 3-Vinylindoles in Chiral Phosphoric Acid-Catalyzed [3 + 2] Cyclization with 3-Indolylmethanols: Monoactivation of the Catalyst to Vinyliminium, Adv. Synth. Catal., 2016, 358, 2017 CrossRef CAS.
- J. Q. Zhang, N. K. Li, S. J. Yin, B. B. Sun, W. T. Fan and X. W. Wang, Chiral N-Heterocyclic Carbene-Catalyzed Asymmetric Michael-Intramolecular Aldol-Lactonization Cascade for Enantioselective Construction of β-Propiolactone-Fused Spiro[cyclopentane-oxindoles], Adv. Synth. Catal., 2017, 359, 1541 CrossRef CAS.
- For earlier work using NHC catalysis see: A. Patra, A. Bhunia, S. R. Yetra, R. G. Gonnade and A. T. Biju, Diastereoselective synthesis of cyclopentanone-fused spirooxindoles by N-heterocyclic carbene-catalyzed homoenolate annulation with isatildenes, Org. Chem. Front., 2015, 2, 1584 RSC.
- L. Wang, S. Li, M. Blümel, R. Puttreddy, A. Peuronen, K. Rissanen and D. Enders, Switchable Access to Different Spirocyclopentane Oxindoles by N-Heterocyclic Carbene Catalyzed Reactions of Isatin-Derived Enals and N-Sulfonyl Ketimines, Angew. Chem., Int. Ed., 2017, 56, 8516 CrossRef CAS.
- J. Zhang, D. Cao, H. Wang, C. Zheng, G. Zhao and Y. Shang, Enantioselective Construction of Spirocyclic Oxindoles via Tandem Michael/Michael Reactions Catalyzed by Multifunctional Quaternary Phosphonium Salt, J. Org. Chem., 2016, 81, 10558 CrossRef CAS.
- Q. Jin, C. Zheng, G. Zhao and G. Zou, Bifunctional Quaternary Ammonium Salts Catalyzed Stereoselective Conjugate Addition of Oxindoles to Electron-Deficient β-Haloalkanes, J. Org. Chem., 2017, 82, 4840 CrossRef CAS.
- (a) G. M. Dubowchik, C. M. Conway and A. W. Xin, Blocking the CGRP Pathway for Acute and Preventive Treatment of Migraine: The Evolution of Success, J. Med. Chem., 2020, 63, 6600 CrossRef CAS; (b) B. M. Crowley, C. A. Stump, D. N. Nguyen, C. M. Potteiger, M. A. McWherter, D. V. Paone, A. G. Quigley, J. G. Bruno, D. Cui and J. C. Culberson, et al., Novel oxazolidinone calcitonin gene-related (CGRP) receptor antagonists for the acute treatment of migraine, Bioorg. Med. Chem. Lett., 2015, 25, 4777 CrossRef CAS.
- B. Xiang, K. M. Belyk, R. A. Reamer and N. Yasuda, Discovery and Application of Doubly Quaternized Cinchona-Alkaloid-Based Phase-Transfer Catalysts, Angew. Chem., Int. Ed., 2014, 53, 8375 CrossRef CAS.
- C. Q. He, A. Simon, Y.-H. Lam, A. P. J. Brunskill, N. Yasuda, J. Tan, A. M. Hyde, E. C. Sherer and K. N. Houk, Model for the Enantioselectivity of Asymmetric Intramolecular Alkylations by Bis-Quaternized Cinchona Alkaloid-Derived Catalysts, J. Org. Chem., 2017, 82, 8645 CrossRef CAS.
- J. A. Daponte, Y. Guo, R. T. Ruck and J. E. Hein, Using an Automated Monitoring Platform for Investigations of Biphasic Reactions, ACS Catal., 2019, 9, 11484 CrossRef CAS.
- X. Zhao, X. Liu, Q. Xiong, H. Mei, B. Ma, L. Lin and X. Feng, The asymmetric synthesis of polycyclic 3-spirooxindole alkaloids via the cascade reaction of 2-isocyanoethylindoles, Chem. Commun., 2015, 51, 16076 RSC.
- A. A. Cobo, B. M. Armstrong, J. C. Fettinger and A. K. Franz, Catalytic Asymmetric Synthesis of Cyclopentene-spirooxindoles Bearing Vinylsilanes Capable of Further Transformations, Org. Lett., 2019, 21, 8196 CrossRef CAS.
- (a) J. A. Xiao, X. L. Cheng, Y. C. Li, Y. M. He, J. L. Li, Z. P. Liu, P. J. Xia, W. Su and H. Yang, Palladium-catalysed ring-opening [3 + 2]-annulation of spirovinylcyclopropyl oxindole to diastereoselectively access spirooxindoles, Org. Biomol. Chem., 2019, 17, 103 RSC. For metal-free approach using cyclopropanes see: (b) D. Pan, C. Mou, N. Zan, Y. Lv, B. A. Song, Y. R. Chi and Z. Jin, NaOH-Promoted Chemoselective Cascade Cyclization of Cyclopropyl Esters with Unsaturated Imines: Access to Bioactive Cyclopenta[c]pyridine derivatives, Org. Lett., 2019, 21, 6624 CrossRef CAS. For Mg catalysed approach: (c) K. Singh, S. Pramanik, T. A. Hamlin, B. Mondal, D. Das and J. Saha, Lewis acid catalyzed annulation of spirocyclic donor-acceptor cyclopropanes with exo-heterocyclic oleifns: access to highly functionalized bis-spirocyclopentane oxindole frameworks, Chem. Commun., 2019, 55, 7069 RSC.
- M. Meazza and R. Rios, Synergistic Catalysis: Enantioselective Ring Expansion of Vinyl Cyclopropanes Combining Four Catalytic Cycles for the Synthesis of Highly Substituted Spirocyclopentanes Bearing up to Four Stereocenters, Chem. – Eur. J., 2016, 22, 9923 CrossRef CAS.
- For examples see: (a) J. N. Desrosiers, L. Hie, S. Biswas, O. V. Zatolochnaya, S. Rodriguez, H. Lee, N. Grinberg, N. Haddad, N. K. Yee and N. K. Garg, et al., Construction of Quaternary Stereocenters by Nickel-Catalyzed Heck Cyclization Reactions, Angew. Chem., Int. Ed., 2016, 55, 11921 CrossRef CAS; (b) W. Ji, Y. A. Liu and X. Liao, Transition-Metal-Free Synthesis of N-Hydroxy Oxindoles by an Aza-Nazarov-Type Reaction Involving Azaoxyallyl Cations, Angew. Chem., Int. Ed., 2016, 55, 13286 CrossRef CAS; (c) W. Kong, Q. Wang and J. Zhu, Water as a Hydride Source in Palladium-Catalyzed Enantioselective Reductive Heck Reactions, Angew. Chem., Int. Ed., 2017, 56, 3987 CrossRef CAS.
- S. Afewerki, G. Ma, I. Ibrahem, L. Liu, J. Sun and A. Córdova, Highly Enantioselective Control of Dynamic Cascade Transformations by Dual Catalysis: Asymmetric Synthesis of Polysubstituted Spirocyclic Oxindoles, ACS Catal., 2015, 5, 1266 CrossRef CAS.
- (a) B. M. Trost, D. Zell, C. Hohn, G. Mata and A. Maruniak, Enantio- and Diastereoselective Synthesis of Chiral Allenes by Palladium-Catalyzed Asymmetric [3 + 2] Cycloaddition Reactions, Angew. Chem., Int. Ed., 2018, 57, 12916 CrossRef CAS; (b) B. M. Trost, Y. Wang and C.-I. (Joey) Hung, Use of α-trifluoromethyl carbanions for palladium-catalysed asymmetric cycloadditions, Nat. Chem., 2020, 12, 294 CrossRef CAS.
- P. Drouhin, T. E. Hurst, A. C. Whitwood and R. J. K. Taylor, Copper-Mediated Construction of Spirocyclic Bis-oxindoles via a Double C-H, Ar-H Coupling Process, Org. Lett., 2014, 16, 4900 CrossRef CAS.
- A. Wetzel, J. Bergman, P. Brandt, M. Larhed and J. Branalt, Regio- and Stereoselective Synthesis of Functionalized Cyclopentene Derivatives via Mizoroki-Heck Reactions, Org. Lett., 2017, 19, 1602 CrossRef CAS.
- T. Roy, P. Brandt, A. Wetzel, J. Bergman, J. Brånalt, J. Sävmarker and M. Larhed, Selective Synthesis of Spirooxindoles by an Intramolecular Heck-Mizoroki Reaction, Org. Lett., 2017, 19, 2738 CrossRef CAS.
- (a) M. Pérez-Gómez, S. Hernández-Ponte, D. Bautista and J. A. García-López, Synthesis of spiro-oxindoles through Pd-catalyzed remote C-H alkylation using α-diazocarbonyl compounds, Chem. Commun., 2017, 53, 2842 RSC. Also see: (b) X. Liu, X. Ma, Y. Huang and Z. Gu, Pd-Catalyzed Heck-Type Cascade Reactions with N-Tosyl Hydrazones: An Efficient Way to Alkenes via in Situ Generated Alkylpalladium, Org. Lett., 2013, 15, 4814 CrossRef CAS; (c) C. Shao, Z. Wu, X. Ji, B. Zhou and Y. Zhang, An approach to spirooxindoles via palladium-catalyzed remote C-H activation and dual alkylation with CH2Br2, Chem. Commun., 2017, 53, 10429 RSC.
- (a) J. R. Frost, S. M. Huber, S. Breitenlechner, C. Bannwarth and T. Bach, Enantiotopos-Selective C-H Oxygenation Catalyzed by a Supramolecular Ruthenium Complex, Angew. Chem., Int. Ed., 2015, 54, 691 CAS; (b) B. Qiu, D. Xu, Q. Sun, J. Lin and W. Sun, Manganese-Catalyzed Asymmetric Oxidation of Methylene C-H of Spirocyclic Oxindoles and Dihydroquinolinones with Hydrogen Peroxide, Org. Lett., 2019, 21, 618 CrossRef CAS.
- (a) M. J. James, P. O'Brien, R. J. K. Taylor and W. P. Unsworth, Selective Synthesis of Six Products from a Single Indolyl α-Diazocarbonyl Precursor, Angew. Chem., Int. Ed., 2016, 55, 9671 CrossRef CAS. For a related reaction in total synthesis of spirobacillenes A and B see: (b) H. Yang, J. Feng and Y. Tang, Biomimetic total syntheses of spirobacillenes A and B, Chem. Commun., 2013, 49, 6442 RSC.
- R. Paniagua-Pérez, E. Madrigal-Bujaidar, D. Molina-Jasso, S. Reyes-Cadena, I. Álvarez-González, L. Sánchez-Chapul and J. Pérez-Gallaga, Antigenotoxic, Antioxidant and Lymphocyte Induction Effects Produced by Pteropodine, Basic Clin. Pharmacol. Toxicol., 2009, 104, 222 CrossRef.
- (a) K. Ding, Y. Lu, Z. Nikolovska-Coleska, S. Qiu, Y. Ding, W. Gao, J. Stuckey, K. Krajewski, P. P. Roller and Y. Tomita, et al., Structure-Based Design of Potent Non-Peptide MDM2 Inhibitors, J. Am. Chem. Soc., 2005, 127, 10130 CrossRef CAS; (b) Y. Zhao, S. Yu, W. Sun, L. Liu, J. Lu, D. McEachern, S. Shargary, D. Bernard, X. Li and T. Zhao, et al., A Potent Small-Molecule Inhibitor of the MDM2-p53 Interaction (MI-888) Achieved Complete and Durable Tumor Regression in Mice, J. Med. Chem., 2013, 56, 5553 CrossRef CAS; (c) Y. Zhao, L. Liu, W. Sun, J. Lu, D. McEachern, X. Li, S. Yu, D. Bernard, P. Ochsenbein and V. Ferey, et al., Diastereomeric Spirooxindoles as Highly Potent and Efficacious MDM2 Inhibitors, J. Am. Chem. Soc., 2013, 135, 7223 CrossRef CAS; (d) A. K. Gupta, M. Bharadwaj, A. Kumar and R. Mehrotra, Spiro-oxindoles as a Promising Class of Small Molecule Inhibitors of p53-MDM2 Interaction Useful in Targeted Cancer Therapy, Top. Curr. Chem., 2017, 375, 3 CrossRef.
- (a) A. Kumar, G. Gupta, A. K. Bishnoi, R. Saxena, K. S. Saini, R. Konwar, S. Kumar and A. Dwivedi, Design and synthesis of new bioisosteres of spirooxindoles (MI-63/219) as anti-breast cancer agents, Bioorg. Med. Chem., 2015, 23, 839 CrossRef CAS; (b) A. Gollner, D. Rudolph, H. Arnhof, M. Bauer, S. M. Blake, G. Boehmelt, X. L. Cockroft, G. Dahmann, P. Ettmayer and T. Gerstberger, et al., Discovery of Novel Spiro[3H-indole-3,2′-pyrrolidin]-2(1H)-one Compounds as Chemically Stable and Orally Active Inhibitors of the MDM2-p53 Interaction, J. Med. Chem., 2016, 59, 10147 CrossRef CAS; (c) A. Aguilar, J. Lu, L. Liu, D. Du, D. Bernard, D. McEachern, S. Przybranowski, X. Li, R. Luo and B. Wen, et al., Discovery of 4-((3′R,4′S,5′R,)-6′′-Chloro-4′-(3-chloro-2-fluorophenyl)-1′ethyl-2′′-oxodispiro[cyclohexane-1,2′-pyrrolidine-3′,3′′-indoline]-5′-carboxamido)bicyclo[2.2.2]octane-1-carboxylic Acid (AA-115/APG-115): A Potent and Orally Active Murine Double Minute 2 (MDM2) Inhibitor in Clinical Development, J. Med. Chem., 2017, 60, 2819 CrossRef CAS; (d) A. Barakat, M. S. Islam, H. M. Ghawas, A. M. Al-Majid, F. F. El-Senduny, F. A. Badria, Y. A. M. M. Elshaier and H. A. Ghabbour, Design and synthesis of new substituted spirooxindoles as potential inhibitors of the MDM2-p53 interaction, Bioorg. Chem., 2019, 86, 598 CrossRef CAS.
- (a) Y. Li, J. Yang, A. Aguilar, D. McEachern, S. Przybranowski, L. Liu, C. Y. Yang, M. Wang, X. Han and S. Wang, Discovery of MD-224 as a First-in-Class, Highly Potent, and Efficacious Proteolysis Targeting Chimera Murine Double Minute 2 Degrader Capable of Achieving Complete and Durable Tumor Regression, J. Med. Chem., 2019, 62, 448 CrossRef CAS; (b) R. P. Wurz and V. J. Cee, Targeted Degradation of MDM2 as a New Approach to Improve the Efficacy of MDM2-p53 Inhibitors, J. Med. Chem., 2019, 62, 445 CrossRef CAS.
- J. Yang, Y. Li, A. Aguilar, Z. Liu, C. Y. Yang and S. Wang, Simple Structural Modifications Converting a Bona fide MDM2 PROTAC Degrader into a Molecular Glue Molecule: A Cautionary Tale in the Design of PROTAC Degraders, J. Med. Chem., 2019, 62, 9471 CrossRef CAS.
- Y. Arun, G. Bhaskar, C. Balachandran, S. Ignacimuthu and P. T. Perumal, Facile one-pot synthesis of novel dispirooxindole-pyrrolidine derivatives and their antimicrobial and anticancer activity against A549 human lung adenocarcinoma cancer cell line, Bioorg. Med. Chem. Lett., 2013, 23, 1839 CrossRef CAS.
- I. V. Efremov, F. F. Vajdos, K. A. Borzilleri, S. Capetta, H. Chen, P. H. Dorff, J. K. Dutra, S. W. Goldstein, M. Mansour and A. McColl, et al., Discovery and Optimization of a Novel Spiropyrrolidine Inhibitor of β-Secretase (BACE1) through Fragment-Based Drug Design, J. Med. Chem., 2012, 55, 9069 CrossRef CAS.
- R. Murugan, S. Anbazhagan and S. Sriman Narayanan, Synthesis and in vivo antidiabetic activity of novel dispiropyrrolidines through [3 + 2] cycloaddition reactions with thiazolidinedione and rhodanine derivatives, Eur. J. Med. Chem., 2009, 44, 3272 CrossRef CAS.
- K. Hagiwara, T. Murakami, G. Xue, Y. Shimizu, E. Takeda, Y. Hashimoto, K. Honda, Y. Kondoh, H. Osada and Y. Tsunetsugu-Yokota, et al., Identification of a novel Vpr-binding compound that inhibits HIV-1 multiplication in macrophages by chemical array, Biochem. Biophys. Res. Commun., 2010, 403, 40 CrossRef CAS.
- S. V. Karthikeyan, B. D. Bala, V. P. A. Raja, S. Perumal, P. Yogeeswari and D. Sriram, A highly atom economic, chemo-, regio- and stereoselective synthesis and evaluation of spiro-pyrrolothiazoles as antitubercular agents, Bioorg. Med. Chem. Lett., 2010, 20, 350 CrossRef CAS.
- (a) C. Marti and E. M. Carreira, Construction of Spiro[pyrrolidine-3,3′-oxindoles] − Recent Applications to the Synthesis of Oxindole Alkaloids, Eur. J. Org. Chem., 2003, 2209 CrossRef CAS; (b) C. V. Galliford and K. A. Scheidt, Pyrrolidinyl-Spirooxindole Natural Products as Inspirations for the Development of Potential Therapeutic Agents, Angew. Chem., Int. Ed., 2007, 46, 8748 CrossRef CAS.
- (a) T. Mukaiyama, K. Ogata, I. Sato and Y. Hayashi, Asymmetric Organocatalyzed Michael Addition of Nitromethane to a 2-Oxoindoline-3-ylidene Acetaldehyde and the Three One-Pot Sequential Synthesis of (−)-Horsfiline and (−)-Coerulescine, Chem. – Eur. J., 2014, 20, 13583 CrossRef CAS; (b) S. De, M. K. Das, S. Bhunia and A. Bisai, Unified Approach to the Spiro(pyrrolidinyl-oxindole) and Hexahydropyrrolo[2,3-b]indole Alkaloids: Total Syntheses of Pseudophrynamines 270 and 272A, Org. Lett., 2015, 17, 5922 CrossRef CAS.
- (a) S. G. Wang, Z. L. Xia, R. Q. Xu, X. J. Liu, C. Zheng and S. L. You, Construction of Chiral Tetrahydro-β-Carbolines: Asymmetric Pictet-Spengler Reaction of Indolyl Dihydropyridines, Angew. Chem., Int. Ed., 2017, 56, 7440 CrossRef CAS; (b) V. Srinivasulu, P. Schilf, S. Ibrahim, M. A. Khanfar, S. M. Sieburth, H. Omar, A. Sebastian, R. A. AlQawasmeh, M. J. O'Connor and T. H. Al-Tel, Multidirectional desymmetrization of pluripotent building block en route to diastereoselective synthesis of complex nature-inspired scaffolds, Nat. Commun., 2018, 9, 4989 CrossRef.
- (a) G. O. Fonseca, Z. J. Wang, O. A. Namjoshi, J. R. Deschamps and J. M. Cook, First stereospecific total synthesis of (−)-affinisine oxindole as well as facile entry into the C(7)-diastereomeric chitosenine stereochemistry, Tetrahedron Lett., 2015, 56, 3052 CrossRef CAS; (b) M. R. Stephen, M. T. Rahman, V. V. N. P. B. Tiruveedhula, G. O. Fonseca, J. R. Deschamps and J. M. Cook, Concise Total Synthesis of (−)-Affinisine Oxindole, (+)-Isoalstonisine, (+)-Alstofoline, (−)-Macrogentine, (+)-Na-Demethylalstonisine, (−)-Alstonoxine A, and (+)-Alstonisine, Chem. – Eur. J., 2017, 23, 15805 CrossRef CAS.
- Q. Yu, P. Guo, J. Jian, Y. Chen and J. Xu, Nine-step total synthesis of (−)-strychnofoline, Chem. Commun., 2018, 54, 1125 RSC.
- Y. K. Xi, H. Zhang, R. X. Li, S. Y. Kang, J. Li and Y. Li, Total Synthesis of Spirotryprostatins through Organomediated Intramolecular Umpolung Cyclization, Chem. – Eur. J., 2019, 25, 3005 CrossRef CAS.
- P. Chen, H. Yang, H. Zhang, W. Chen, Z. Zhang, J. Zhang, H. Li, X. Wang, X. Xie and X. She, Total Synthesis of (−)-Gardmultimine A, Org. Lett., 2020, 5, 2022–2025 CrossRef.
- (a) M. Amat, C. Ramos, M. Pérez, E. Molins, P. Florindo, M. M. M. Santos and J. Bosch, Enantioselective formal synthesis of ent-rhynchophylline and ent-isorhynchophylline, Chem. Commun., 2013, 49, 1954 RSC. Also see: (b) M. Pérez, C. Ramos, L. Massi, S. Gazzola, C. Taglienti, N. Yayik, E. Molins, A. Viayna, F. J. Luque and J. Bosch, et al., Enantioselective Synthesis of Spiro[indolizidine-1,3′-oxindoles], Org. Lett., 2017, 19, 4050 CrossRef.
- (a) X.-H. Chen, Q. Wei, S.-W. Luo, H. Xiao and L.-Z. Gong, Organocatalytic Synthesis of Spiro[pyrrolidin-3,3′-oxindoles] with High Enantiopurity and Structural Diversity, J. Am. Chem. Soc., 2009, 131, 13819 CrossRef CAS. For 3,2′-spiropyrrolidines see: (b) F. Shi, Z. L. Tao, S. W. Luo, S. J. Tu and L. Z. Gong, Scaffold-Inspired Enantioselective Synthesis of Biologically Important Spiro[pyrrolidin-3,2′-oxindoles] with Structural Diversity through Catalytic Isatin-Derived 1,3-Dipolar Cycloadditions, Chem. – Eur. J., 2012, 18, 6885 CrossRef CAS.
- For a related chiral auxiliary approach see: (a) P. R. Sebahar and R. M. Williams, The Asymmetric Total Synthesis of (+)- and (−)-Spirotryprostatin B, J. Am. Chem. Soc., 2000, 122, 5666 CrossRef CAS; (b) T. Onishi, P. R. Sebahar and R. M. Williams, Concise, Asymmetric Total Synthesis of Spirotryprostatin A, Org. Lett., 2003, 5, 3135 CrossRef CAS.
- (a) J. Li, J. Wang, Z. Xu and S. Zhu, Combinatorial Synthesis of Functionalized Spirooxindole-Pyrrolidine/Pyrrolizidine/Pyrrolothiazole Derivatives via Three-Component 1,3-Dipolar Cycloaddition Reactions, ACS Comb. Sci., 2014, 16, 506 CrossRef CAS; (b) L. Cui, G. Zhu, S. Liu, X. Bao, X. Zhao, J. Qu and B. Wang, Construction of indolenine-substituted spiro[pyrrolidine-2,3′-oxindoles] from 2-alkenylindolenines and isatin-derived azomethine ylides, Tetrahedron, 2018, 74, 2369 CrossRef CAS; (c) J. Yue, S. Chen, X. Zuo, X. L. Liu, S. W. Xu and Y. Zhou, Diversity-oriented one-pot multicomponent synthesis of chromanone-based 3,3′-pyrrolidinyl-spirooxindoles via a 1,3-dipolar cycloaddition reaction, Tetrahedron Lett., 2019, 60, 137 CrossRef CAS; (d) M. S. Islam, H. M. Ghawas, F. F. El-Senduny, A. M. Al-Majid, Y. A. M. M. Elshaier, F. A. Badria and A. Barakat, Synthesis of new thiazolo-pyrrolidine-(spirooxindole) tethered to 3-acylindole as anticancer agents, Bioorg. Chem., 2019, 82, 423 CrossRef CAS; (e) L. Wang, X. M. Shi, W. P. Dong, L. P. Zhu and R. Wang, Efficient construction of highly functionalized spiro[γ-butyrolactone-pyrrolidin-3,3′-oxindole] tricyclic skeletons via an organocatalytic 1,3-dipolar cycloaddition, Chem. Commun., 2013, 49, 3458 RSC; (f) F. Salahi, M. J. Taghizadeh, H. Arvinnezhad, M. Moemeni, K. Jadidi and B. Notash, An efficient, one-pot, three-component procedure for the synthesis of chiral spirooxindolopyrrolizidines via catalytic highly enantioselective 1,3-dipolar cycloaddition, Tetrahedron Lett., 2014, 55, 1515 CrossRef CAS; (g) X. Meng, Y. Du, Q. Zhang, A. Yu, Y. Zhang, J. Jia and X. Liu, Direct Functionalization of Azepane via Azomethine Ylides: A Highly Efficient Synthesis of Spirooxindoles Bearing a 1-Azabicyclo[5.3.0]decane Moiety, Asian J. Org. Chem., 2017, 6, 1719 CrossRef CAS; (h) Y. Du, A. Yu, J. Jia, Y. Zhang and X. Meng, Direct N-H/α,α,β,β-C(sp3)-H functionalization of piperidine via an azomethine ylide route: synthesis of spirooxindoles bearing 3-substituted oxindoles, Chem. Commun., 2017, 53, 1684 RSC; (i) X. Zhang, M. Liu, W. Qiu, J. Evans, M. Kaur, J. P. Jasinski and W. Zhang, One-Pot Synthesis of Polycyclic Spirooxindoles via Montmorillonite K10-Catalyzed C-H Functionalization of Cyclic Amines, ACS Sustainable Chem. Eng., 2018, 6, 5574 CrossRef CAS.
- (a) Y. Huang, H. L. Fang, Y. X. Huang, J. Sun and C. G. Yan, Synthesis of 7′-Arylidenespiro[indoline-3,1′-pyrrolizines] and 7′-Arylidenespiro[indene-2,1′-pyrrolizines] via [3 + 2] Cycloaddition and β-C-H Functionalized Pyrrolidine, J. Org. Chem., 2019, 84, 12437 CrossRef CAS; (b) I. B. Kutyashev, M. V. Ulitko, A. Y. Barkov, N. S. Zimnitskiy, V. Y. Korotaev and V. Y. Sosnovskikh, A regio- and stereocontrolled approach to the synthesis of 4-CF3-substituted spiro[chromeno[3,4-c]pyrrolidine-oxindoles] via reversible [3 + 2] cycloaddition of azomethine ylides generated from isatins and sarcosine to 3-nitro-2-(trifluoromethyl)-2H-chromenes, New J. Chem., 2019, 43, 18495 RSC; (c) Z. Zhang, W. Sun, G. Zhu, J. Yang, M. Zhang, L. Hong and R. Wang, Chiral phosphoric acid catalyzed enantioselective 1,3-dipolar cycloaddition reaction of azlactones, Chem. Commun., 2016, 52, 1377 RSC; (d) W. Sun, G. Zhu, C. Wu, G. Li, L. Hong and R. Wang, Organocatalytic Diastereo- and Enantioselective 1,3-Dipolar Cycloaddition of Azlactones and Methyleneindolinones, Angew. Chem., Int. Ed., 2013, 52, 8633 CrossRef CAS; (e) A. S. Filatov, N. A. Knyazev, A. P. Molchanov, T. L. Panikorovsky, R. R. Kostikov, A. G. Larina, V. M. Boitsov and A. V. Stepakov, Synthesis of Functionalized 3-Spiro[cyclopropa[a]pyrrolizine]- and 3-Spiro[3-azabicyclo[3.1.0]hexane]oxindoles from Cyclopropenes and Azomethine Ylides via [3 + 2]-Cycloaddition, J. Org. Chem., 2017, 82, 959 CrossRef CAS; (f) X.-C. Yang, J.-Y. Liu, Z. Liu, X.-Q. Hu and P.-F. Xu, Quaternary Carbon Center Forming [3 + 2] Cyclization Reaction by Adjusting the Substituents of Substrates, J. Org. Chem., 2019, 84, 13871 CrossRef CAS.
- (a) P. Wu, H. Gao, J. Sun and C. G. Yan, Isatin hybrids and their anti-tuberculosis activity, Chin. Chem. Lett., 2017, 28, 329 CrossRef CAS; (b) Y. Huang, Y. X. Huang, J. Sun and C. G. Yan, A [3 + 2] cycloaddition reaction for the synthesis of spiro[indoline-3,3′-pyrrolidines] and evaluation of cytotoxicity towards cancer cells, New J. Chem., 2019, 43, 8903 RSC.
- C. S. Wang, R. Y. Zhu, J. Zheng, F. Shi and S. J. Tu, Enantioselective Construction of Spiro[indoline-3,2′-pyrrole] Framework via Catalytic Asymmetric 1,3-Dipolar Cycloadditions Using Allenes as Equivalents of Alkynes, J. Org. Chem., 2015, 80, 512 CrossRef CAS.
- (a) G. Zhu, B. Wang, X. Bao, H. Zhang, Q. Wei and J. Qu, Catalytic asymmetric construction of spiro[pyrrolidine-2,3′-oxindole] scaffolds through chiral phosphoric acid-catalyzed 1,3-dipolar cycloaddition involving 3-amino oxindoles, Chem. Commun., 2015, 51, 15510–15513 RSC; (b) Q. Wei, G. Zhu, H. Zhang, J. Qu and B. Wang, 1,3-Dipolar Cycloaddition of Azomethine Ylides Involving 3-Aminooxindoles: Versatile Construction of Dispiro[pyrrolidine-2,3′-oxindole] Scaffolds, Eur. J. Org. Chem., 2016, 5335 CrossRef CAS; (c) G. Zhu, S. Liu, S. Wu, L. Peng, J. Qu and B. Wang, Assembly of Indolenines, 3-Amino Oxindoles, and Aldehydes into Indolenine-Substituted Spiro[pyrrolidin-2,3′-oxindoles] via 1,3-Dipolar Cycloaddition with Divergent Diastereoselectivities, J. Org. Chem., 2017, 82, 4317 CrossRef CAS; (d) G. Zhu, Q. Wei, H. Chen, Y. Zhang, W. Shen, J. Qu and B. Wang, Asymmetric [3 + 2] Cycloaddition of 3-Amino Oxindole-Based Azomethine Ylides and α,β-Enones with Divergent Diastereocontrol on the Spiro[pyrrolidine-oxindoles], Org. Lett., 2017, 19, 1862 CrossRef CAS; (e) G. Zhu, S. Wu, X. Bao, L. Cui, Y. Zhang, J. Qu, H. Chen and B. Wang, Asymmetric [3 + 2] cycloaddition of 3-amino oxindole-based azomethine ylides with α,β-ynones: a straightforward approach to spirooxindoles incorporating 2,5-dihydropyrroles and pyrroles, Chem. Commun., 2017, 53, 4714 RSC; (f) J. He, R.-G. Sun, L. Fan, S.-Y. Tian, T.-P. Huang, Z. Chen and L. Chen, 4-(N,N-Dimethylamino)pyridine (DMAP)-Catalyzed 1,3-Dipolar Cycloaddition of 3-Aminooxindole-Based Azomethine Ylides with α,β-Unsaturated Acyl Phosphonates for the Construction of Spiropyrrolidinyl-2,3′-oxindoles, Synthesis, 2019, 51, 1353 CrossRef CAS; (g) T. Huang, L. Liu, Q. Wang, M. Wu and D. Kong, 1,3-Dipolar Cycloaddition of 3-Amino Oxindole-Based Azomethine Ylides and O-Vinylphosphonylated Salicylaldehydes for Diastereoselective Synthesis of Oxindole Spiro-P,N-polycyclic Heterocycles, Synthesis, 2020, 52, 1387 CrossRef CAS.
- (a) J. Sun, G. L. Shen, Y. Huang and C. G. Yan, Formation of diverse polycyclic spirooxindoles via three-component reaction of isoquinolinium salts, isatins and malononitrile, Sci. Rep., 2017, 7, 41024 CrossRef; (b) Y. Huang, W. Min, Q. W. Wu, J. Sun, D. H. Shi and C. G. Yan, Facile one-pot synthesis of spirooxindole-pyrrolidine derivatives and their antimicrobial and acetylcholinesterase inhibitory activities, New J. Chem., 2018, 42, 16211 RSC.
- (a) X. Wang, P. Yang, Y. Zhang, C. Z. Tang, F. Tian, L. Peng and L. X. Wang, Isatin, N,N′-Cyclic Azomethine Imine 1,3-Dipole and Abnormal [3 + 2]-Cycloaddition with Maleimide in the Presence of 1,4-Diazabicyclo[2.2.2]octane, Org. Lett., 2017, 19, 646 CrossRef CAS; (b) X.-J. Song, H.-X. Ren, M. Xiang, C.-Y. Li, F. Tian and L.-X. Wang, Base Catalyzed Abnormal [3 + 2]-Cycloaddition between Isatin N,N′-Cyclic Azomethine Imine 1,3-Dipole and 3-Methyleneoxindole for the One-Step Construction of Tetracyclic Bispirooxindoles, J. Org. Chem., 2020, 85, 3921 CrossRef CAS.
- J. Day, M. Uroos, R. A. Castledine, W. Lewis, B. McKeever-Abbas and J. Dowden, Alkaloid inspired spirocyclic oxindoles from 1,3-dipolar cycloaddition of pyridinium ylides, Org. Biomol. Chem., 2013, 11, 6502 RSC.
- (a) J. A. Xiao, H. G. Zhang, S. Liang, J. W. Ren, H. Yang and X. Q. Chen, Synthesis of Pyrrolo(spiro-[2.3′]-oxindole)-spiro-[4.3′′]-oxindole via 1,3-Dipolar Cycloaddition of Azomethine Ylides with 3-Acetonylideneoxindole, J. Org. Chem., 2013, 78, 11577 CrossRef CAS; (b) Y. L. Qian, B. Li, P. J. Xia, J. Wang, H. Y. Xiang and H. Yang, Diastereospecific entry to pyrrolidinyldispirooxindole skeletons via three-component 1,3-dipolar cycloadditions, Tetrahedron, 2018, 74, 6821 CrossRef CAS; (c) T. T. Feng, Y. Gong, Q. Di Wei, G. L. Wang, H. H. Liu, M. Y. Tian, X. L. Liu, Z. Y. Chen and Y. Zhou, Diversity-oriented Construction of Chromanone-fused Polycyclic Pyrrolidinyl-dispirooxindoles, J. Heterocycl. Chem., 2018, 55, 1136 CrossRef CAS; (d) Y. Huang, Y. X. Huang, J. Sun and C. G. Yan, Diastereoselective synthesis of dispirooxindoles via, [3 + 2] cycloaddition of azomethine ylides to 3-phenacylideneoxindoles and evaluation of their cytotoxicity, RSC Adv., 2018, 8, 23990 RSC; (e) J. Guo, Y. Zhao, D. Fang, Q. Wang and Z. Bu, Diastereoselective construction of pyrrolo[2,1-a]isoquinoline-based bispirooxindoles through a three-component [3 + 2] cycloaddition, Org. Biomol. Chem., 2018, 16, 6025 RSC; (f) S. Bhandari, S. Sana, B. Sridhar and N. Shankaraiah, Microwave-Assisted One-Pot [3 + 2] Cycloaddition of Azomethine Ylides and 3-Alkenyl Oxindoles: A Facile Approach to Pyrrolidine-Fused Bis-Spirooxindoles, ChemistrySelect, 2019, 4, 1727 CrossRef CAS; (g) W. Dai, X. L. Jiang, Q. Wu, F. Shi and S. J. Tu, Diastereo- and Enantioselective Construction of 3,3′-Pyrrolidinyldispirooxindole Framework via Catalytic Asymmetric 1,3-Dipolar Cycloadditions, J. Org. Chem., 2015, 80, 5737 CrossRef CAS; (h) K. Suman, L. Srinu and S. Thennarasu, Lewis Acid Catalyzed Unprecedented [3 + 2] Cycloaddition Yields 3,3′-Pyrrolidinyldispirooxindoles Containing Four Contiguous Chiral Stereocenters with Two Contiguous Quaternary Spirostereocenters, Org. Lett., 2014, 16, 3732 CrossRef CAS; (i) D. Shukla and S. A. Babu, Pd-Catalyzed Diastereoselective Intramolecular Amide α-C−H Arylation in Sterically Hindered Monospirooxindole Motifs, Adv. Synth. Catal., 2019, 361, 2075 CrossRef CAS; (j) N. Shahrestani, K. Tovfighmadar, M. Eskandari, K. Jadidi, B. Notash and P. Mirzaei, Synthesis of Highly Enantioenriched Bis-spirooxindole Pyrrolizidine/Pyrrolidines through Asymmetric [3 + 2] Cycloaddition Reaction, Asian J. Org. Chem., 2020, 9, 822 CrossRef CAS.
- (a) T. Arai, H. Ogawa, A. Awata, M. Sato, M. Watabe and M. Yamanaka, PyBidine-Cu(OTf)2-Catalyzed Asymmetric [3 + 2] Cycloaddition with Imino Esters: Harmony of Cu-Lewis Acid and Imidazolidine-NH Hydrogen Bonding in Concerto Catalysis, Angew. Chem., Int. Ed., 2015, 54, 1595 CrossRef CAS; (b) W. L. Yang, Y. Z. Liu, S. Luo, X. Yu, J. S. Fossey and W. P. Deng, The copper-catalyzed asymmetric construction of a dispiropyrrolidine skeleton via 1,3-dipolar cycloaddition of azomethine ylides to α-alkylidene succinimides, Chem. Commun., 2015, 51, 9212 RSC.
- L. Shu, Z. Li, C. Gu and D. Fishlock, Synthesis of a Spiroindolinone Pyrrolidinecarboxamide MDM2 Antagonist, Org. Process Res. Dev., 2013, 17, 247 CrossRef CAS.
- J. P. Macdonald, B. H. Shupe, J. D. Schreiber and A. K. Franz, Counterion effects in the catalytic stereoselective synthesis of 2,3′-pyrrolidinyl spirooxindoles, Chem. Commun., 2014, 50, 5242 RSC.
- M. Ma, Y. Zhu, Q. Sun, X. Li, J. Su, L. Zhao, Y. Zhao, S. Qiu, W. Yan and K. Wang, et al., The asymmetric synthesis of CF3-containing spiro[pyrrolidin-3,2′-oxindole] through the organocatalytic 1,3-dipolar cycloaddition reaction, Chem. Commun., 2015, 51, 8789 RSC.
- Q. Sun, X. Li, J. Su, L. Zhao, M. Ma, Y. Zhu, Y. Zhao, R. Zhu, W. Yan and K. Wang, et al., The Squaramide-Catalyzed 1,3-Dipolar Cycloaddition of Nitroalkenes with N-2,2,2-Trifluoroethylisatin Ketimines: An Approach for the Synthesis of 5′-Trifluoromethyl-spiro[pyrrolidin-3,2′-oxindoles], Adv. Synth. Catal., 2015, 357, 3187 CrossRef CAS.
- (a) A. Ponce, I. Alonso, J. Adrio and J. C. Carretero, Stereoselective Ag-Catalyzed 1,3-Dipolar Cycloaddition of Activated Trifluoromethyl-Substituted Azomethine Ylides, Chem. – Eur. J., 2016, 22, 4952 CrossRef CAS; (b) H. Z. Gui, Y. N. Gao, Y. Wei and M. Shi, Highly Efficient and Diastereoselective Construction of Trifluoromethyl-Containing Spiro[pyrrolidin-3,2′-oxindole] by a Catalyst-free Mutually Activated [3 + 2] Cycloaddition Reaction, Chem. – Eur. J., 2018, 24, 10038 CrossRef CAS; (c) X. Y. Wu, Y. N. Gao and M. Shi, Phosphine-Catalyzed [3 + 2] Annulation of N-2,2,2-Trifluoroethylisatin Ketimines with γ-Substituted Allenoates: Synthesis of Spiro[indoline-3,2′-pyrrole], Eur. J. Org. Chem., 2019, 1620 CrossRef CAS.
- (a) Z. H. Wang, Z. J. Wu, D. F. Yue, W. F. Hu, X. M. Zhang, X. Y. Xu and W. C. Yuan, Organocatalytic asymmetric [3 + 2] cycloaddition of N-2,2,2-trifluoroethylisatin ketimines with 3-alkenyl-5-arylfuran-2(3H)-ones, Chem. Commun., 2016, 52, 11708 RSC; (b) C. Wang, D. Wen, H. Chen, Y. Deng, X. Liu, X. Liu, L. Wang, F. Gao, Y. Guo and M. Sun, et al., The catalytic asymmetric synthesis of CF3-containing spiro-oxindole-pyrrolidine-pyrazolone compounds through squaramide-catalyzed 1,3-dipolar cycloaddition, Org. Biomol. Chem., 2019, 17, 5514 RSC; (c) C. Zou, Y. Han, C. Zeng, T. Y. Zhang, J. Ye and G. Song, Remote regioselective organocatalytic asymmetric [3 + 2] cycloaddition of N-2,2,2-trifluoroethyl isatin ketimines with cyclic 2,4-dienones, Chin. Chem. Lett., 2020, 31, 377 CrossRef CAS; (d) Y. Yi, Y. Hua, H. Lu, L. Liu and M. Wang, Brønsted Base and Lewis Acid Cooperatively Catalyzed Asymmetric exo′-Selective [3 + 2] Cycloaddition of Trifluoromethylated Azomethine Ylides and Methyleneindolinones, Org. Lett., 2020, 22, 2527 CrossRef CAS; (e) B. Li, F. Gao, X. Feng, M. Sun, Y. Guo, D. Wen, Y. Deng, J. Huang, K. Wang and W. Yan, Highly efficient enantioselective synthesis of bispiro[benzofuran-oxindole-pyrrolidine]s through organocatalytic cycloaddition, Org. Chem. Front., 2019, 6, 1567 RSC; (f) X. Liu, D. Lu, J.-H. Wu, J.-P. Tan, C. Jiang, G. Gao and T. Wang, Stereoselective Synthesis of CF3-Containing Spirooxindoles via 1,3-Dipolar Cycloaddition by Dipeptide-Based Phosphonium Salt Catalysis, Adv. Synth. Catal., 2020, 362, 1490 CrossRef CAS; (g) Y. Zhi, K. Zhao, C. Von Essen, K. Rissanen and D. Enders, Thiourea-Catalyzed Domino Michael-Mannich [3 + 2] Cycloadditions: A Strategy for the Asymmetric Synthesis of 3,3′-Pyrrolidinyl-dispirooxindoles, Synlett, 2017, 28, 2876 CrossRef CAS; (h) T. L. An and D. M. Du, Chiral Squaramide Catalyzed Asymmetric [3 + 2] Cycloaddition Reaction for Synthesis of Trifluoromethylated Barbituric Acid Derivatives, ChemistrySelect, 2019, 4, 11302 CrossRef CAS; (i) F.-Y. Chen, L. Xiang, G. Zhan, H. Liu, B. Kang, S.-C. Zhang, C. Peng and B. Han, Highly stereoselective organocatalytic synthesis of pyrrolidinyl spirooxindoles containing halogenated contiguous quaternary carbon stereocenters, Tetrahedron Lett., 2020, 61, 151806 CrossRef CAS.
- J. X. Zhang, H. Y. Wang, Q. W. Jin, C. W. Zheng, G. Zhao and Y. J. Shang, Thiourea-Quaternary Ammonium Salt Catalyzed Asymmetric 1,3-Dipolar Cycloaddition of Imino Esters To Construct Spiro[pyrrolidin-3,3′-oxindoles], Org. Lett., 2016, 18, 4774 CrossRef CAS.
- J. H. Siitonen, S. Lira, M. Yousufuddin and L. Kürti, Total synthesis of isatindigotindoline C, Org. Biomol. Chem., 2020, 18, 2051 RSC.
- P. B. Alper, C. Meyers, A. Lerchner, D. R. Siegel and E. M. Carreira, Facile, Novel Methodology for the Synthesis of Spiro[pyrrolidin-3,3′-oxindoles]: Catalyzed Ring Expansion Reactions of Cyclopropanes by Aldimines, Angew. Chem., Int. Ed., 1999, 38, 3186 CrossRef CAS.
- (a) A. Lerchner and E. M. Carreira, First Total Synthesis of (±)-Strychnofoline via a Highly Selective Ring-Expansion Reaction, J. Am. Chem. Soc., 2002, 124, 14826 CrossRef CAS; (b) A. Lerchner and E. M. Carreira, Synthesis of (±)-Strychnofoline via a Highly Convergent Selective Annulation Reaction, Chem. – Eur. J., 2006, 12, 8208 CrossRef CAS; (c) C. Meyers and E. M. Carreira, Total Synthesis of (−)-Spirotryprostatin B, Angew. Chem., Int. Ed., 2003, 42, 694 CrossRef CAS; (d) C. Marti and E. M. Carreira, Total Synthesis of (−)-Spirotryprostatin B: Synthesis and Related Studies, J. Am. Chem. Soc., 2005, 127, 11505–11515 CrossRef CAS; (e) C. P. Seath, J. W. B. Fyfe, J. J. Molloy and A. J. B. Watson, Synthesis of Oxindoles and Benzofuranones via Oxidation of 2-Heterocyclic BMIDAs, Synthesis, 2017, 49, 891 CAS.
- (a) V. Helan, A. Mills, D. Drewry and D. Grant, A Rapid Three-Component MgI2-Mediated Synthesis of 3,3-Pyrollidinyl Spirooxindoles, J. Org. Chem., 2010, 75, 6693 CrossRef CAS; (b) E. M. Buev, V. S. Moshkin and V. Y. Sosnovskikh, Reactivity of spiroanthraceneoxazolidines with cyclopropanes: An approach to the oxindole alkaloid scaffold, Tetrahedron Lett., 2018, 59, 3409 CrossRef CAS.
- Z. Zhang, W. Zhang, F. Kang, F. C. F. Ip, N. Y. Ip and R. Tong, Asymmetric Total Syntheses of Rhynchophylline and Isorhynchophylline, J. Org. Chem., 2019, 84, 11359 CrossRef CAS.
- A. A. Akaev, E. V. Villemson, N. S. Vorobyeva, A. G. Majouga, E. M. Budynina and M. Y. Melnikov, 3-(2-Azidoethyl)oxindoles: Advanced Building Blocks for One-Pot Assembly of Spiro[pyrrolidine-3,3′-oxindoles], J. Org. Chem., 2017, 82, 5689 CrossRef CAS.
- S. Hajra, S. K. Abu Saleh, A. Hazra and M. S. Singh, Organocatalytic Domino Reaction of Spiroaziridine Oxindoles and Malononitrile for the Enantiopure Synthesis of Spiro[dihydropyrrole-3,3′-oxindoles], J. Org. Chem., 2019, 84, 8194 CrossRef CAS.
- T. R. Li, B. Y. Cheng, S. Q. Fan, Y. N. Wang, L. Q. Lu and W. J. Xiao, Highly Stereoselective [3 + 2] Cycloadditions of Chiral Palladium-Containing N1–1,3-Dipoles: A Divergent Approach to Enantioenriched Spirooxindoles, Chem. – Eur. J., 2016, 22, 6243 CrossRef CAS.
- S. Hajra, S. S. Bhosale, A. Hazra and N. Kanaujia, The one pot asymmetric synthesis of 3,3′-pyrrolidonyl spiroxindoles via a regio- and stereoselective domino reaction, Org. Biomol. Chem., 2019, 17, 8140 RSC.
- A. A. Akaev, S. I. Bezzubov, V. G. Desyatkin, N. S. Vorobyeva, A. G. Majouga, M. Y. Melnikov and E. M. Budynina, Stereocontrolled [3 + 2] Cycloaddition of Donor-Acceptor Cyclopropanes to Iminooxindoles: Access to Spiro[oxindole-3,2′-pyrrolidines], J. Org. Chem., 2019, 84, 3340 CrossRef CAS.
- X. B. Huang, X. J. Li, T. T. Li, B. Chen, W. D. Chu, L. He and Q. Z. Liu, Palladium-Catalyzed Highly Enantioselective Cycloaddition of Vinyl Cyclopropanes with Imines, Org. Lett., 2019, 21, 1713 CrossRef CAS.
- P. Yuvaraj and B. S. R. Reddy, Synthesis of 3-spiropyrrolidine-3-spirooxindoles from Baylis-Hillman adducts of chromone with azomethine ylides via [3 + 2] cycloaddition reaction, Tetrahedron Lett., 2013, 54, 821 CrossRef CAS.
- Q. He, W. Du and Y. C. Chen, Asymmetric [3 + 2] Annulations to Construct 1,2-Bispirooxindoles Incorporating a Dihydropyrrolidine Motif, Adv. Synth. Catal., 2017, 359, 3782 CrossRef CAS.
- X. Tang, Y. J. Gao, H. Q. Deng, J. J. Lei, S. W. Liu, L. Zhou, Y. Shi, H. Liang, J. Qiao and L. Guo, et al., Catalyst-free [3 + 2] cyclization of dihydroisoquinoline imines and isatin-derived Morita-Baylis-Hillman carbonates via 1,5-electrocyclization: synthesis of tetrahydroisoquinoline-fused spirooxindoles, Org. Biomol. Chem., 2018, 16, 3362 RSC.
- D. Jiang, S. Dong, W. Tang, T. Lu and D. Du, N-Heterocyclic, Carbene-Catalyzed Formal [3 + 2] Annulation of α-Bromoenals with 3-Aminooxindoles: A Stereoselective Synthesis of Spirooxindole γ-Butyrolactams, J. Org. Chem., 2015, 80, 11593 CrossRef CAS.
- K.-Q. Chen, Y. Li, C.-L. Zhang, D.-Q. Sun and S. Ye, N-Heterocyclic, carbene-catalyzed [3 + 2] annulation of bromoenals with 3-aminooxindoles: highly enantioselective synthesis of spirocyclic oxindolo-γ-lactams, Org. Biomol. Chem., 2016, 14, 2007 RSC.
- (a) C. Wang, S. Zhu, G. Wang, Z. Li and X. P. Hui, Enantioselective Synthesis of Spiro[indoline-3,2′-pyrroles] through N-Heterocyclic-Carbene-Catalyzed Formal [3 + 2] Annulation, Eur. J. Org. Chem., 2016, 5653 CrossRef CAS. For detailed mechanistic study see: (b) Y. Li, Z. Zhang and C. Liang, Understanding the mechanism and stereoselectivity of NHC-catalyzed [3 + 2] cycloaddition of 3-bromoenals and isatin N-Boc ketimines, Org. Biomol. Chem., 2018, 16, 9251 RSC.
- X. Y. Chen, J. W. Xiong, Q. Liu, S. Li, H. Sheng, C. von Essen, K. Rissanen and D. Enders, Control of N-Heterocyclic Carbene Catalyzed Reactions of Enals: Asymmetric Synthesis of Oxindole-γ-Amino Acid Derivatives, Angew. Chem., Int. Ed., 2018, 57, 300 CrossRef CAS.
- For a review see: W. Y. Han, J. Q. Zhao, J. Zuo, X. Y. Xu, X. M. Zhang and W. C. Yuan, Recent Advances of α-Isothiocyanato Compounds in the Catalytic Asymmetric Reaction, Adv. Synth. Catal., 2015, 357, 3007 CrossRef CAS.
- Y. M. Cao, F. F. Shen, F. T. Zhang and R. Wang, Catalytic Asymmetric Michael Addition/Cyclization of Isothiocyanato Oxindoles: Highly Efficient and Versatile Approach for the Synthesis of 3,2′-Pyrrolidinyl Mono- and Bi-spirooxindole Frameworks, Chem. – Eur. J., 2013, 19, 1184 CrossRef CAS.
- (a) Q. Chen, J. Liang, S. Wang, D. Wang and R. Wang, An asymmetric approach toward chiral multicyclic spirooxindoles from isothiocyanato oxindoles and unsaturated pyrazolones by a chiral tertiary amine thiourea catalyst, Chem. Commun., 2013, 49, 1657 RSC; (b) In 2014, Yuan showed an example of the same reaction catalysed by quinine: B. D. Cui, S. W. Li, J. Zuo, Z. J. Wu, X. M. Zhang and W. C. Yuan, Quinine-catalyzed asymmetric domino Michael-cyclization reaction for the synthesis of spirocyclic oxindoles bearing two spiro quaternary centers and three consecutive stereocenters, Tetrahedron, 2014, 70, 1895 CrossRef CAS.
- (a) W. Y. Han, S. W. Li, Z. J. Wu, X. M. Zhang and W. C. Yuan, 3-Isothiocyanato Oxindoles Serving as Powerful and Versatile Precursors to Structurally Diverse Dispirocyclic Thiopyrrolidineoxindoles through a Cascade Michael/Cyclization Process with Amino-Thiocarbamate Catalysts, Chem. – Eur. J., 2013, 19, 5551 CrossRef CAS; (b) X. L. Liu, W. Y. Han, X. M. Zhang and W. C. Yuan, Highly Efficient and Stereocontrolled Construction of 3,3′-Pyrrolidonyl Spirooxindoles via Organocatalytic Domino Michael/Cyclization Reaction, Org. Lett., 2013, 15, 1246 CrossRef CAS.
- (a) Z. K. Fu, J. Y. Pan, D. C. Xu and J. W. Xie, Organocatalytic domino Michael/cyclization reaction: efficient synthesis of multi-functionalized tetracyclic spirooxindoles with multiple stereocenters, RSC Adv., 2014, 4, 51548 RSC; (b) F. Tan, L. Q. Lu, Q. Q. Yang, W. Guo, Q. Bian, J. R. Chen and W. J. Xiao, Enantioselective Cascade Michael Addition/Cyclization Reactions of 3-Nitro-2H-Chromenes with 3-Isothiocyanato Oxindoles: Efficient Synthesis of Functionalized Polycyclic Spirooxindoles, Chem. – Eur. J., 2014, 20, 3415 CrossRef CAS; (c) S. Kayal and S. Mukherjee, Catalytic Asymmetric Michael Addition/Cyclization Cascade Reaction of 3-Isothiocyanatooxindoles with Nitro Olefins, Eur. J. Org. Chem., 2014, 6696 CrossRef CAS; (d) J. Q. Zhao, M. Q. Zhou, Z. J. Wu, Z. H. Wang, D. F. Yue, X. Y. Xu, X. M. Zhang and W. C. Yuan, Asymmetric Michael/Cyclization Cascade Reaction of 3-Isothiocyanato Oxindoles and 3-Nitroindoles with Amino-Thiocarbamate Catalysts: Enantioselective Synthesis of Polycyclic Spirooxindoles, Org. Lett., 2015, 17, 2238 CrossRef CAS; (e) J. Q. Zhao, Z. J. Wu, M. Q. Zhou, X. Y. Xu, X. M. Zhang and W. C. Yuan, Zn-Catalyzed Diastereo- and Enantioselective Cascade Reaction of 3-Isothiocyanato Oxindoles and 3-Nitroindoles: Stereocontrolled Syntheses of Polycyclic Spirooxindoles, Org. Lett., 2015, 17, 5020 CrossRef CAS; (f) L. Wang, D. Yang, D. Li, X. Liu, Q. Zhao, R. Zhu, B. Zhang and R. Wang, Catalytic Asymmetric [3 + 2] Cyclization Reactions of 3-Isothiocyanato Oxindoles and Alkynyl Ketones Via an in Situ Generated Magnesium Catalyst, Org. Lett., 2015, 17, 4260 CrossRef CAS; (g) Y. Lin, L. Liu and D. M. Du, Squaramide-catalyzed asymmetric Michael/cyclization cascade reaction of 3-isothiocyanato oxindoles with chalcones for synthesis of pyrrolidinyl spirooxindoles, Org. Chem. Front., 2017, 4, 1229 RSC; (h) J. Q. Zhao, X. J. Zhou, Y. Zhou, X. Y. Xu, X. M. Zhang and W. C. Yuan, Diastereo- and Enantioselective Dearomative [3 + 2] Cycloaddition Reaction of 2-Nitrobenzofurans with 3-Isothiocyanato Oxindoles, Org. Lett., 2018, 20, 909 CrossRef CAS; (i) L. L. Zhang, B. C. Da, S. H. Xiang, S. Zhu, Z. Y. Yuan, Z. Guo and B. Tan, Organocatalytic double arylation of 3-isothiocyanato oxindoles: Stereocontrolled synthesis of complex spirooxindoles, Tetrahedron, 2019, 75, 1689 CrossRef CAS; (j) H. Gui, X. Wu, Y. Wei and M. Shi, A Formal Condensation and [4 + 1] Annulation Reaction of 3-Isothiocyanato Oxindoles with Aza-o-Quinone Methides, Adv. Synth. Catal., 2019, 361, 5466 CrossRef CAS; (k) M. Bai, Y. Z. Chen, B. D. Cui, X. Y. Xu and W. C. Yuan, Thiourea-catalyzed asymmetric domino Michael-cyclization reaction of 3-isothiocyanato oxindoles with β,γ-unsaturated α-keto esters for the synthesis of spirocyclic oxindoles, Tetrahedron, 2019, 75, 2155 CrossRef CAS; (l) L. Liu, B. L. Zhao and D. M. Du, Organocatalytic Asymmetric Michael/Cyclization Cascade Reaction of 3-Isothiocyanato Oxindoles with Maleimides for the Efficient Construction of Pyrrolidonyl Spirooxindoles, Eur. J. Org. Chem., 2016, 4711 CrossRef CAS; (m) R. Chowdhury, M. Kumar and S. K. Ghosh, Organocatalyzed enantioselective Michael addition/cyclization cascade reaction of 3-isothiocyanato oxindoles with arylidene malonates, Org. Biomol. Chem., 2016, 14, 11250 RSC; (n) H.-W. Zhao, T. Tian, B. Li, Z. Yang, H.-L. Pang, W. Meng, X.-Q. Song and X.-Q. Chen, Diastereoselective Synthesis of Dispirobarbiturates through Et3N-Catalyzed [3 + 2] Cycloaddition of Barbiturate-Based Olefins with 3-Isothiocyanato Oxindoles, J. Org. Chem., 2015, 80, 10380 CrossRef CAS; (o) S. Chen, G. L. Wang, S. W. Xu, M. Y. Tian, M. Zhang, X. L. Liu and W. C. Yuan, Regio- and stereoselective [3 + 2] cycloaddition reaction: access to isoxazole-dispirobisoxindoles featuring three contiguous stereocenters, Org. Biomol. Chem., 2019, 17, 6551 RSC.
- See ref. 212; (a) H. Wu, L. L. Zhang, Z. Q. Tian, Y. D. Huang and Y. M. Wang, Highly Efficient Enantioselective Construction of Bispirooxindoles Containing Three Stereocenters through an Organocatalytic Cascade Michael-Cyclization Reaction, Chem. – Eur. J., 2013, 19, 1747 CrossRef CAS; (b) F. Tan, H. G. Cheng, B. Feng, Y. Q. Zou, S. W. Duan, J. R. Chen and W. J. Xiao, Highly Enantioselective Organocatalytic Michael Addition/Cyclization Cascade Reaction of Ylideneoxindoles with Isothiocyanato Oxindoles: A Formal [3 + 2] Cycloaddition Approach to Optically Active Bispirooxindole Derivatives, Eur. J. Org. Chem., 2013, 2071 CrossRef CAS; (c) W. R. Zhu, Q. Chen, N. Lin, K. Bin Chen, Z. W. Zhang, G. Fang, J. Weng and G. Lu, Organocatalytic Michael/cyclization cascade reactions of 3-isothiocyanato oxindoles with 3-trifluoroethylidene oxindoles: an approach for the synthesis of 3′-trifluoromethyl substituted 3,2′-pyrrolidinyl-bispirooxindoles, Org. Chem. Front., 2018, 5, 1375 RSC.
- M. Monecke and T. Lindel, Tackling the Spiro Tetracyclic Skeleton of Cyanogramide: Incorporation of a Hydantoin Moiety, Org. Lett., 2018, 20, 7969 CrossRef CAS.
- B. D. Cui, J. Zuo, J. Q. Zhao, M. Q. Zhou, Z. J. Wu, X. M. Zhang and W. C. Yuan, Tandem Michael Addition-Ring Transformation Reactions of 3-Hydroxyoxindoles/3-Aminooxindoles with Olefinic Azlactones: Direct Access to Structurally Diverse Spirocyclic Oxindoles, J. Org. Chem., 2014, 79, 5305 CrossRef CAS.
- L. Chen, Z. J. Wu, M. L. Zhang, D. F. Yue, X. M. Zhang, X. Y. Xu and W. C. Yuan, Organocatalytic Asymmetric Michael/Cyclization Cascade Reactions of 3-Hydroxyoxindoles/3-Aminooxindoles with α,β-Unsaturated Acyl Phosphonates for the Construction of Spirocyclic Oxindole-γ-lactones/lactams, J. Org. Chem., 2015, 80, 12668 CrossRef CAS.
- For a related reaction see: B. Cui, Y. Chen, J. Shan, L. Qin, C. Yuan, Y. Wang, W. Han, N. Wan and Y. Chen, An enantioselective synthesis of spiro-oxindole-based 3,4-dihydropyrroles via a Michael/cyclization cascade of 3-aminooxindoles with 2-enoylpyridines, Org. Biomol. Chem., 2017, 15, 8518 RSC.
- P. Yang, X. Wang, F. Chen, Z. B. Zhang, C. Chen, L. Peng and L. X. Wang, Organocatalytic Enantioselective Michael/Cyclization Domino Reaction between 3-Amideoxindoles and α,β-Unsaturated Aldehydes: One-Pot Preparation of Chiral Spirocyclic Oxindole-γ-lactams, J. Org. Chem., 2017, 82, 3908 CrossRef CAS.
- X. C. Yang, M. M. Liu, F. Mathey, H. Yang, Y. Z. Hua and M. C. Wang, Access to Chiral 2,5-Pyrrolidinyl Dispirooxindoles via Dinuclear Zinc-Catalyzed Asymmetric Cascade Reactions, J. Org. Chem., 2019, 84, 7762 CrossRef CAS.
- P. F. Zheng, Q. Ouyang, S. L. Niu, L. Shuai, Y. Yuan, K. Jiang, T. Y. Liu and Y. C. Chen, Enantioselective [4 + 1] Annulation Reactions of α-Substituted Ammonium Ylides To Construct Spirocyclic Oxindoles, J. Am. Chem. Soc., 2015, 137, 9390 CrossRef CAS.
- R. Le Goff, M. Sanselme, A. M. Lawson, A. Daïch and S. Comesse, Highly Stereoselective Domino Oxa-Michael/Aza-Michael/Cyclization: Synthesis of Bicyclic Lactams and Spiroox-indole Skeleton, Eur. J. Org. Chem., 2015, 7244 CrossRef CAS.
- X. Huang, M. Liu, K. Pham, X. Zhang, W. Bin Yi, J. P. Jasinski and W. Zhang, Organocatalytic One-Pot Asymmetric Synthesis of Thiolated Spiro-γ-lactam Oxindoles Bearing Three Stereocenters, J. Org. Chem., 2016, 81, 5362 CrossRef CAS.
- H. Xu, H. W. Liu, H. S. Lin and G. W. Wang, Solvent-free iodine-promoted synthesis of 3,2′-pyrrolinyl spirooxindoles from alkylidene oxindoles and enamino esters under ball-milling conditions, Chem. Commun., 2017, 53, 12477 RSC.
- J. W. Ren, Q. L. Zhao, J. A. Xiao, P. J. Xia, H. Y. Xiang, X. Q. Chen and H. Yang, A One-Pot Ring-Opening/Ring-Closure Sequence for the Synthesis of Polycyclic Spirooxindoles, Chem. – Eur. J., 2019, 25, 4673 CrossRef CAS.
- (a) Y. Lin, B. L. Zhao and D. M. Du, Organocatalytic Asymmetric Synthesis of 3,3′-Pyrrolidinyl-bispirooxindoles via Michael/N-Hemiketalization Cascade Reaction between 3-Aminooxindoles and Isatin-Derived β,γ-Unsaturated α-Keto Esters, J. Org. Chem., 2018, 83, 7741 CrossRef CAS; (b) K. Zhao, Y. Zhi, X. Li, R. Puttreddy, K. Rissanen and D. Enders, Asymmetric synthesis of 3,3′-pyrrolidinyl-dispirooxindoles via a one-pot organocatalytic Mannich/deprotection/aza-Michael sequence, Chem. Commun., 2016, 52, 2249 RSC.
- B. L. Zhao and D. M. Du, Asymmetric Synthesis of Spirooxindoles with Seven Stereocenters via Organocatalyzed One-pot Three-component Sequential Cascade Reactions, Adv. Synth. Catal., 2019, 361, 3412 CrossRef CAS.
- (a) T. Kang, P. Zhao, J. Yang, L. Lin, X. Feng and X. Liu, Asymmetric Catalytic Double Michael Additions for the Synthesis of Spirooxindoles, Chem. – Eur. J., 2018, 24, 3703 CrossRef CAS; (b) W. He, J. Hu, P. Wang, L. Chen, K. Ji, S. Yang, Y. Li, Z. Xie and W. Xie, Highly Enantioselective Tandem Michael Addition of Tryptamine-Derived Oxindoles to Alkynones: Concise Synthesis of Strychnos Alkaloids, Angew. Chem., Int. Ed., 2018, 57, 3806 CrossRef CAS.
- H. Sasai, S. Takizawa, M. Kusaba, K. Kishi, B. Jianfei and T. Suzuki, Facile Synthesis of Spirooxindoles via an Enantioselective Organocatalyzed Sequential Reaction of Oxindoles with Ynone, Heterocycles, 2017, 95, 761 CrossRef.
- T. Cong, H. Wang, X. Li, H. H. Wu and J. Zhang, Chiral bifunctional bisphosphine enabled enantioselective tandem Michael addition of tryptamine-derived oxindoles to ynones, Chem. Commun., 2019, 55, 9176 RSC.
- F. Beltran, I. Fabre, I. Ciofini and L. Miesch, Direct Spirocyclization from Keto-sulfonamides: An Approach to Azaspiro Compounds, Org. Lett., 2017, 19, 5042 CrossRef CAS.
- T. Zha, X. Tong, Y. Deng, F. Peng and Z. Shao, Catalytic Asymmetric and Divergent Synthesis of Tricyclic and Tetracyclic Spirooxindoles: Controllable Site-Selective Electrophilic Halocyclization of 1,6-Enynes, Org. Lett., 2019, 21, 6068 CrossRef CAS.
- (a) G. Y. Chen, F. Zhong and Y. Lu, Asymmetric Allylic Alkylation of Isatin-Derived Morita-Baylis-Hillman Carbonates with Nitroalkanes, Org. Lett., 2012, 14, 3955 CrossRef CAS; (b) L. Liu, D. Wu, S. Zheng, T. Li, X. Li, S. Wang, J. Li, H. Li and W. Wang, Synthesis of Highly Functionalized Chiral 3,3′-Disubstituted Oxindoles via an Organocatalytic Enantioselective Michael Addition of Nitroalkanes to Indolylidenecyanoacetates, Org. Lett., 2012, 14, 134 CrossRef CAS.
- S. Shimizu, T. Tsubogo, P. Xu and S. Kobayashi, Calcium-Catalyzed Asymmetric Synthesis of 3-Tetrasubstituted Oxindoles: Efficient Construction of Adjacent Quaternary and Tertiary Chiral Centers, Org. Lett., 2015, 17, 2006 CrossRef CAS.
- For a related reaction of in situ derived imines in a domino aza-Michael/lactamisation including a preliminary asymmetric example see: J. Zhu, S. Fang, S. Jin, R. Ma, T. Lu and D. Du, Application of isatin-derived saturated esters in the synthesis of 3,3′-spirooxindole γ-butyrolactams, Org. Biomol. Chem., 2019, 17, 8745 RSC.
- K. Ohmatsu, Y. Ando, T. Nakashima and T. Ooi, A Modular Strategy for the Direct Catalytic Asymmetric α-Amination of Carbonyl Compounds, Chem, 2016, 1, 802 CAS.
- G.-Y. Ran, X.-X. Yang, J.-F. Yue, W. Du and Y.-C. Chen, Asymmetric Allylic Alkylation with Deconjugated Carbonyl Compounds: Direct Vinylogous Umpolung Strategy, Angew. Chem., Int. Ed., 2019, 58, 9210 CrossRef CAS.
- H. Hu, F. Teng, J. Liu, W. Hu, S. Luo and Q. Zhu, Enantioselective Synthesis of 2-Oxindole Spirofused Lactones and Lactams by Heck/Carbonylative Cylization Sequences: Method Development and Applications, Angew. Chem., Int. Ed., 2019, 58, 9225 CrossRef CAS.
- (a) S. Crosignani, P. Page, M. Missotten, V. Colovray, C. Cleva, J.-F. Arrighi, J. Atherall, J. Macritchie, T. Martin and Y. Humbert, et al., Discovery of a New Class of Potent, Selective, and Orally Bioavailable CRTH2 (DP2) Receptor Antagonists for the Treatment of Allergic Inflammatory Diseases, J. Med. Chem., 2008, 51, 2227 CrossRef CAS; (b) S. Crosignani, C. Jorand-Lebrun, P. Page, G. Campbell, V. Colovray, M. Missotten, Y. Humbert, C. Cleva, J. F. Arrighi and M. Gaudet, et al., Optimization of the Central Core of Indolinone-Acetic Acid-Based CRTH2 (DP2) Receptor Antagonists, ACS Med. Chem. Lett., 2011, 2, 644 CrossRef CAS.
- J. Xu, L. D. Shao, D. Li, X. Deng, Y. C. Liu, Q. S. Zhao and C. Xia, Construction of Tetracyclic 3-Spirooxindole through Cross-Dehydrogenation of Pyridinium: Applications in Facile Synthesis of (±)-Corynoxine and (±)-Corynoxine B, J. Am. Chem. Soc., 2014, 136, 17962 CrossRef CAS.
- X. Chen, H. Chen, X. Ji, H. Jiang, Z. J. Yao and H. Liu, Asymmetric One-Pot Sequential Mannich/Hydroamination Reaction by Organo- and Gold Catalysts: Synthesis of Spiro[pyrrolidin-3,2′-oxindole] Derivatives, Org. Lett., 2013, 15, 1846 CrossRef CAS.
- X. P. Yin, X. P. Zeng, Y. L. Liu, F. M. Liao, J. S. Yu, F. Zhou and J. Zhou, Asymmetric Triple Relay Catalysis: Enantioselective Synthesis of Spirocyclic Indolines through a One-Pot Process Featuring an Asymmetric 6π Electrocyclization, Angew. Chem., Int. Ed., 2014, 53, 13740 CrossRef CAS.
- S. Hajra and B. Jana, Quinine-Based Trifunctional Organocatalyst for Tandem Aza-Henry Reaction-Cyclization: Asymmetric Synthesis of Spiroxindole-Pyrrolidine/Piperidines, Org. Lett., 2017, 19, 4778 CrossRef CAS.
- C. Liu, F.-X. Tan, J. Zhou, H.-Y. Bai, T.-M. Ding, G.-D. Zhu, S.-Y. Zhang and H. Chemo-, Site-, and Enantioseletive para C-H Aminoalkylation of N-Monosubstituted Aniline Derivatives Affording 3-Amino-2-oxindoles, Org. Lett., 2020, 22, 2173 CrossRef CAS.
- N. Sharma, Z. Li, U. K. Sharma and E. V. Van Der Eycken, Facile Access to Functionalized Spiro[indoline-3,2′-pyrrole]-2,5′-diones via Post-Ugi Domino Buchwald-Hartwig/Michael Reaction, Org. Lett., 2014, 16, 3884 CrossRef CAS.
- S. J. Chambers, G. Coulthard, W. P. Unsworth, P. O'Brien and R. J. K. Taylor, From Heteroaromatic Acids and Imines to Azaspirocycles: Stereoselective Synthesis and 3D Shape Analysis, Chem. – Eur. J., 2016, 22, 6496 CrossRef CAS.
- (a) J. T. R. Liddon, A. K. Clarke, R. J. K. Taylor and W. P. Unsworth, Preparation and Reactions of Indoleninyl Halides: Scaffolds for the Synthesis of Spirocyclic Indole Derivatives, Org. Lett., 2016, 18, 6328 CrossRef CAS. (Also includes the synthesis of racemic spirocyclopentane oxindoles). For related recent work see: (b) C. Li, L. Xue, J. Zhou, Y. Zhao, G. Han, J. Hou, Y. Song and Y. Liu, Copper-Catalyzed Trifluoromethylation of Ynones Coupled with Dearomatizing Spirocyclization of Indoles: Access to CF3-Containing Spiro[cyclopentane-1,3′-indole], Org. Lett., 2020, 22, 3291 CrossRef CAS.
- S. M. Nicolle, W. Lewis, C. J. Hayes and C. J. Moody, Stereoselective Synthesis of Functionalized Pyrrolidines by the Diverted N−H Insertion Reaction of Metallocarbenes with β-Aminoketone Derivatives, Angew. Chem., Int. Ed., 2016, 55, 3749 CrossRef CAS.
- A. C. S. Reddy, P. M. Reddy and P. Anbarasan, Diastereoselective Palladium Catalyzed Carbenylative Amination of ortho-Vinylanilines with 3-Diazoindolin-2-ones, Adv. Synth. Catal., 2020, 362, 801 CrossRef CAS.
- G. Zeng, Y. Li, B. Qiao, X. Zhao and Z. Jiang, Photoredox asymmetric catalytic enantioconvergent substitution of 3-chlorooxindoles, Chem. Commun., 2019, 55, 11362 RSC.
- There are a number of works where 5-membered oxygen spirocycles are synthesised as well as nitrogen heterocycles, these will be referenced again in the appropriate section but not further discussed.
- (a) S. Chowdhury, M. Chafeev, S. Liu, J. Sun, V. Raina, R. Chui, W. Young, R. Kwan, J. Fu and J. A. Cadieux, Discovery of XEN907, a spirooxindole blocker of NaV1.7 for the treatment of pain, Bioorg. Med. Chem. Lett., 2011, 21, 3676 CrossRef CAS; (b) Y. P. Goldberg, N. Price, R. Namdari, C. J. Cohen, M. H. Lamers, C. Winters, J. Price, C. E. Young, H. Verschoof and R. Sherrington, et al., Treatment of Nav1.7-mediated pain in inherited erythromelalgia using a novel sodium channel blocker, Pain, 2012, 153, 80–85 CrossRef CAS; (c) S. K. Bagal, M. L. Chapman, B. E. Marron, R. Prime, R. I. Storer and N. A. Swain, Recent progress in sodium channel modulators for pain, Bioorg. Med. Chem. Lett., 2014, 24, 3690 CrossRef CAS.
- (a) A. K. Franz, P. D. Dreyfuss and S. L. Schreiber, Synthesis and Cellular Profiling of Diverse Organosilicon Small Molecules, J. Am. Chem. Soc., 2007, 129, 1020 CrossRef CAS; (b) R. T. Moon, T. L. Biechele, S. Haggarty and D. Fass, Molecular Inhibitors of the WNT/Beta-Catenin Pathway, PCT Int. ApplWO2010075282A1, 2010 Search PubMed; (c) S. Rana, E. C. Blowers, C. Tebbe, J. I. Contreras, P. Radhakrishnan, S. Kizhake, T. Zhou, R. N. Rajule, J. L. Arnst and A. R. Munkarah, et al., Isatin Derived Spirocyclic Analogues with α-Methylene-γ-butyrolactone as Anticancer Agents: A Structure-Activity Relationship Study, J. Med. Chem., 2016, 59, 5121 CrossRef CAS.
- N. D. Heindel and J. A. Minatelli, Synthesis and Antibacterial and Anticancer Evaluations of α-Methylene-γ-butyrolactones, J. Pharm. Sci., 1981, 70, 84 CrossRef CAS.
- A. Grossmann, S. Bartlett, M. Janecek, J. T. Hodgkinson and D. R. Spring, Diversity-Oriented Synthesis of Drug-Like Macrocyclic Scaffolds Using an Orthogonal Organo- and Metal Catalysis Strategy, Angew. Chem., Int. Ed., 2014, 53, 13093 CrossRef CAS.
- E. D. Styduhar, A. D. Huters, N. A. Weires and N. K. Garg, Enantiospecific Total Synthesis of N-Methylwelwitindolinone D Isonitrile, Angew. Chem., Int. Ed., 2013, 52, 12422 CrossRef CAS.
- (a) M. C. Nakhla and J. L. Wood, Total Synthesis of (±)-Aspergilline A, J. Am. Chem. Soc., 2017, 139, 18504 CrossRef CAS. For an asymmetric synthesis of a related core structure see: (b) L. Caruana, M. Fochi, M. C. Franchini, S. Ranieri, A. Mazzanti and L. Bernardi, Asymmetric synthesis of 3,4-annulated indoles through an organocatalytic cascade approach, Chem. Commun., 2014, 50, 445 RSC.
- H. Liu, L. Chen, K. Yuan and Y. Jia, A Ten-Step Total Synthesis of Speradine C, Angew. Chem., Int. Ed., 2019, 58, 6362 CrossRef CAS.
- M. A. Maskeri, M. J. O'Connor, A. A. Jaworski, A. V. Davies and K. A. Scheidt, A Cooperative Hydrogen Bond Donor-Brønsted Acid System for the Enantioselective Synthesis of Tetrahydropyrans, Angew. Chem., Int. Ed., 2018, 57, 17225 CrossRef CAS.
- T. Wei and D. J. Dixon, Catalytic stereoselective total synthesis of a spiro-oxindole alkaloid and the pentacyclic core of tryptoquivalines, Chem. Commun., 2018, 54, 12860 RSC.
- G. Bergonzini and P. Melchiorre, Dioxindole in Asymmetric Catalytic Synthesis: Routes to Enantioenriched 3-Substituted 3-Hydroxyoxindoles and the Preparation of Maremycin A, Angew. Chem., Int. Ed., 2012, 51, 971 CrossRef CAS.
- (a) M. Silvi, I. Chatterjee, Y. Liu and P. Melchiorre, Controlling the Molecular Topology of Vinylogous Iminium Ions by Logical Substrate Design: Highly Regio- and Stereoselective Aminocatalytic 1,6-Addition to Linear 2,4-Dienals, Angew. Chem., Int. Ed., 2013, 52, 10780 CrossRef CAS. For a related reaction for cyclopentane synthesis see: (b) X. Tian and P. Melchiorre, Control of Remote Stereochemistry in the Synthesis of Spirocyclic Oxindoles: Vinylogous Organocascade Catalysis, Angew. Chem., Int. Ed., 2013, 52, 5360 CrossRef CAS.
- C. Zheng, W. Yao, Y. Zhang and C. Ma, Chiral Spirooxindole-Butenolide Synthesis through Asymmetric N-Heterocyclic Carbene-Catalyzed Formal (3 + 2) Annulation of 3-Bromoenals and Isatins, Org. Lett., 2014, 16, 5028 CrossRef CAS.
- J. L. Li, B. Sahoo, C. G. Daniliuc and F. Glorius, Conjugate Umpolung of β,β-Disubstituted Enals by Dual Catalysis with an N-Heterocyclic Carbene and a Brønsted Acid: Facile Construction of Contiguous Quaternary Stereocenters, Angew. Chem., Int. Ed., 2014, 53, 10515 CrossRef CAS.
- J. Cao, S. Dong, D. Jiang, P. Zhu, H. Zhang, R. Li, Z. Li, X. Wang, W. Tang and D. Du, β-Functionalization of Indolin-2-one-Derived Aliphatic Acids for the Divergent Synthesis of Spirooxindole γ-Butyrolactones, J. Org. Chem., 2017, 82, 4186 CrossRef CAS.
- (a) X. Y. Chen, K. Q. Chen, D. Q. Sun and S. Ye, N-Heterocyclic, carbene-catalyzed oxidative [3 + 2] annulation of dioxindoles and enals: cross coupling of homoenolate and enolate, Chem. Sci., 2017, 8, 1936 RSC; (b) Z. Y. Song, K. Q. Chen, X. Y. Chen and S. Ye, Diastereo- and Enantioselective Synthesis of Spirooxindoles with Contiguous Tetrasubstituted Stereocenters via Catalytic Coupling of Two Tertiary Radicals, J. Org. Chem., 2018, 83, 2966 CrossRef CAS; (c) For a related report at a similar time see: S. Mukherjee, S. Joseph, A. Bhunia, R. G. Gonnade, S. R. Yetra and A. T. Biju, Enantioselective synthesis of spiro γ-butyrolactones by N-heterocyclic carbene (NHC)-catalyzed formal [3 + 2] annulation of enals with 3-hydroxy oxindoles, Org. Biomol. Chem., 2017, 15, 2013 RSC.
- S. Y. Zhu, H. Zhang, Q. W. Ma, D. Liu and X. P. Hui, Oxidative NHC catalysis: direct activation of β sp3 carbons of saturated acid chlorides, Chem. Commun., 2019, 55, 298 RSC.
- (a) Z. Jin, S. Chen, Y. Wang, P. Zheng, S. Yang and Y. R. Chi, β-Functionalization of Carboxylic Anhydrides with β-Alkyl Substituents through Carbene Organocatalysis, Angew. Chem., Int. Ed., 2014, 53, 13506 CrossRef CAS; (b) J. Xu, S. Yuan, M. Miao and Z. Chen, 1-Hydroxybenzotriazole-Assisted, N-Heterocyclic Carbene Catalyzed β-Functionalization of Saturated Carboxylic Esters: Access to Spirooxindole Lactones, J. Org. Chem., 2016, 81, 11454 CrossRef CAS; (c) Y. Gao, Y. Ma, C. Xu, L. Li, T. Yang, G. Sima, Z. Fu and W. Huang, Potassium 2-oxo-3-enoates as Effective and Versatile Surrogates for α, β-Unsaturated Aldehydes in NHC-Catalyzed Asymmetric Reactions, Adv. Synth. Catal., 2018, 360, 479 CrossRef CAS; (d) B. Liu, G. Luo, H. Wang, L. Hao, S. Yang, Z. Jin and Y. R. Chi, Carbene-Catalyzed Direct Functionalization of the β-sp3-Carbon Atoms of α-Chloroaldehydes, Chem. – Eur. J., 2019, 25, 12719 CrossRef CAS.
- Indeed for many of the previously discussed papers, where protected 3-aminooxindoles were used, this can be replaced with 3-hydroxyoxindole to produce the analogous oxygenated product. See ref. 218, 219 and 230.
- S. Ming, B. L. Zhao and D. M. Du, Chiral squaramide-catalysed enantioselective Michael/cyclization cascade reaction of 3-hydroxyoxindoles with α,β-unsaturated N-acylated succinimides, Org. Biomol. Chem., 2017, 15, 6205 RSC.
- (a) X. Q. Zhu, J. S. Wu and J. W. Xie, Stereoselective construction of Bi-spirooxindole frameworks via a Michael addition/cyclization and an unexpected redox/oxidative coupling/cyclization, Tetrahedron, 2016, 72, 8327 CrossRef CAS; (b) Y. S. Zhu, W. B. Wang, B. B. Yuan, Y. N. Li, Q. L. Wang and Z. W. Bu, A DBU-catalyzed Michael-Pinner-isomerization cascade reaction of 3-hydroxyoxindoles with isatylidene malononitriles: access to highly functionalized bispirooxindoles containing a fully substituted dihydrofuran motif, Org. Biomol. Chem., 2017, 15, 984 RSC; (c) N. Gupta, G. Bhojani, R. Tak, A. Jakhar, N. ul H. Khan, S. Chatterjee and R. I. Kureshy, Highly Diastereoselective Syntheses of Spiro-Oxindole Dihydrofuran Derivatives in Aqueous Media and Their Antibacterial Activity, ChemistrySelect, 2017, 2, 10902 CrossRef CAS.
- M. Balha, B. Mondal and S. C. Pan, Organocatalytic asymmetric synthesis of dihydrofuran-spirooxindoles from benzylidene malononitriles and dioxindoles, Org. Biomol. Chem., 2019, 17, 6557 RSC.
- Z. T. Yang, J. Zhao, W. L. Yang and W. P. Deng, Enantioselective Construction of CF3-Containing Spirooxindole γ-Lactones via Organocatalytic Asymmetric Michael/Lactonization, Org. Lett., 2019, 21, 1015 CrossRef CAS.
- For an alternative Michael addition/Cyclisation see: C. K. Tang, Z. Y. Zhou, A. B. Xia, L. Bai, J. Liu, D. Q. Xu and Z. Y. Xu, Combining Organocatalysis and Iodine Catalysis: One-Pot Sequential Catalytic Synthesis of Chiral Spirodihydrobenzofuran Pyrazolones and Spirodihydrobenzofuran Oxindoles, Org. Lett., 2018, 20, 5840 CrossRef CAS.
- (a) J. W. Ren, L. Zheng, Z. P. Ye, Z. X. Deng, Z. Z. Xie, J. A. Xiao, F. W. Zhu, H. Y. Xiang, X. Q. Chen and H. Yang, Organocatalytic, Enantioselective, Polarity-Matched Ring-Reorganization Domino Sequence Based on the 3-Oxindole Scaffold, Org. Lett., 2019, 21, 2166 CrossRef CAS. For a related cascade reaction. (b) B. L. Zhao and D. M. Du, Squaramide-Catalyzed Enantioselective Cascade Approach to Bispirooxindoles with Multiple Stereocenters, Adv. Synth. Catal., 2016, 358, 3992 CrossRef CAS.
- X. L. Jiang, S. J. Liu, Y. Q. Gu, G. J. Mei and F. Shi, Catalytic Asymmetric [4 + 1] Cyclization of ortho-Quinone Methides with 3-Chlorooxindoles, Adv. Synth. Catal., 2017, 359, 3341 CrossRef CAS.
- (a) L. Cerisoli, M. Lombardo, C. Trombini and A. Quintavalla, The First Enantioselective Organocatalytic Synthesis of 3-Spiro-α-Alkylidene-γ-Butyrolactone Oxindoles, Chem. – Eur. J., 2016, 22, 3865 CrossRef CAS. For another enantioselective aldol/cyclisation see: (b) W. Guo, X. Wang, B. Zhang, S. Shen, X. Zhou, P. Wang, Y. Liu and C. Li, Facile Synthesis of Chiral Spirooxindole-Based Isoelectronic Acids and 5–1H-Pyrrol-2-ones through Cascade Reactions with Bifunctional Organocatalysts, Chem. – Eur. J., 2014, 20, 8545 CrossRef CAS. For aldol/Michael see: (c) D. Trubitsõn, S. Žari, S. Kaabel, M. Kudrjashova, K. Kriis, I. Järving, T. Pehk and T. Kanger, Asymmetric Organocatalytic Cascade Synthesis of Tetrahydro-furanyl Spirooxindoles, Synthesis, 2018, 50, 314 CrossRef.
- (a) H. Chen, H. Liu, S. H. Zhao, S. B. Cheng, X. Y. Xu, W. C. Yuan and X. M. Zhang, Enantioselective Arylation of 3-Carboxamide Oxindoles with Quinone Monoimines and Synthesis of Chiral Spirooxindole-benzofuranones, Synlett, 2019, 30, 1067 CrossRef CAS; (b) J. Li, Y. Liu, C. Li and X. Jia, Syntheses of Spirocyclic Oxindole-Butenolides by Using Three-Component Cycloadditions of Isocyanides, Allenoates, and Isatins, Chem. – Eur. J., 2011, 17, 7409 CrossRef CAS; (c) Z. Tang, Z. Liu, Y. An, R. Jiang, X. Zhang, C. Li, X. Jia and J. Li, Isocyanide-Based Multicomponent Bicyclization with Substituted Allenoate and Isatin: Synthesis of Unusual Spirooxindole Containing [5.5]-Fused Heterocycle, J. Org. Chem., 2016, 81, 9158 CrossRef CAS; (d) K. Zhang, H. Han, L. Wang, Z. Zhang, Q. Wang, W. Zhang and Z. Bu, An unexpected cascade reaction of 3-hydroxyoxindoles with coumarin-3-carboxylates to construct 2,3-dihydrobenzofuran spirooxindoles, Chem. Commun., 2019, 55, 13681 RSC; (e) H. Bin Yang, Y. Z. Zhao, R. Sang, Y. Wei and M. Shi, Asymmetric Synthesis of Bioxindole-Substituted Hexahydrofuro[2,3-b]furans via Hydroquinine Anthraquinone-1,4-diyl Diether-Catalyzed Domino Annulation of Acylidenoxindoles/Isatins, Acylidenoxindoles and Allenoates, Adv. Synth. Catal., 2014, 356, 3799 CrossRef.
- S. De, M. K. Das, A. Roy and A. Bisai, Synthesis of 2-Oxindoles Sharing Vicinal All-Carbon Quaternary Stereocenters via Organocatalytic Aldol Reaction, J. Org. Chem., 2016, 81, 12258 CrossRef CAS.
- J. A. Sclafani, J. Chen, D. V. Levy, H. Reese, M. Dimitri, P. Mudipalli, M. Christie, C. J. Neville, M. Olsen and R. P. Bakale, The First Asymmetric Pilot-Scale Synthesis of TV-45070, Org. Process Res. Dev., 2017, 21, 1616 CrossRef CAS.
- Q. L. Wang, L. Peng, F. Y. Wang, M. L. Zhang, L. N. Jia, F. Tian, X. Y. Xu and L. X. Wang, An organocatalytic asymmetric sequential allylic alkylation-cyclization of Morita-Baylis-Hillman carbonates and 3-hydroxyoxindoles, Chem. Commun., 2013, 49, 9422 RSC.
- (a) Y. L. Liu, X. Wang, Y. L. Zhao, F. Zhu, X. P. Zeng, L. Chen, C. H. Wang, X. L. Zhao and J. Zhou, One-Pot Tandem Approach to Spirocyclic Oxindoles Featuring Adjacent Spiro-Stereocenters, Angew. Chem., Int. Ed., 2013, 52, 13735 CrossRef CAS; (b) P. K. Warghude, A. S. Sabale and R. G. Bhat, Access to highly enantioselective and diastereoselective spirooxindole dihydrofuran fused pyrazolones, Org. Biomol. Chem., 2020, 18, 1794 RSC.
- S. Jayakumar, S. Muthusamy, M. Prakash and V. Kesavan, Enantioselective Synthesis of Spirooxindole α-exo-Methylene-γ-butyrolactones from 3-OBoc-Oxindoles, Eur. J. Org. Chem., 2014, 1893 CrossRef CAS.
- (a) F. Le Hu, Y. Wei and M. Shi, Phosphine-catalyzed asymmetric [4 + 1] annulation of activated α,β-unsaturated ketones with Morita-Baylis-Hillman carbonates: enantioselective synthesis of spirooxindoles containing two adjacent quaternary stereocenters, Chem. Commun., 2014, 50, 8912 RSC. For a related diastereoselective reaction see: (b) R. Zhou, K. Zhang, Y. Chen, Q. Meng, Y. Liu, R. Li and Z. He, P(NMe2)3-mediated reductive [1 + 4] annulation of isatins with enones: a facile synthesis of spirooxindole-dihydrofurans, Chem. Commun., 2015, 51, 14663 RSC.
- For a related enantioselective transformation see: (a) N. J. Zhong, F. Wei, Q. Q. Xuan, L. Liu, D. Wang and Y. J. Chen, Highly diastereo- and enantioselective [3 + 2] annulation of isatin-derived Morita-Baylis-Hillman carbonates with trifluoropyruvate catalyzed by tertiary amines, Chem. Commun., 2013, 49, 11071 RSC.
- Z. C. Chen, P. Chen, Z. Chen, Q. Ouyang, H. P. Liang, W. Du and Y. C. Chen, Organocatalytic Enantioselective 1,3-Difunctionalizations of Morita-Baylis-Hillman Carbonates, Org. Lett., 2018, 20, 6279 CrossRef CAS.
- (a) Y. Murata, M. Takahashi, F. Yagishita, M. Sakamoto, T. Sengoku and H. Yoda, Construction of Spiro-Fused 2-Oxindole/α-Methylene-γ-Butyrolactone Systems with Extremely High Enantioselectivity via Indium-Catalyzed Amide Allylation of N-Methyl Isatin, Org. Lett., 2013, 15, 6182 CrossRef CAS. Also see: (b) M. Takahashi, Y. Murata, F. Yagishita, M. Sakamoto, T. Sengoku and H. Yoda, Catalytic Enantioselective Amide Allylation of Isatins and Its Application in the Synthesis of 2-Oxindole Derivatives Spiro-Fused to the α-Methylene-γ-Butyrolactone Functionality, Chem. – Eur. J., 2014, 20, 11091 CrossRef CAS.
- For a related Pd-catalysed allylation see: S. Jayakumar, N. Kumarswamyreddy, M. Prakash and V. Kesavan, Palladium Catalyzed Asymmetric Allylation of 3-OBoc-Oxindoles: An Efficient Synthesis of 3-Allyl-3-hydroxyoxindoles, Org. Lett., 2015, 17, 1066 CrossRef CAS.
- H. Zhang, Q. Yao, W. Cao, S. Ge, J. Xu, X. Liu and X. Feng, Catalytic enantioselective ene-type reactions of vinylogous hydrazone: construction of α-methylene-γ-butyrolactone derivatives, Chem. Commun., 2018, 54, 12511 RSC.
- B. M. Trost and K. Hirano, Dinuclear Zinc Catalyzed Asymmetric Spirannulation Reaction: An Umpolung Strategy for Formation of α-Alkylated-α-Hydroxyoxindoles, Org. Lett., 2012, 14, 2446 CrossRef CAS.
- (a) M. M. Liu, X. C. Yang, Y. Z. Hua, J. B. Chang and M. C. Wang, Synthesis of Chiral Bispirotetrahydrofuran Oxindoles by Cooperative Bimetallic-Catalyzed Asymmetric Cascade Reaction, Org. Lett., 2019, 21, 2111 CrossRef CAS. Also see: (b) Y. H. Miao, Y. Z. Hua and M. C. Wang, Dinuclear zinc cooperative catalytic three-component reactions for highly enantioselective synthesis of 3,3′-dihydrofuran spirooxindoles, Org. Biomol. Chem., 2019, 17, 7172 RSC.
- Y.-J. Guo, X. Guo, D.-Z. Kong, H. Lu, L. Liu, Y.-Z. Hua and M.-C. Wang, Catalytic Asymmetric Synthesis of Tetrahydrofuran Spirooxindoles via a Dinuclear Zinc Catalyst, J. Org. Chem., 2020, 85, 4195 CrossRef CAS.
- (a) J. Liu, X. Xu, J. Li, B. Liu, H. Jiang and B. Yin, Palladium-catalyzed dearomatizing 2,5-alkoxyarylation of furan rings: diastereospecific access to spirooxindoles, Chem. Commun., 2016, 52, 9550 RSC; (b) J. Liu, H. Peng, L. Lu, X. Xu, H. Jiang and B. Yin, Diastereospecific and Enantioselective Access to Dispirooxindoles from Furfurylcyclobutanols by Means of a Pd-Catalyzed Arylative Dearomatization/Ring Expansion Cascade, Org. Lett., 2016, 18, 6440 CrossRef CAS.
- D. F. Li, Y. Gu, J. R. Zhang, K. Liu and L. M. Zhao, Diastereoselective Construction of Spiro-furo[3,2-c]benzopyranoxindoles through a Cu(OTf)2/AcOH Cooperative Promoted Bicyclization Reaction, J. Org. Chem., 2019, 84, 879 CrossRef CAS.
- J. L. Nallasivam and T. K. Chakraborty, Titanocene(III)-Mediated 5-exo-trig Radical Cyclization: En Route to Spirooxindole-Based Tetrahydrofuran and Bicyclic Lactone, J. Org. Chem., 2019, 84, 16124 CrossRef CAS.
- (a) E. L. McInturff, J. Mowat, A. R. Waldeck and M. J. Krische, Ruthenium-Catalyzed Hydrohydroxyalkylation of Acrylates with Diols and α-Hydroxycarbonyl Compounds To Form Spiro- and α-Methylene-γ-butyrolactones, J. Am. Chem. Soc., 2013, 135, 17230 CrossRef CAS; (b) T. Luong, S. Chen, K. Qu, E. L. McInturff and M. J. Krische, Ruthenium(0)-Catalyzed C-C Coupling of Alkynes and 3-Hydroxy-2-oxindoles: Direct C-H Vinylation of Alcohols, Org. Lett., 2017, 19, 966 CrossRef CAS.
- C. S. Buxton, D. C. Blakemore and J. F. Bower, Reductive Coupling of Acrylates with Ketones and Ketimines by a Nickel-Catalyzed Transfer-Hydrogenative Strategy, Angew. Chem., Int. Ed., 2017, 56, 13824 CrossRef CAS.
- B. M. Trost, Z. Jiao and C. I. Hung, Elaborating Complex Heteroaryl-Containing Cycles via Enantioselective Palladium-Catalyzed Cycloadditions, Angew. Chem., Int. Ed., 2019, 58, 15154 CrossRef CAS.
- S. M. Nicolle, W. Lewis, C. J. Hayes and C. J. Moody, Stereoselective Synthesis of Highly Substituted Tetrahydrofurans through Diverted Carbene O-H Insertion Reaction, Angew. Chem., Int. Ed., 2015, 54, 8485 CrossRef CAS.
- (a) G. Karthik, T. Rajasekaran, B. Sridhar and B. V. Subba Reddy, Highly regio- and diastereoselective three-component reaction of acyclic/cyclic donor-acceptor carbenoids for the synthesis of angularly fused furocoumarins and spirooxindolylfurocoumarins, Tetrahedron, 2014, 70, 8148 CrossRef CAS; (b) B. V. S. Reddy, G. Karthik, T. Rajasekaran and B. Sridhar, Stereoselective Synthesis of Highly Functionalized Dispirooxindoles through [3 + 2] Cycloaddition of Carbonyl Ylides with 3-Arylideneoxindoles, Eur. J. Org. Chem., 2015, 2038 CrossRef; (c) B. V. S. Reddy, E. P. Reddy, B. Sridhar and Y. J. Rao, Rhodium-catalyzed cycloaddition of carbonyl ylides for the synthesis of spiro[furo[2,3-a]xanthene-2,3′-indolin]-2′-one scaffolds, RSC Adv., 2016, 6, 50497 RSC; (d) S. Murarka, C. Golz, C. Strohmann, A. Antonchick and H. Waldmann, Biology-Oriented Synthesis of 3,3-Spiro(2-tetrahydrofuranyl)oxindoles, Synthesis, 2016, 49, 87 CrossRef; (e) A. C. Hunter, S. C. Schlitzer, J. C. Stevens, B. Almutwalli and I. Sharma, A Convergent Approach to Diverse Spiroethers through Stereoselective Trapping of Rhodium Carbenoids with Gold-Activated Alkynols, J. Org. Chem., 2018, 83, 2744 CrossRef CAS; (f) N. P. Massaro, J. C. Stevens, A. Chatterji and I. Sharma, Stereoselective Synthesis of Diverse Lactones through a Cascade Reaction of Rhodium Carbenoids with Ketoacids, Org. Lett., 2018, 20, 7585 CrossRef CAS; (g) G. Xu, S. Tang, Y. Shao and J. Sun, B(C6F5)3-Catalyzed formal (4 + 1)-annulation of ortho-quinone methides with diazoacetates: access to 2,3-dihydrobenzofurans, Chem. Commun., 2019, 55, 9096–9099 RSC; (h) S. Muthusamy, A. Prabu and E. Suresh, Copper-catalyzed synthesis of spiro-indolofurobenzopyrans: tandem reactions of diazoamides and O-propargyl salicylaldehydes, Org. Biomol. Chem., 2019, 17, 8088 RSC.
- L. Y. Mei, Y. Wei, Q. Xu and M. Shi, Diastereo- and Enantioselective Construction of Oxindole-Fused Spirotetrahydrofuran Scaffolds through Palladium-Catalyzed Asymmetric [3 + 2] Cycloaddition of Vinyl Cyclopropanes and Isatins, Organometallics, 2013, 32, 3544 CrossRef CAS.
- J. A. Xiao, Y. C. Li, Z. J. Luo, X. L. Cheng, Z. X. Deng, W. Q. Chen, W. Su and H. Yang, Construction of Bispirooxindole Heterocycles via Palladium-Catalyzed Ring-Opening Formal [3 + 2]-Cycloaddition of Spirovinylcyclopropyl Oxindole and 3-Oxindole Derivatives, J. Org. Chem., 2019, 84, 2297 CrossRef CAS.
- (a) B. M. Sharma, M. Yadav, R. G. Gonnade and P. Kumar, Unified Approach to Fused and Spirocyclic Oxindoles through Lewis-Acid-Promoted Opening of Spiroepoxyoxindoles with Allylsilanes: Application to the Formal Synthesis of (±)-Physovenine, Eur. J. Org. Chem., 2017, 2603 CrossRef CAS; (b) For a related reaction using Sc(OTf)3 as catalyst see: S. Hajra, S. Roy and S. Maity, Reversal of Selectivity in C3-Allylation and Formal [3 + 2]-Cycloaddition of Spiro-epoxyoxindole: Unified Synthesis of Spiro-furanooxindole, (±)-N-Methylcoerulescine, (±)-Physovenine, and 3a-Allylhexahydropyrrolo[2,3-b]indole, Org. Lett., 2017, 19, 1998–2001 CrossRef CAS Also see ref. 63.
- (a) S. Hajra, S. Maity and S. Roy, Regioselective Friedel-Crafts Reaction of Electron-Rich Benzenoid Arenes and Spiroepoxyoxindole at the Spiro-Centre: Efficient Synthesis of Benzofuroindolines and 2H-Spiro[benzofuran]-3,3′-oxindoles, Adv. Synth. Catal., 2016, 358, 2300 CrossRef CAS. For a related report see: (b) S. Hajra, S. Maity, S. Roy, R. Maity and S. Samanta, Brønsted Acid Promoted Regioselective C-3 Arylation and Heteroarylation of Spiro-epoxyoxindoles for the Construction of All Carbon Quaternary Centres: A Detailed Study, Eur. J. Org. Chem., 2019, 969 CrossRef CAS.
- B. A. Laevens, J. Tao and G. K. Murphy, Iodide-Mediated Synthesis of Spirooxindolo Dihydrofurans from Iodonium Ylides and 3-Alkylidene-2-oxindoles, J. Org. Chem., 2017, 82, 11903 CrossRef CAS.
- H. Wu, Y. P. He, L. Xu, D. Y. Zhang and L. Z. Gong, Asymmetric Organocatalytic Direct C(sp2)-H/C(sp3)-H Oxidative Cross-Coupling by Chiral Iodine Reagents, Angew. Chem., Int. Ed., 2014, 53, 3466 CrossRef CAS.
- (a) Y. Cao, X. Zhang, G. Lin, D. Zhang-Negrerie and Y. Du, Chiral Aryliodine-Mediated Enantioselective Organocatalytic Spirocyclization: Synthesis of Spirofurooxindoles via Cascade Oxidative C-O and C-C Bond Formation, Org. Lett., 2016, 18, 5580 CrossRef CAS. For related work by Du see: (b) X. Zhen, X. Wan, W. Zhang, Q. Li, D. Zhang-Negrerie and Y. Du, Synthesis of Spirooxindoles from N-Arylamide Derivatives via Oxidative C(sp2)-C(sp3) Bond Formation Mediated by PhI(OMe)2 Generated in Situ, Org. Lett., 2019, 21, 890 CrossRef CAS.
- (a) C. Serradeil-Le Gal, C. Lacour, G. Valette, G. Garcia, L. Foulon, G. Galindo, L. Bankir, B. Pouzet, G. Guillon and C. Barberis, et al., Characterization of SR 121463A, a highly potent and selective, orally active vasopressin V2 receptor antagonist, J. Clin. Invest., 1996, 98, 2729 CrossRef CAS; (b) H. Venkatesan, M. C. Davis, Y. Altas, J. P. Snyder and D. C. Liotta, Total Synthesis of SR 121463 A, a Highly Potent and Selective Vasopressin V2 Receptor Antagonist, J. Org. Chem., 2001, 66, 3653 CrossRef CAS.
- J.-J. Liu and Z. Zhang, Spiroindolinone Derivatives, PCT Int. ApplWO2008055812A1, 2008 Search PubMed.
- Y. Zhang, J. Zhang, H. Xie, T. Yu, H. Luo, Q. Ren, X. Wu, C. Fu, S. Li, Y. Lei and B. Hu, Preparation of Pyrrolidine Derivatives as Hepatitis C Inhibitors for Treating Hepatitis C Virus Infection, PCT Int. ApplCN103420991, 2013 Search PubMed.
- A. Fensome, W. R. Adams, A. L. Adams, T. J. Berrodin, J. Cohen, C. Huselton, A. Illenberger, J. C. Kern, V. A. Hudak and M. A. Marella, et al., Design, Synthesis, and SAR of New Pyrrole-Oxindole Progesterone Receptor Modulators Leading to 5-(7-Fluoro-3,3-dimethyl-2-oxo-2,3-dihydro-1H-indol-5-yl)-1-methyl-1H-pyrrole-2-carbonitrile (WAY-255348), J. Med. Chem., 2008, 51, 1861 CrossRef CAS.
- (a) H. Lin and S. J. Danishefsky, Gelsemine: A Thought-Provoking Target for Total Synthesis, Angew. Chem., Int. Ed., 2003, 42, 36 CrossRef CAS; (b) A. Ghosh and R. G. Carter, Recent Syntheses and Strategies toward Polycyclic Gelsemium Alkaloids, Angew. Chem., Int. Ed., 2019, 58, 681 CrossRef CAS.
- (a) J. K. Lam, S. B. Joseph and C. D. Vanderwal, A Zincke aldehyde approach to gelsemine, Tetrahedron Lett., 2015, 56, 3165 CrossRef CAS; (b) X. Chen, S. Duan, C. Tao, H. Zhai and F. G. Qiu, Total synthesis of (+)-gelsemine via an organocatalytic Diels-Alder approach, Nat. Commun., 2015, 6, 7204 CrossRef.
- R. Samineni, J. Madapa, S. Pabbaraja and G. Mehta, Stitching Oxindoles and Ynones in a Domino Process: Access to Spirooxindoles and Application to a Short Synthesis of Spindomycin B, Org. Lett., 2017, 19, 6152 CrossRef CAS.
- M. Gicquel, C. Gomez, P. Retailleau, A. Voituriez and A. Marinetti, Synthesis of 3,3′-Spirocyclic Oxindoles via Phosphine Catalyzed [4 + 2] Cyclizations, Org. Lett., 2013, 15, 4002 CrossRef CAS.
- B. X. Xiao, B. Jiang, X. Song, W. Du and Y. C. Chen, Phosphine-catalysed asymmetric dearomative formal [4 + 2] cycloadditions of 3-benzofuranyl vinyl ketones, Chem. Commun., 2019, 55, 3097 RSC.
- (a) D. B. Ramachary, C. Venkaiah and R. Madhavachary, Asymmetric Synthesis of Druglike Six-Membered Spirooxindoles through an Amino Enyne Catalysis, Org. Lett., 2013, 15, 3042 CrossRef CAS. For a related reaction using 3-methylene oxindoles to react with malonitriles under Mg catalysis see: (b) H. Mei, L. Lin, B. Shen, X. Liu and X. Feng, Highly enantioselective desymmetrization of prochiral cyclic α,α-dicyanoalkenes via the direct vinylogous Michael/cyclization domino reaction, Org. Chem. Front., 2018, 5, 2505 RSC. For reaction of related malonitrile oxindoles with allenes: (c) R. Chen, X. Fan, Z. Xu and Z. He, Facile Synthesis of Spirooxindole-Cyclohexenes via Phosphine-Catalyzed [4 + 2] Annulation of α-Substituted Allenoates, Chin. J. Chem., 2017, 35, 1469 CrossRef CAS.
- (a) H. Huang, M. Bihani and J. C. G. Zhao, Stereoselective synthesis of spirooxindole derivatives using an organocatalyzed tandem Michael-Michael reaction, Org. Biomol. Chem., 2016, 14, 1755 RSC; (b) Z. Zhou, Q. He, Y. Jiang, Q. Ouyang, W. Du and Y. C. Chen, Double Thiol-Chiral Brønsted Base Catalysis: Asymmetric Cross Rauhut-Currier Reaction and Sequential [4 + 2] Annulation for Assembly of Different Activated Olefins, Org. Lett., 2019, 21, 7184 CrossRef CAS.
- (a) X. L. He, H. R. Zhao, X. Song, B. Jiang, W. Du and Y. C. Chen, Asymmetric Barton-Zard Reaction To Access 3-Pyrrole-Containing Axially Chiral Skeletons, ACS Catal., 2019, 9, 4374 CrossRef CAS; (b) L. Peng, K. Li, C. Xie, S. Li, D. Xu, W. Qin and H. Yan, Organocatalytic Asymmetric Annulation of ortho-Alkynylanilines: Synthesis of Axially Chiral Naphthyl-C2-indoles, Angew. Chem., Int. Ed., 2019, 58, 17199 CrossRef CAS.
- (a) X. Zuo, X. L. Liu, J. X. Wang, Y. M. Yao, Y. Y. Zhou, Q. Di Wei, Y. Gong and Y. Zhou, Organocatalytic Reaction of Chromone-Oxindole Synthon: Access to Chromanone-Based Spirocyclohexaneoxindoles with Five Adjacent Stereocenters, J. Org. Chem., 2019, 84, 6679 CrossRef CAS; (b) X. L. Liu, G. Zhou, Y. Gong, Z. Yao, X. Zuo, W. H. Zhang and Y. Zhou, Stereocontrolled Synthesis of Bispirooxindole-Based Hexahydroxanthones with Five Contiguous Stereocenters, Org. Lett., 2019, 21, 2528 CrossRef CAS; (c) S. Q. Chang, X. Zou, Y. Gong, X. W. He, X. L. Liu and Y. Zhou, Stereocontrolled construction of six vicinal stereogenic centers on a hexahydroxanthone framework through a formal [2 + 1 + 3] annulation, Chem. Commun., 2019, 55, 14003 RSC; (d) X. L. Liu, Q. Di Wei, X. Zuo, S. W. Xu, Z. Yao, J. X. Wang and Y. Zhou, Organocatalytic Michael/Michael Cycloaddition Enabled Asymmetric Construction of Hexahydroxanthones with Skeletal Diversity, Adv. Synth. Catal., 2019, 361, 2836 CrossRef CAS; (e) X. L. Liu, Y. Gong, S. Chen, X. Zuo, Z. Yao and Y. Zhou, Bifunctional oxindole-chromone 4C building block directed asymmetric synthesis of bispirocyclic hexahydroxanthones featuring five contiguous stereocenters and two side-by-side oxindoles, Org. Chem. Front., 2019, 6, 1603 RSC; (f) M. Zhang, J. X. Wang, S. Q. Chang, X. L. Liu, X. Zuo and Y. Zhou, Highly efficient enantioselective synthesis of bispiro[benzofuran-oxindole/benzofuran-chromanone]s through organocatalytic inter-/intramolecular Michael cycloaddition, Chin. Chem. Lett., 2020, 31, 381 CrossRef CAS.
- W. Ren, X. Y. Wang, J. J. Li, M. Tian, J. Liu, L. Ouyang and J. H. Wang, Efficient construction of biologically important functionalized polycyclic spiro-fused carbocyclicoxindoles via an asymmetric organocatalytic quadruple-cascade reaction, RSC Adv., 2017, 7, 1863 RSC.
- (a) A. K. Ghosh and B. Zhou, Enantioselective synthesis of spiro[cyclohexane-1,3′-indolin]-2′-ones containing multiple stereocenters via organocatalytic Michael/aldol cascade reactions, Tetrahedron Lett., 2013, 54, 2311 CrossRef CAS; (b) S. Roy, M. Amireddy and K. Chen, Organocatalytic formal [5 + 1] annulation: diastereoselective cascade synthesis of functionalized six-membered spirocyclic indane-1,3-diones/oxindoles via Michael-aldol reaction, Tetrahedron, 2013, 69, 8751 CrossRef CAS; (c) S. Xu, C. Li, X. Jia and J. Li, AlCl3-Promoted Selective Michael Addition with Allenoate and Methyleneindolinone: Synthesis of Spirocyclic Oxindole by Using Allenoate as a Four-Carbon Component Building Block, J. Org. Chem., 2014, 79, 11161 CrossRef CAS; (d) M. Vishwanath, P. Vinayagam, V. P. R. Gajulapalli and V. Kesavan, Asymmetric Organocatalytic Assembly of Oxindoles Fused with Spiro-3,4-dihydropyrans with Three Contiguous Stereocenters Consisting of Vicinal Quaternary Centers, Asian J. Org. Chem., 2016, 5, 613 CrossRef CAS. For a desymmetrising aldol reaction see: (e) B. B. Sun, Y. F. Zhang, J. Q. Zhang, S. J. Yin, W. T. Fan, H. Y. Li and X. W. Wang, Cinchona Alkaloid Derived Primary Amine Catalyzed Intramolecular Desymmetrizing Aldolization Reaction of Diacetonyloxindoles, Eur. J. Org. Chem., 2017, 2871 CrossRef CAS.
- These examples include reactions with more than 2 steps or conjugate additions followed by a different cyclisation mode: (a) B. Zhou, Y. Yang, J. Shi, Z. Luo and Y. Li, Synthesis of Six-Membered Spirocyclic Oxindoles with Five Consecutive Stereocenters in an Asymmetric Organocatalytic One-Pot Michael/Michael/Aldol Addition Sequence, J. Org. Chem., 2013, 78, 2897 CrossRef CAS; (b) Z. Zhou, X. Feng, X. Yin and Y. C. Chen, Direct Remote Asymmetric Bisvinylogous 1,4-Additions of Cyclic 2,5-Dienones to Nitroalkenes, Org. Lett., 2014, 16, 2370 CrossRef CAS; (c) R. Zhou, Q. Wu, M. Guo, W. Huang, X. He, L. Yang, F. Peng, G. He and B. Han, Organocatalytic cascade reaction for the asymmetric synthesis of novel chroman-fused spirooxindoles that potently inhibit cancer cell proliferation, Chem. Commun., 2015, 51, 13113 RSC; (d) Q. S. Sun, H. Lin, X. Sun and X. W. Sun, Highly efficient synthesis of enantioenriched fully-substituted spirocyclohexane oxindoles via a Michael-Michael-aldol cascade reaction, Tetrahedron Lett., 2016, 57, 5673 CrossRef CAS. Radical pathway: (e) H. R. Wu, L. Cheng, D. L. Kong, H. Y. Huang, C. L. Gu, L. Liu, D. Wang and C. J. Li, FeCl3-Mediated Radical Tandem Reactions of 3-Benzyl-2-oxindoles with Styrene Derivatives for the Stereoselective Synthesis of Spirocyclohexene Oxindoles, Org. Lett., 2016, 18, 1382 CrossRef CAS.
- B. Wang, X. H. Wang, W. Huang, J. Zhou, H. P. Zhu, C. Peng and B. Han, Protecting Group-Directed Diastereodivergent Synthesis of Chiral Tetrahydronaphthalene-Fused Spirooxindoles via Bifunctional Tertiary Amine Catalysis, J. Org. Chem., 2019, 84, 10349 CrossRef.
- (a) Y. Liu, M. Nappi, E. Arceo, S. Vera and P. Melchiorre, Asymmetric Catalysis of Diels-Alder Reactions with in Situ Generated Heterocyclic ortho-Quinodimethanes, J. Am. Chem. Soc., 2011, 133, 15212 CrossRef CAS; (b) B. Tan, G. Hernández-Torres and C. F. Barbas, Highly Efficient Hydrogen-Bonding Catalysis of the Diels-Alder Reaction of 3-Vinylindoles and Methyleneindolinones Provides Carbazolespirooxindole Skeletons, J. Am. Chem. Soc., 2011, 133, 12354 CrossRef CAS.
- Hydrogen bonding catalysis: (a) L. J. Huang, J. Weng, S. Wang and G. Lu, Organocatalytic Diels-Alder Reaction of 2-Vinylindoles with Methyleneindolinones: An Efficient Approach to Functionalized Carbazolespirooxindoles, Adv. Synth. Catal., 2015, 357, 993 CrossRef CAS; (b) J. W. Ren, J. Wang, J. A. Xiao, J. Li, H. Y. Xiang, X. Q. Chen and H. Yang, L-Pyroglutamic, Sulphonamide as Hydrogen-Bonding Organocatalyst: Enantioselective Diels-Alder Cyclization to Construct Carbazolespirooxindoles, J. Org. Chem., 2017, 82, 6441 CrossRef CAS. TfOH catalysed domino reactions: (c) R. Y. Yang, J. Sun, Y. Tao, Q. Sun and C. G. Yan, TfOH-Catalyzed One-Pot Domino Reaction for Diastereoselective Synthesis of Polysubstituted Tetrahydrospiro[carbazole-1,3′-indoline]s, J. Org. Chem., 2017, 82, 13277 CrossRef CAS; (d) R. Y. Yang, J. Sun, Q. Sun and C. G. Yan, Selective Synthesis of 3-(9H-Carbazol-2-yl)indolin-2-ones and Spiro[tetrahydrocarbazole-3,3′-oxindoles] via a HOTf Catalyzed Three-Component Reaction, J. Org. Chem., 2018, 83, 5909 CrossRef CAS; (e) J. Guo, X. Bai, Q. Wang and Z. Bu, Diastereoselective Construction of Indole-Bridged Chroman Spirooxindoles through a TfOH-Catalyzed Michael Addition-Inspired Cascade Reaction, J. Org. Chem., 2018, 83, 3679 CrossRef CAS. Ag catalysed aminoenyne formation: (f) P. Sharma, N. P. Kumar, N. H. Krishna, D. Prasanna, B. Sridhar and N. Shankaraiah, Silver(I)-catalysed domino alkyne-annulation/Diels-Alder reaction: a mild synthetic approach to tetrahydrospiro[carbazole-4,3′-indoline] scaffolds, Org. Chem. Front., 2016, 3, 1503 RSC. Iminium ion catalysis: (g) Z. H. You, Y. H. Chen, Y. Tang and Y. K. Liu, Organocatalytic Asymmetric Synthesis of Spiro-Bridged and Spiro-Fused Heterocyclic Compounds Containing Chromane, Indole, and Oxindole Moieties, Org. Lett., 2018, 20, 6682 CrossRef CAS. Cu mediated 3-component reaction: (h) S.-C. Zhan, J. Sun, R.-Z. Liu and C.-G. Yan, Diastereoselective construction of carbazole-based spirooxindoles via the Levy three-component reaction, Org. Biomol. Chem., 2020, 18, 163 RSC.
- H. Zheng, P. He, Y. Liu, Y. Zhang, X. Liu, L. Lin and X. Feng, Efficient synthesis of carbazolespirooxindole skeletons via asymmetric Diels-Alder reaction of 3-vinylindoles and methyleneindolinones, Chem. Commun., 2014, 50, 8794 RSC.
- Y. Wang, M. S. Tu, L. Yin, M. Sun and F. Shi, Brønsted Acid Catalyzed Asymmetric Diels-Alder Reactions: Stereoselective Construction of Spiro[tetrahydrocarbazole-3,3′-oxindole] Framework, J. Org. Chem., 2015, 80, 3223 CrossRef CAS.
- H. Miyamoto, T. Hirano, Y. Okawa, A. Nakazaki and S. Kobayashi, Stereoselective synthesis of spirocyclic oxindoles based on a one-pot Ullmann coupling/Claisen rearrangement and its application to the synthesis of a hexahydropyrrolo[2,3-b]indole alkaloid, Tetrahedron, 2013, 69, 9481 CrossRef CAS.
- G. Li, T. Liang, L. Wojtas and J. C. Antilla, An Asymmetric Diels-Alder Reaction Catalyzed by Chiral Phosphate Magnesium Complexes: Highly Enantioselective Synthesis of Chiral Spirooxindoles, Angew. Chem., Int. Ed., 2013, 52, 4628 CrossRef CAS.
- (a) J. Zheng, L. Lin, K. Fu, H. Zheng, X. Liu and X. Feng, Synthesis of Optically Pure Spiro[cyclohexane-oxindoline] Derivatives via Catalytic Asymmetric Diels-Alder Reaction of Brassard-Type Diene with Methyleneindolines, J. Org. Chem., 2015, 80, 8836 CrossRef CAS. Also see: (b) Y. F. Zhang, S. J. Yin, M. Zhao, J. Q. Zhang, H. Y. Li and X. W. Wang, Dinuclear zinc-catalyzed desymmetric intramolecular aldolization: an enantioselective construction of spiro[cyclohexanone-oxindole] derivatives, RSC Adv., 2016, 6, 30683 RSC.
- For a related diastereoselective reaction mediated by AcOH see: R. Y. Yang, J. Sun and C. G. Yan, HOAc-Mediated Domino Diels-Alder Reaction for Synthesis of Spiro[cyclohexane-1,3′-indolines] in Ionic Liquid [Bmim]Br, ACS Omega, 2018, 3, 5406 CrossRef CAS.
- (a) S. Muthusamy and V. Kesavan, Asymmetric Cycloaddition Reactions of Oxindole α-Keto Esters via Cascade Dienamine-Enamine and Trienamine Strategies, Eur. J. Org. Chem., 2019, 4046 CrossRef CAS. For a similar reaction of 2,4-dienals see: (b) A. S. Marques, J. Marrot, I. Chataigner, V. Coeffard, G. Vincent and X. Moreau, In Situ Generation of Cyclopentadienol Intermediates from 2,4-Dienals. Application to the Synthesis of Spirooxindoles via a Domino Polycyclization, Org. Lett., 2018, 20, 792 CrossRef CAS.
- H. Zheng, Y. Wang, C. Xu, Q. Xiong, L. Lin and X. Feng, Diversified Cycloisomerization/Diels-Alder Reactions of 1,6-Enynes through Bimetallic Relay Asymmetric Catalysis, Angew. Chem., Int. Ed., 2019, 58, 5327 CrossRef CAS.
- Y. Luo, H. Zhang, S. Wang, Y. Zhou, S. Dong and X. Feng, Asymmetric Catalytic Diverse Ring Opening/Cycloadditions of Cyclobutenones with (E)-Alkenyloxindoles and (E)-Dioxopyrrolidines, Org. Lett., 2020, 22, 2645 CrossRef CAS.
- L. J. Huang, J. Weng, S. Wang and G. Lu, Organocatalytic Diels-Alder Reaction of 2-Vinylindoles with Methyleneindolinones: An Efficient Approach to Functionalized Carbazolespirooxindoles, Adv. Synth. Catal., 2015, 357, 993 CrossRef CAS.
- (a) B. K. Min, D. Y. Seo, J. Y. Ryu, J. Lee and J. N. Kim, Synthesis of Spirocyclohexadieneyl-2-Oxindoles by 6π-Electrocyclization of Trienes Derived from Wittig Reaction of Morita-Baylis-Hillman Carbonates and α,β-Unsaturated Aldehydes, Bull. Korean Chem. Soc., 2018, 39, 115 CrossRef CAS. For related reaction to bispirooxindoles: (b) B. K. Min, S. Lee, H. J. Roh, J. Y. Ryu, J. Lee and J. N. Kim, Synthesis of dispirocyclohexadiene bisoxindole from Morita-Baylis-Hillman carbonate of isatin, Tetrahedron Lett., 2017, 58, 3251 CrossRef CAS.
- (a) J. R. Huang, M. Sohail, T. Taniguchi, K. Monde and F. Tanaka, Formal (4 + 1) Cycloaddition and Enantioselective Michael-Henry Cascade Reactions To Synthesize Spiro[4,5]decanes and Spirooxindole Polycycles, Angew. Chem., Int. Ed., 2017, 56, 5853 CrossRef CAS. For work on related oxygenated heterocycles see: (b) M. Sohail and F. Tanaka, Dynamic stereoselective annulation via aldol-oxa-cyclization cascade reaction to afford spirooxindole pyran polycycles, Commun. Chem., 2019, 2, 73 CrossRef.
- K. H. Kim, H. R. Moon, J. Lee and J. N. Kim, Palladium-Catalyzed Construction of Spirooxindoles by Arylative Cyclization of 3-(γ,δ-Disubstituted)allylidene-2-Oxindoles, Adv. Synth. Catal., 2015, 357, 701 CrossRef CAS . Also see ref. 81.
- (a) H. Yoon, A. Lossouarn, F. Landau and M. Lautens, Pd-Catalyzed Spirocyclization via C-H Activation and Benzyne Insertion, Org. Lett., 2016, 18, 6324 CrossRef CAS; (b) M. Pérez-Gómez and J. A. García-López, Trapping σ-Alkyl-Palladium(II) Intermediates with Arynes Encompassing Intramolecular C−H Activation: Spirobiaryls through Pd-Catalyzed Cascade Reactions, Angew. Chem., Int. Ed., 2016, 55, 14389 CrossRef.
- (a) H. Yoon, M. Rölz, F. Landau and M. Lautens, Palladium-Catalyzed Spirocyclization through C−H Activation and Regioselective Alkyne Insertion, Angew. Chem., Int. Ed., 2017, 56, 10920 CrossRef CAS; (b) I. Franzoni, H. Yoon, J.-A. García-López, A. I. Poblador-Bahamonde and M. Lautens, Exploring the mechanism of the Pd-catalyzed spirocyclization reaction: a combined DFT and experimental study, Chem. Sci., 2018, 9, 1496 RSC. For a review see: (c) A. D. Marchese, E. M. Larin, B. Mirabi and M. Lautens, Metal-Catalyzed Approaches toward the Oxindole Core, Acc. Chem. Res., 2020, 53, 1605 CrossRef CAS.
- X. Luo, Y. Xu, G. Xiao, W. Liu, C. Qian, G. Deng, J. Song, Y. Liang and C. Yang, Palladium-Catalyzed Tandem Reaction of Three Aryl Iodides Involving Triple C-H Activation, Org. Lett., 2018, 20, 2997 CrossRef CAS.
- D. Arunprasath, B. D. Bala and G. Sekar, Stereoselective Construction of α-Tetralone-Fused Spirooxindoles via Pd-Catalyzed Domino Carbene Migratory Insertion/Conjugate Addition Sequence, Org. Lett., 2017, 19, 5280 CrossRef CAS.
- D.-Y. Zhang, L. Xu, H. Wu and L.-Z. Gong, Chiral Iodine-Catalyzed Dearomatizative Spirocyclization for the Enantioselective Construction of an All-Carbon Stereogenic Center, Chem. – Eur. J., 2015, 21, 10314 CrossRef CAS.
- (a) A. V. Chate, S. P. Kamdi, A. N. Bhagat, C. K. Jadhav, A. Nipte, A. P. Sarkate, S. V. Tiwari and C. H. Gill, Design, Synthesis and SAR Study of Novel Spiro [Pyrimido[5,4-b]Quinoline-10,5′-Pyrrolo[2,3-d]Pyrimidine] Derivatives as Promising Anticancer Agents, J. Heterocycl. Chem., 2018, 55, 2297 CrossRef CAS; (b) J. Li, N. Wu, Y. Tian, J. Zhang and S. Wu, Aminopyridyl/Pyrazinyl Spiro[indoline-3,4′-piperidine]-2-ones As Highly Selective and Efficacious c-Met/ALK Inhibitors, ACS Med. Chem. Lett., 2013, 4, 806 CrossRef CAS; (c) F. Shirai, A. Mizutani, Y. Yashiroda, T. Tsumura, Y. Kano, Y. Muramatsu, T. Chikada, H. Yuki, H. Niwa and S. Sato, et al., Design and Discovery of an Orally Efficacious Spiroindolinone-Based Tankyrase Inhibitor for the Treatment of Colon Cancer, J. Med. Chem., 2020, 63, 4183 CrossRef CAS.
- F. Shirai, T. Tsumura, Y. Yashiroda, H. Yuki, H. Niwa, S. Sato, T. Chikada, Y. Koda, K. Washizuka and N. Yoshimoto, et al., Discovery of Novel Spiroindoline Derivatives as Selective Tankyrase Inhibitors, J. Med. Chem., 2019, 62, 3407 CrossRef CAS.
- H. Richter, A. L. Satz, M. Bedoucha, B. Buettelmann, A. C. Petersen, A. Harmeier, R. Hermosilla, R. Hochstrasser, D. Burger and B. Gsell, et al., DNA-Encoded Library-Derived DDR1 Inhibitor Prevents Fibrosis and Renal Function Loss in a Genetic Mouse Model of Alport Syndrome, ACS Chem. Biol., 2019, 14, 37 CrossRef CAS.
- J. Xu, X. Xie, N. Ye, J. Zou, H. Chen, M. A. White, P.-Y. Shi and J. Zhou, Design, Synthesis, and Biological Evaluation of Substituted 4,6-Dihydrospiro[[1,2,3]triazolo[4,5-b]pyridine-7,3′-indoline]-2′,5(3H)-dione Analogues as Potent NS4B Inhibitors for the Treatment of Dengue Virus Infection, J. Med. Chem., 2019, 62, 7941 CrossRef CAS.
- (a) G. C. Bignan, K. Battista, P. J. Connolly, M. J. Orsini, J. Liu, S. A. Middleton and A. B. Reitz, Preparation of 3-spirocyclic indolin-2-ones as ligands for the ORL-1 receptor, Bioorg. Med. Chem. Lett., 2005, 15, 5022 CrossRef CAS; (b) S. Ansorge, U. Bank, K. Noordhoff, M. Täger and F. Striggow, Novel Alanyl-Amino Peptidase Inhibitors for Functionally Influencing Different Cells and Treating Immunological, Inflammatory, Neuronal, and Other Diseases, PCT Int. ApplWO2005037257A2, 2005 Search PubMed.
- E. Hayashi and S. Yamada, Pharmacological studies on surugatoxin, the toxic principle from Japanese ivory mollusc (Babylonia japonica), Br. J. Pharmacol., 1975, 53, 207 CrossRef CAS.
- See ref. 16 and (a) M. Rottmann, C. McNamara, B. K. S. Yeung, M. C. S. Lee, B. Zou, B. Russell, P. Seitz, D. M. Plouffe, N. V. Dharia and J. Tan, et al., Spiroindolones, a Potent Compound Class for the Treatment of Malaria, Science, 2010, 329, 1175 CrossRef CAS; (b) N. J. White, S. Pukrittayakamee, A. P. Phyo, R. Rueangweerayut, F. Nosten, P. Jittamala, A. Jeeyapant, J. P. Jain, G. Lefèvre and R. Li, et al., Spiroindolone KAE609 for Falciparum and Vivax Malaria, N. Engl. J. Med., 2014, 371, 403 CrossRef; (c) H. Turner, Spiroindolone NITD609 is a novel antimalarial drug that targets the P-type ATPase PfATP4, Future Med. Chem., 2016, 8, 227 CrossRef CAS; (d) T. D. Ashton, S. M. Devine, J. J. Möhrle, B. Laleu, J. N. Burrows, S. A. Charman, D. J. Creek and B. E. Sleebs, The Development Process for Discovery and Clinical Advancement of Modern Antimalarials, J. Med. Chem., 2019, 62, 10526 CrossRef CAS.
- H. Takada, N. Kumagai and M. Shibasaki, Stereoselective Total Synthesis of KAE609 via Direct Catalytic Asymmetric Alkynylation to Ketimine, Org. Lett., 2015, 17, 4762 CrossRef CAS.
- H. Zheng, X. Liu, C. Xu, Y. Xia, L. Lin and X. Feng, Regio- and Enantioselective Aza-Diels-Alder Reactions of 3-Vinylindoles: A Concise Synthesis of the Antimalarial Spiroindolone NITD609, Angew. Chem., Int. Ed., 2015, 54, 10958 CrossRef CAS.
- For piperazines see: (a) K. Ramakumar, T. Maji, J. J. Partridge and J. A. Tunge, Synthesis of Spirooxindoles via the tert-Amino Effect, Org. Lett., 2017, 19, 4014 CrossRef CAS; (b) L.-L. Wang, T. Jiang, P.-H. Li, R.-J. Sun and Z. Zuo, Asymmetric Syntheses of Spirooxindole-dihydroquinazolinones by Cyclization Reactions between N-substituted Anthranilamides and Isatins, Adv. Synth. Catal., 2018, 360, 4832 CrossRef CAS; (c) R. Ye and C. G. Yan, Construction of Spiro[indoline-3,3′-pyridazines] and Spiro[indene-2,3′-pyridazines] via TEMPO-Mediated Oxidative Aza-Diels-Alder Reactions, Eur. J. Org. Chem., 2019, 5882 CrossRef CAS. For morpholines see: (d) M. Montesinos-Magraner, C. Vila, R. Cantõn, G. Blay, I. Fernández, M. C. Muñoz and J. R. Pedro, Organocatalytic Asymmetric Addition of Naphthols and Electron-Rich Phenols to Isatin-Derived Ketimines: Highly Enantioselective Construction of Tetrasubstituted Stereocenters, Angew. Chem., Int. Ed., 2015, 54, 6320 CrossRef CAS; (e) P. W. Xu, C. Chen, J. K. Liu, Y. T. Song, F. Zhou, J. Yan and J. Zhou, One-Pot Sequential [3 + 3] Dipolar Cycloaddition of Aldehyde or Ketone and Hydroxylamine with Spirocyclopropyl Oxindole, J. Org. Chem., 2018, 83, 12763 CrossRef CAS; (f) S. Hajra, A. Hazra and S. K. Abu Saleh, One-Pot Synthesis of Enantiopure Spiro[3,4-dihydrobenzo[b,][1,4]oxazine-2,3′-oxindole] via Regio- and Stereoselective Tandem Ring Opening/Cyclization of Spiroaziridine Oxindoles with Bromophenols, J. Org. Chem., 2019, 84, 10412 CrossRef CAS. Urea: (g) M. Stucchi, G. Lesma, F. Meneghetti, G. Rainoldi, A. Sacchetti and A. Silvani, Organocatalytic Asymmetric Biginelli-like Reaction Involving Isatin, J. Org. Chem., 2016, 81, 1877 CrossRef CAS. Carbamate: (h) W. Guo, L. Li, Q. Ding, X. Lin, X. Liu, K. Wang, Y. Liu, H. Fan and C. Li, Synthesis of Chiral Trispirocyclic Oxindoles via Organic-Base/Au(I)-Catalyzed Sequential Reactions, ACS Catal., 2018, 8, 10180 CrossRef CAS. Other: (i) G. Zhu, W. Sun, C. Wu, G. Li, L. Hong and R. Wang, Base-Catalyzed Diastereoselective [3 + 3] Annulation of 3-Isothiocyanatooxindoles and Azomethine Imines, Org. Lett., 2013, 15, 4988 CrossRef CAS.
- X. N. Zhang, G. Q. Chen, X. Dong, Y. Wei and M. Shi, Phosphine-Catalyzed Asymmetric [4 + 2] Annulation of Vinyl Ketones with Oxindole-Derived α,β-Unsaturated Imines: Enantioselective Syntheses of 2′,3′-Dihydro-1′H-spiro[indoline-3,4′-pyridin]-2-ones, Adv. Synth. Catal., 2013, 355, 3351 CrossRef CAS.
- B. V. S. Reddy, M. Swain, S. M. Reddy, J. S. Yadav and B. Sridhar, Gold-Catalyzed 5-endo-dig Cyclization of 2-[(2-Aminophenyl)ethynyl]phenylamine with Ketones for the Synthesis of Spiroindolone and Indolo[3,2-c]quinolone Scaffolds, Eur. J. Org. Chem., 2014, 3313 CrossRef.
- S. Jayakumar, K. Louven, C. Strohmann and K. Kumar, A Tunable and Enantioselective Hetero-Diels-Alder Reaction Provides Access to Distinct Piperidinoyl Spirooxindoles, Angew. Chem., Int. Ed., 2017, 56, 15945 CrossRef CAS.
- (a) W. Cao, X. Liu, J. Guo, L. Lin and X. Feng, Asymmetric Tandem 1,5-Hydride Shift/Ring Closure for the Synthesis of Chiral Spirooxindole Tetrahydroquinolines, Chem. – Eur. J., 2015, 21, 1632 CrossRef CAS; (b) Z. Mao, F. Mo and X. Lin, Diastereo- and Enantioselective Assembly of Spirooxindole Tetrahydroquinoline Skeletons through Asymmetric Binary Acid Catalyzed Hydride Transfer-Cyclization, Synlett, 2016, 27, 546 CAS; (c) S. S. Li, S. Zhu, C. Chen, K. Duan, Q. Liu and J. Xiao, Hydride Transfer Involved Redox-Neutral Cascade Cyclizations for Construction of Spirocyclic Bisoxindoles Featuring a [3,4]-Fused Oxindole Moiety, Org. Lett., 2019, 21, 1058 CrossRef CAS.
- G. J. Mei, D. Li, G. X. Zhou, Q. Shi, Z. Cao and F. Shi, A catalytic asymmetric construction of a tetrahydroquinoline-based spirooxindole framework via a diastereo- and enantioselective decarboxylative [4 + 2] cycloaddition, Chem. Commun., 2017, 53, 10030 RSC.
- L. Hao, C. Chuen, R. Ganguly and Y. Chi, NHC-Catalyzed Ester Activation: Access to Sterically Congested Spirocyclic Oxindoles via Reaction of α-Aryl Esters and Unsaturated Imines, Synlett, 2013, 24, 1197 CrossRef CAS.
- D. Xie, L. Yang, Y. Lin, Z. Zhang, D. Chen, X. Zeng and G. Zhong, Rapid Access to Spirocylic Oxindoles: Application of Asymmetric N-Heterocyclic Carbene-Catalyzed [3 + 3] Cycloaddition of Imines to Oxindole-Derived Enals, Org. Lett., 2015, 17, 2318 CrossRef CAS.
- W. Q. Jia, H. M. Zhang, C. L. Zhang, Z. H. Gao and S. Ye, N-Heterocyclic, carbene-catalyzed [4 + 2] annulation of α,β-unsaturated carboxylic acids: enantioselective synthesis of dihydropyridinones and spirocyclic oxindolodihydropyridinones, Org. Chem. Front., 2016, 3, 77 RSC.
- J. Xu, S. Yuan and M. Miao, N-Heterocyclic, Carbene Catalyzed [4 + 2] Annulation Reactions with in Situ Generated Heterocyclic ortho-Quinodimethanes, Org. Lett., 2016, 18, 3822 CrossRef CAS.
- C. He, Z. Li, J. Xu and H. Ren, Asymmetric Synthesis of Spirocyclic Oxindole δ-Lactams via NHC-Catalyzed Formal [2 + 4] Annulation of Aliphatic Aldehydes with Oxindole-Derived α,β-Unsaturated Ketimines, J. Org. Chem., 2019, 84, 12177 CrossRef CAS.
- (a) Q. Liu, X. Y. Chen, S. Li, K. Rissanen and D. Enders, N-Heterocyclic, Carbene Catalyzed Asymmetric Synthesis of Pentacyclic Spirooxindoles via [3 + 3] Annulations of Isatin-Derived Enals and Cyclic N-Sulfonyl Ketimines, Adv. Synth. Catal., 2019, 361, 1991 CrossRef CAS. For a further example see: (b) H. Lu, C. Y. Tan, H. X. Zhang, J. L. Zhang, J. Y. Liu, H. Y. Li and P. F. Xu, Participation of β-Ketothioamides in N-Heterocyclic Carbene-Catalyzed [3 + 3] Spiroannulation: Asymmetric Synthesis of Functionalized Spiro-piperidinone Derivatives, J. Org. Chem., 2018, 83, 15245 CrossRef CAS.
- F. Shi, G.-J. Xing, R.-Y. Zhu, W. Tan and S. Tu, A Catalytic Asymmetric Isatin-Involved Povarov Reaction: Diastereo- and Enantioselective Construction of Spiro[indolin-3,2′-quinoline] Scaffold, Org. Lett., 2013, 15, 128 CrossRef CAS.
- For other reports of the use of the Povarov reaction to spiropiperidines see: (a) H. Gao, J. Sun and C. G. Yan, Povarov Reaction of β-Enamino Esters and Isatin-3-imines for Diastereoselective Synthesis of Spiro[indoline-3,2′-quinolines], Synthesis, 2014, 46, 489 Search PubMed; (b) G. Bianchini, P. Ribelles, D. Becerra, M. T. Ramos and J. C. Menéndez, Efficient synthesis of 2-acylquinolines based on an aza-vinylogous Povarov reaction, Org. Chem. Front., 2016, 3, 412 RSC.
- (a) H. H. Zhang, X. X. Sun, J. Liang, Y. M. Wang, C. C. Zhao and F. Shi, Catalytic asymmetric Povarov reaction of isatin-derived 2-azadienes with 3-vinylindoles, Org. Biomol. Chem., 2014, 12, 9539 RSC. Also see: (b) Z. Mao, F. Mo and X. Lin, Diastereo- and Enantioselective Assembly of Spirooxindole Tetrahydroquinoline Skeletons through Asymmetric Binary Acid Catalyzed Hydride Transfer-Cyclization, Synlett, 2016, 27, 546 CAS.
- H. Mao, A. Lin, Y. Tang, Y. Shi, H. Hu, Y. Cheng and C. Zhu, Organocatalytic oxa/aza-Michael-Michael Cascade Strategy for the Construction of Spiro [Chroman/Tetrahydroquinoline-3,3′-oxindole] Scaffolds, Org. Lett., 2013, 15, 4062 CrossRef CAS.
- (a) Y. You, B. D. Cui, M. Q. Zhou, J. Zuo, J. Q. Zhao, X. Y. Xu, X. M. Zhang and W. C. Yuan, Organocatalytic Asymmetric Michael/Friedel-Crafts Cascade Reaction of 3-Pyrrolyl-oxindoles and α,β-Unsaturated Aldehydes for the Construction of Chiral Spiro[5,6-dihydropyrido[1,2-a]pyrrole-3,3′-oxindoles], J. Org. Chem., 2015, 80, 5951 CrossRef CAS. Also see: (b) W. T. Fan, N. K. Li, L. Xu, C. Qiao and X. W. Wang, Organo-Catalyzed Asymmetric Michael-Hemiketalization-Oxa-Pictet-Spengler Cyclization for Bridged and Spiro Heterocyclic Skeletons: Oxocarbenium Ion as a Key Intermediate, Org. Lett., 2017, 19, 6626 CrossRef CAS; (c) Z. Gao, J. Zhang, H. Yang and G. Jiang, Brønsted Acid-Promoted Friedel-Crafts Alkylation/Cyclization of (7-Hydroxynaphthalenyl)pyrrole or (2-Hydroxyphenyl)pyrroles with Isatins for the Construction of Pyrrolospirooxindole Derivatives, J. Org. Chem., 2018, 83, 11407 CrossRef CAS; (d) N. K. Li, W. T. Fan, J. Q. Zhang, B. B. Sun, J. B. Chen and X. W. Wang, Diastereodivergent synthesis of bispirooxindoles via asymmetric Friedel-Crafts/aldol cascade reaction: co-catalyst effects on diastereoselective outcomes, Chem. Commun., 2018, 54, 2260 RSC.
- (a) W. Yang and D.-M. Du, Cinchona-based squaramide-catalysed cascade aza-Michael-Michael addition: enantioselective construction of functionalized spirooxindole tetrahydroquinolines, Chem. Commun., 2013, 49, 8842 RSC; (b) Y. M. Huang, C. W. Zheng and G. Zhao, Organocatalyzed aza-Michael-Michael cascade reactions to construct spirooxindole tetrahydroquinolines with all-carbon chiral centers, RSC Adv., 2013, 3, 16999 RSC; (c) Y. Tan, E. L. Feng, Q. S. Sun, H. Lin, X. Sun, G. Q. Lin and X. W. Sun, Enantioselective synthesis of spirooxindole benzoquinolizines via organo-catalyzed cascade reactions, Org. Biomol. Chem., 2017, 15, 778 RSC; (d) W. Dai, H. Lu, X. Li, F. Shi and S. J. Tu, Diastereo- and Enantioselective Construction of a Bispirooxindole Scaffold Containing a Tetrahydro-β-carboline Moiety through an Organocatalytic Asymmetric Cascade Reaction, Chem. – Eur. J., 2014, 20, 11382 CrossRef CAS; (e) Y. Liu, J. Xue, Z. Sun, D. Liu, Y. Xing and Y. Li, Substituent-Controlled Selective Synthesis of Spirooxindoles and Oxazolyloxindoles via Two Tandem Reactions, Asian J. Org. Chem., 2016, 5, 43 CrossRef CAS; (f) Q. Zhao, C. Peng, H. Huang, S. J. Liu, Y. J. Zhong, W. Huang, G. He and B. Han, Asymmetric synthesis of tetrahydroisoquinoline-fused spirooxindoles as Ras-GTP inhibitors that inhibit colon adenocarcinoma cell proliferation and invasion, Chem. Commun., 2018, 54, 8359 RSC.
- M. C. Yang, C. Peng, H. Huang, L. Yang, X. H. He, W. Huang, H. L. Cui, G. He and B. Han, Organocatalytic Asymmetric Synthesis of Spiro-oxindole Piperidine Derivatives That Reduce Cancer Cell Proliferation by Inhibiting MDM2-p53 Interaction, Org. Lett., 2017, 19, 6752 CrossRef CAS.
- (a) Y. Liao, M. Bai, S. Yu, M. Zhang, F. Hu, X. Xu, W. Yuan and X. Zhang, Construction of Novel Tetrahydro-β-carboline-1-thione Spirooxindoles by Brønsted Acid Mediated Formal [3 + 3] Cyclization of 3-Indolylmethanols with 3-Isothiocyanato Oxindoles, J. Heterocycl. Chem., 2017, 54, 1311 CrossRef CAS; (b) S. Kawamura, K. Dosei, E. Valverde, K. Ushida and M. Sodeoka, N-Heterocycle-Forming, Amino/Carboperfluoroalkylations of Aminoalkenes by Using Perfluoro Acid Anhydrides: Mechanistic Studies and Applications Directed Toward Perfluoroalkylated Compound Libraries, J. Org. Chem., 2017, 82, 12539 CrossRef CAS; (c) L. Qi, H. Hou, F. Ling, L. Fang, W. Luo and W. Zhong, Cinchona Alkaloid Thiourea Catalyzed Asymmetric Synthesis and Anticancer Activity Evaluation of Tetrahydro-β-spirooxindoles, Heterocycles, 2018, 96, 1119 CrossRef CAS; (d) G. Zhan, M. L. Shi, W. J. Lin, Q. Ouyang, W. Du and Y. C. Chen, Direct Asymmetric Aza-Vinylogous-Type Michael Additions of Nitrones from Isatins to Nitroalkenes, Chem. – Eur. J., 2017, 23, 6286 CrossRef CAS.
- For examples see: (a) S. J. Kalita, B. Das and D. C. Deka, A Quick, Simple and Clean Synthesis of Spiro(indoline-3,4′-pyrazolo[4′, 3′:5,6]pyrido[2, 3-d]pyrimidines) in Water through a Novel One-Pot Multicomponent Reaction, ChemistrySelect, 2017, 2, 5701 CrossRef CAS; (b) A. Mondal, B. Naskar, S. Goswami, C. Prodhan, K. Chaudhuri and C. Mukhopadhyay, I2 catalyzed access of spiro[indoline-3,4′-pyridine] appended amine dyad: new ON-OFF chemosensors for Cu2+ and imaging in living cells, Org. Biomol. Chem., 2018, 16, 302 RSC; (c) M. Esmaeilpour, A. R. Sardarian and H. Firouzabadi, Theophylline Supported on Modified Silica-Coated Magnetite Nanoparticles as a Novel, Efficient, Reusable Catalyst in Green One-Pot Synthesis of Spirooxindoles and Phenazines, ChemistrySelect, 2018, 3, 9236 CrossRef CAS; (d) Q. Niu, J. Xi, L. Li, L. Li, C. Pan, M. Lan and L. Rong, Isatins 3-C annulation vs ring-opening: Two different pathways for synthesis of spiro compounds via multicomponent reactions, Tetrahedron Lett., 2019, 60, 151181 CrossRef CAS.
- (a) Q. N. Zhu, Y. C. Zhang, M. M. Xu, X. X. Sun, X. Yang and F. Shi, Enantioselective Construction of Tetrahydroquinolin-5-one-Based Spirooxindole Scaffold via an Organocatalytic Asymmetric Multicomponent [3 + 3] Cyclization, J. Org. Chem., 2016, 81, 7898 CrossRef CAS; (b) P. Zhou, B. Hu, L. Lu, R. Huang and F. Yu, One-Pot Synthesis of Functionalised 4-Spiro-1,4-Dihydropyridines Via [1 + 2 + 1 + 2]-Cyclisation, J. Chem. Res., 2017, 41, 513 CrossRef CAS.
- Y. H. Wang, J. S. Tian, P. W. Tan, Q. Cao, X. X. Zhang, Z. Y. Cao, F. Zhou, X. Wang and J. Zhou, Regiodivergent Intramolecular Nucleophilic Addition of Ketimines for the Diverse Synthesis of Azacycles, Angew. Chem., Int. Ed., 2020, 59, 1634 CrossRef CAS.
- Y. Bin Shen, L. X. Wang, Y. M. Sun, F. Y. Dong, L. Yu, Q. Liu and J. Xiao, Hexafluoroisopropanol-Mediated Redox-Neutral α-C(sp3)-H Functionalization of Cyclic Amines via Hydride Transfer, J. Org. Chem., 2020, 85, 1915 CrossRef.
- M. Holmquist, G. Blay, M. C. Muñoz and J. R. Pedro, Aza-Henry Reaction of Isatin Ketimines with Methyl 4-Nitrobutyrate en Route to Spiro[piperidine-3,3′-oxindoles], Adv. Synth. Catal., 2015, 357, 3857 CrossRef CAS.
- (a) Y. Zhu, Y. Li, Q. Meng and X. Li, An organocatalytic enantioselective vinylogous Mannich reaction of α,α-dicyanoolefins with isatin N-Boc ketimines, Org. Chem. Front., 2016, 3, 709 RSC; (b) S. Nakamura, K. Matsuzaka, T. Hatanaka and Y. Funahashi, Enantioselective Vinylogous Mannich Reaction of Acyclic Vinylketene Silyl Acetals with Ketimines Using Chiral Bis(imidazoline)-Cu(II) Catalysts, Org. Lett., 2020, 22, 2868 CrossRef CAS. For a phase transfer catalysed version see: (c) C. Cheng, X. Lu, L. Ge, J. Chen, W. Cao, X. Wu and G. Zhao, Effective asymmetric vinylogous Mannich reaction of isatin imines with α,α-dicyanoolefins in the presence of a simple chiral amide phosphonium bifunctional phase transfer catalyst, Org. Chem. Front., 2017, 4, 101 RSC.
- Z. Chang, C. Ye, J. Fu, P. Chigumbu, X. Zeng, Y. Wang, C. Jiang and X. Han, Enantioselective Synthesis of Oxindole-Derived α-Aryl-β-Amino Acid Derivatives and δ-Lactams with Homophthalic Anhydrides, Adv. Synth. Catal., 2019, 361, 5516 CrossRef CAS.
- K. R. Senwar, P. Sharma, T. S. Reddy, M. K. Jeengar, V. L. Nayak, V. G. M. Naidu, A. Kamal and N. Shankaraiah, Spirooxindole-derived morpholine-fused-1,2,3-triazoles: Design, synthesis, cytotoxicity and apoptosis inducing studies, Eur. J. Med. Chem., 2015, 102, 413 CrossRef CAS.
- G. Schwertz, M. C. Witschel, M. Rottmann, U. Leartsakulpanich, P. Chitnumsub, A. Jaruwat, W. Amornwatcharapong, W. Ittarat, A. Schäfer and R. A. Aponte, et al., Potent Inhibitors of Plasmodial Serine Hydroxymethyltransferase (SHMT) Featuring a Spirocyclic Scaffold, ChemMedChem, 2018, 13, 931 CrossRef CAS.
- (a) P. R. Eastwood, J. Gonzalez Rodriguez and V. Giulio Matassa, New Substituted Indolin2-one. Derivatives and Their Use as P39 Mitogen-Activated Kinase-Inhibitors, PCT Int. ApplWO2009132774A1, 2009 Search PubMed; (b) P. Eastwood, J. González, E. Gómez, F. Caturla, N. Aguilar, M. Mir, J. Aiguadé, V. Matassa, C. Balagué and A. Orellana, et al., Indolin-2-one p38α inhibitors III: Bioisosteric amide replacement, Bioorg. Med. Chem. Lett., 2011, 21, 6253 CrossRef CAS.
- S. J. Han, F. Vogt, S. Krishnan, J. A. May, M. Gatti, S. C. Virgil and B. M. Stoltz, A Diastereodivergent Synthetic Strategy for the Syntheses of Communesin F and Perophoramidine, Org. Lett., 2014, 16, 3316 CrossRef CAS.
- J. Z. Huang, C. L. Zhang, Y. F. Zhu, L. L. Li, D. F. Chen, Z. Y. Han and L. Z. Gong, Organocatalytic Highly Enantioselective Substitution of 3-(1-Tosylalkyl)indoles with Oxindoles Enables the First Total Synthesis of (+)-Trigolutes B, Chem. – Eur. J., 2015, 21, 8389 CrossRef CAS.
- For related total synthesis: B. N. Reddy and C. V. Ramana, A concise approach for central core of trigolutes: Total synthesis of trigolute B and 3-epi-trigolute B and analogues, Tetrahedron, 2017, 73, 888 CrossRef CAS.
- S. Zhao, J. B. Lin, Y. Y. Zhao, Y. M. Liang and P. F. Xu, Hydrogen-Bond-Directed Formal [5 + 1] Annulations of Oxindoles with Ester-Linked Bisenones: Facile Access to Chiral Spirooxindole δ-Lactones, Org. Lett., 2014, 16, 1802 CrossRef CAS.
- (a) K. Zhao, Y. Zhi, T. Shu, A. Valkonen, K. Rissanen and D. Enders, Organocatalytic Domino Oxa-Michael/1,6-Addition Reactions: Asymmetric Synthesis of Chromans Bearing Oxindole Scaffolds, Angew. Chem., Int. Ed., 2016, 55, 12104 CrossRef CAS. For a recent diastereoselective oxa-Michael reaction see: (b) M. Pasha, M. Sohail and F. Tanaka, Intramolecular Oxa-Michael Reactions of Aldols Generated from Enones and Isatins to Afford Spirooxindole Tetrahydropyrans, Heterocycles, 2020, 101, 339 CrossRef CAS.
- (a) L. Zhu, Q. Chen, D. Shen, W. Zhang, C. Shen, X. Zeng and G. Zhong, Enantioselective Construction of Spirocyclic Oxindole Derivatives with Multiple Stereocenters via an Organocatalytic Michael/Aldol/Hemiacetalization Cascade Reaction, Org. Lett., 2016, 18, 2387 CrossRef CAS. Also see: (b) N. Kumarswamyreddy and V. Kesavan, Enantioselective Synthesis of Dihydrospiro[indoline-3,4′-pyrano[2,3-c]pyrazole] Derivatives via Michael/Hemiketalization Reaction, Org. Lett., 2016, 18, 1354 CrossRef CAS; (c) L. Qiao, Z. W. Duan, X. N. Wu, D. H. Li, Q. Q. Gu and Y. K. Liu, Organocatalytic Diversity-Oriented Asymmetric Synthesis of Structurally and Stereochemically Complex Heterocycles, Org. Lett., 2018, 20, 1630 CrossRef CAS.
- (a) J.-L. Han and C.-H. Chang, An asymmetric assembly of spirooxindole dihydropyranones through a direct enantioselective organocatalytic vinylogous aldol-cyclization cascade reaction of 3-alkylidene oxindoles with isatins, Chem. Commun., 2016, 52, 2322 RSC; (b) J. L. Han, Y. Da Tsai and C. H. Chang, Asymmetric Synthesis of Spirooxindole δ-Lactones with Vicinal Tertiary and Quaternary Stereocenters via Regio-, Diastereo-, and Enantioselective Organocatalytic Vinylogous Aldol-cyclization Cascade Reaction, Adv. Synth. Catal., 2017, 359, 4043 CrossRef CAS.
- J. L. Hu, F. Sha, Q. Li and X. Y. Wu, Highly enantioselective Michael/cyclization tandem reaction between dimedone and isatylidene malononitriles, Tetrahedron, 2018, 74, 7148 CrossRef CAS.
- For a further enantioselective example of a related scaffold: (a) S. Konda, S. Jakkampudi, H. D. Arman and J. C. G. Zhao, Enantioselective synthesis of spiro[4H-pyran-3,3′-oxindole] derivatives catalyzed by cinchona alkaloid thioureas: Significant water effects on the enantioselectivity, Synth. Commun., 2019, 49, 2971 CAS. For racemic synthesis of related scaffolds see selected examples: (b) M. Zhang, W. Yang, M. Qian, T. Zhao, L. Yang and C. Zhu, Iodine-promoted three-component reaction for the synthesis of spirooxindoles, Tetrahedron, 2018, 74, 955 CrossRef CAS; (c) A. S. Hussen, A. P. Pandey and A. Sharma, Mechanochemical- (Hand-Grinding-) Assisted Domino Synthesis of Fused Pyran-Spirooxindoles under Solvent- and Catalyst-Free Condition, ChemistrySelect, 2018, 3, 11505 CrossRef CAS; (d) D. Y. Seo, S. Lee, B. K. Min and J. N. Kim, Synthesis of Spirooxindoles Bearing Benzopyrano[2,3-b]indole Moiety by NBS-Mediated Oxidative Coupling Reaction, Bull. Korean Chem. Soc., 2018, 39, 1011 CrossRef CAS Also see ref. 371.
- (a) M. Liu, X. Zhang, X. Huang, G. Dhawan, J. Evans, M. Kaur, J. P. Jasinski and W. Zhang, Recyclable Organocatalyst for One-Pot Asymmetric Synthesis of Dihydrofuranone and Tetrahydropyranone Spirooxindoles, Eur. J. Org. Chem., 2019, 150 CrossRef CAS; (b) Z. H. Wang, X. Y. Zhang, C. W. Lei, J. Q. Zhao, Y. You and W. C. Yuan, Highly enantioselective sequential vinylogous aldol reaction/transesterification of methyl-substituted olefinic butyrolactones with isatins for the construction of chiral spirocyclic oxindole-dihydropyranones, Chem. Commun., 2019, 55, 9327 RSC.
- (a) H. L. Cui and F. Tanaka, Catalytic Enantioselective Formal Hetero-Diels-Alder Reactions of Enones with Isatins to Give Spirooxindole Tetrahydropyranones, Chem. – Eur. J., 2013, 19, 6213 CrossRef CAS; (b) D. B. Ramachary, M. Shiva Prasad, S. Vijaya Laxmi and R. Madhavachary, Asymmetric synthesis of drug-like spiro[chroman-3,3′-indolin]-2′-ones through aminal-catalysis, Org. Biomol. Chem., 2014, 12, 574 RSC; (c) M. Chennapuram, I. A. Owolabi, C. Seki, Y. Okuyama, E. Kwon, K. Uwai, M. Tokiwa, M. Takeshita and H. Nakano, New Hybrid-type Squaramide-Fused Amino Alcohol Organocatalyst for Enantioselective Domino Michael Addition/Cyclization Reaction of Oxoindolines with Cyclic 1,3-Diketones, ACS Omega, 2018, 3, 11718 CrossRef CAS.
- (a) Y. Bin Shen, S. S. Li, X. Liu, L. Yu, Q. Liu and J. Xiao, Dearomative [4 + 2] Cycloaddition of Oxindole-Embedded ortho-Quinone Methides with 2,5-Dialkylfurans, Adv. Synth. Catal., 2019, 361, 1453 CrossRef; (b) Y. Bin Shen, S. S. Li, X. Liu, L. Yu, Y. M. Sun, Q. Liu and J. Xiao, Formal [4 + 2] Annulation of Oxindole-Embedded ortho-Quinone Methides with 1,3-Dicarbonyls: Synthesis of Spiro[Chromen-4,3′-Oxindole] Scaffolds, J. Org. Chem., 2019, 84, 3990 CrossRef.
- H. Shi, L. Wang, S. S. Li, Y. Liu and L. Xu, Divergent syntheses of spirooxindoles from oxindole-embedded four-membered synthon via cycloaddition reactions, Org. Chem. Front., 2020, 7, 747 RSC.
- (a) Y. Jiang, S. W. Yu, Y. Yang, Y. Le Liu, X. Y. Xu, X. M. Zhang and W. C. Yuan, Synthesis of polycyclic spirooxindoles via an asymmetric catalytic one-pot stepwise Aldol/chloroetherification/aromatization procedure, Org. Biomol. Chem., 2018, 16, 6647 RSC; (b) M. Luo, X. Zhu, R. Liu, S. Yu and W. Wei, FeCl3-Promoted Annulation of 2-Haloindoles: Switchable Synthesis of Spirooxindole-chromeno[2,3-b]indoles and Spirooxindole-chromeno[3,2-b]indoles, J. Org. Chem., 2020, 85, 3638 CrossRef CAS.
- (a) J. Peng, G. Y. Ran, W. Du and Y. C. Chen, Divergent Cyclization Reactions of Morita-Baylis-Hillman Carbonates of 2-Cyclohexenone and Isatylidene Malononitriles, Org. Lett., 2015, 17, 4490 CrossRef CAS; (b) A. Yadav, J. Banerjee, S. K. Arupula, S. M. Mobin and S. Samanta, Lewis-Base-Catalyzed Domino Reaction of Morita-Baylis-Hillman Carbonates of Isatins with Enolizable Cyclic Carbonyl Compounds: Stereoselective Access to Spirooxindole-Pyrans, Asian J. Org. Chem., 2018, 7, 1595 CrossRef CAS.
- (a) Y. Que, T. Li, C. Yu, X. S. Wang and C. Yao, Enantioselective Assembly of Spirocyclic Oxindole-dihydropyranones through NHC-Catalyzed Cascade Reaction of Isatins with N-Hydroxybenzotriazole Esters of α,β-Unsaturated Carboxylic Acid, J. Org. Chem., 2015, 80, 3289 CrossRef CAS. Also see: (b) J. T. Cheng, X. Y. Chen, Z. H. Gao and S. Ye, N-Heterocyclic Carbene Catalyzed Generation and [4 + 2] Annulation of Unsubstituted Dienolate - Enantioselective Synthesis of Spirocyclic Oxindolodihydropyranones, Eur. J. Org. Chem., 2015, 1047 CrossRef CAS.
- W. Zhang, J. Xu, J. Cao, C. Fang, J. Zhu, T. Lu and D. Du, N-heterocyclic carbene-mediated formal [3 + 3] annulation of isatin-derived α, β-unsaturated acids: Access to functionalized 3,4′-spirooxindole δ-lactones, Tetrahedron, 2017, 73, 3249 CrossRef CAS.
- J.-B. Lin, X.-N. Cheng, X.-D. Tian, G.-Q. Xu, Y.-C. Luo and P.-F. Xu, A C1-symmetric N-heterocyclic carbene catalysed oxidative spiroannulation of isatin-derived enals: highly enantioselective synthesis of spirooxindole δ-lactones, RSC Adv., 2018, 8, 15444 RSC.
- Z. J. Zhang, L. Zhang, R. L. Geng, J. Song, X. H. Chen and L. Z. Gong, N-Heterocyclic, Carbene/Copper Cooperative Catalysis for the Asymmetric Synthesis of Spirooxindoles, Angew. Chem., Int. Ed., 2019, 58, 12190 CrossRef CAS.
- J. Zheng, L. Lin, K. Fu, Y. Zhang, X. Liu and X. Feng, Chem. – Eur. J., 2014, 20, 14493–14498 CrossRef CAS.
- J. Wang, Q. Zhang, Y. Li, X. Liu, X. Li and J.-P. Cheng, Bi(OAc)3/chiral phosphoric acid catalyzed enantioselective allylation of isatins, Chem. Commun., 2020, 56, 261 RSC.
- R. Sato, T. Tosaka, H. Masu and T. Arai, Catalytic Asymmetric Synthesis of Chiral Bis(indolyl)methanes Using a Ts-PyBidine-Nickel Complex, J. Org. Chem., 2019, 84, 14248 CrossRef CAS.
- B. V. Subba Reddy, V. Swathi, M. Swain, M. P. Bhadra, B. Sridhar, D. Satyanarayana and B. Jagadeesh, Stereoselective Synthesis of Spiro[tetrahydropyran-3,3′-oxindole] Derivatives Employing Prins Cascade Strategy, Org. Lett., 2014, 16, 6267 CrossRef CAS.
- S. Jia, Y. Lei, L. Song, A. G. Krishna Reddy, D. Xing and W. Hu, Diastereoselective Intramolecular Aldol-Type Trapping of Zwitterionic Intermediates by Ketones for the Synthesis of Spiro[chroman-4,3′-oxindole] Derivatives, Adv. Synth. Catal., 2017, 359, 58 CrossRef CAS.
- N. Probst, G. Grelier, N. E. Ghermani, V. Gandon, M. Alami and S. Messaoudi, Intramolecular Pd-Catalyzed Anomeric C(sp3)-H Activation of Glycosyl Carboxamides, Org. Lett., 2017, 19, 5038 CrossRef CAS.
- S. R. Kandimalla and G. Sabitha, Diversity-Oriented Synthesis of Oxacyclic Spirooxindole Derivatives through Ring-Closing Enyne Metathesis and Intramolecular Pauson-Khand (2 + 2 + 1) Cyclization of Oxindole Enynes, Adv. Synth. Catal., 2017, 359, 3444 CrossRef CAS.
- See ref. 314b and (a) J. Qu, L. Fang, X. D. Ren, Y. Liu, S. S. Yu, L. Li, X. Q. Bao, D. Zhang, Y. Li and S. G. Ma, Bisindole Alkaloids with Neural Anti-inflammatory Activity from Gelsemium elegans, J. Nat. Prod., 2013, 76, 2203 CrossRef CAS; (b) W. Zhang, W. Xu, G. Y. Wang, X. Y. Gong, N. P. Li, L. Wang and W. C. Ye, Gelsekoumidines A and B: Two Pairs of Atropisomeric Bisindole Alkaloids from the Roots of Gelsemium elegans, Org. Lett., 2017, 19, 5194 CrossRef CAS; (c) N. P. Li, M. Liu, X. J. Huang, X. Y. Gong, W. Zhang, M. J. Cheng, W. C. Ye, L. Wang and A.-E. Gelsecorydines, Five Gelsedine-Corynanthe-Type Bisindole Alkaloids from the Fruits of Gelsemium elegans, J. Org. Chem., 2018, 83, 5707 CrossRef CAS.
- (a) O.-G. Berge, A. Claesson and B.-M. Swahn, New Compounds, PCT Int. ApplWO2001005790A1, 2001 Search PubMed; (b) J.-J. Liu, J. W. Tilley and Z. Zhang, 3,3-Spiroindolinone Derivatives, PCT Int. ApplWO2008141917A1, 2008 Search PubMed.
- S. Pellegrino, M. Ruscica, P. Magni, G. Vistoli and M. L. Gelmi, Antiproliferative activity on human prostate carcinoma cell lines of new peptidomimetics containing the spiroazepinoindolinone scaffold, Bioorg. Med. Chem., 2013, 21, 5470 CrossRef CAS.
- (a) Y. Liu, Z. Sun, S. Li, K. Xiang, Y. Zhang and Y. Li, Mg(ClO4)2-promoted [4 + 3] cycloaddition of oxindole derivatives with conjugated dienes: concise synthesis of spirocycloheptane oxindole derivatives, RSC Adv., 2016, 6, 26954 RSC; (b) Z. J. Jia, G. Shan, C. G. Daniliuc, A. P. Antonchick and H. Waldmann, Enantioselective Synthesis of the Spirotropanyl Oxindole Scaffold through Bimetallic Relay Catalysis, Angew. Chem., Int. Ed., 2018, 57, 14493 CrossRef CAS; (c) M. Balha and S. C. Pan, Organocatalytic Asymmetric Synthesis of Bridged Acetals with Spirooxindole Skeleton, J. Org. Chem., 2018, 83, 14703 CrossRef CAS; (d) B. Hu, X. Zhang, Y. Mo, J. Li, L. Lin, X. Liu and X. Feng, Catalytic Asymmetric Tandem Cycloisomerization/[5 + 2] Cycloaddition Reaction of N-Aryl Nitrone Alkynes with Methyleneindolinones, Org. Lett., 2020, 22, 1034 CrossRef CAS.
- (a) S. Diethelm and E. M. Carreira, Total Synthesis of (±)-Gelsemoxonine, J. Am. Chem. Soc., 2013, 135, 8500 CrossRef CAS; (b) S. Diethelm and E. M. Carreira, Total Synthesis of Gelsemoxonine through a Spirocyclopropane Isoxazolidine Ring Contraction, J. Am. Chem. Soc., 2015, 137, 6084 CrossRef CAS.
- E. T. Newcomb, P. C. Knutson, B. A. Pedersen and E. M. Ferreira, Total Synthesis of Gelsenicine via a Catalyzed Cycloisomerization Strategy, J. Am. Chem. Soc., 2016, 138, 108 CrossRef CAS.
- (a) T. Harada, J. Shimokawa and T. Fukuyama, Unified Total Synthesis of Five Gelsedine-Type Alkaloids: (−)-Gelsenicine, (−)-Gelsedine, (−)-Gelsedilam, (−)-14-Hydroxygelsenicine, and (−)-14,15-Dihydroxygelsenicine, Org. Lett., 2016, 18, 4622 CrossRef CAS; (b) P. Wang, Y. Gao and D. Ma, Divergent Entry to Gelsedine-Type Alkaloids: Total Syntheses of (−)-Gelsedilam, (−)-Gelsenicine, (−)-Gelsedine, and (−)-Gelsemoxonine, J. Am. Chem. Soc., 2018, 140, 11608 CrossRef CAS.
- A. Saito, N. Kogure, M. Kitajima and H. Takayama, Total Synthesis of (−)-14-Hydroxygelsenicine and Six Biogenetically Related Gelsemium Alkaloids, Org. Lett., 2019, 21, 7134 CrossRef CAS.
- (a) L. Wang, S. Li, M. Blümel, A. R. Philipps, A. Wang, R. Puttreddy, K. Rissanen and D. Enders, Asymmetric Synthesis of Spirobenzazepinones with Atroposelectivity and Spiro-1,2-Diazepinones by NHC-Catalyzed [3 + 4] Annulation Reactions, Angew. Chem., Int. Ed., 2016, 55, 11110 CrossRef CAS. For a mechanistic study see: (b) Y. Li and Z. Zhang, Mechanisms and Stereoselectivities of NHC-Catalyzed [3 + 4] Cycloaddition Reaction between Isatin-Derived Enal and N-(ortho-Chloromethyl)aryl Amide, Eur. J. Org. Chem., 2019, 2989 CrossRef CAS.
- W. Li, H. Yuan, Z. Liu, Z. Zhang, Y. Cheng and P. Li, NHC-Catalyzed Enantioselective [4 + 3] Cycloaddition of Ortho-Hydroxyphenyl Substituted Para-Quinone Methides with Isatin-Derived Enals, Adv. Synth. Catal., 2018, 360, 2460 CrossRef CAS.
- Q. Liu, S. Li, X. Y. Chen, K. Rissanen and D. Enders, Asymmetric Synthesis of Spiro-oxindole-ε-lactones through N-Heterocyclic Carbene Catalysis, Org. Lett., 2018, 20, 3622 CrossRef CAS.
- Z.-H. Gao, K.-Q. Chen, Y. Zhang, L.-M. Kong, Y. Li and S. Ye, Enantioselective N-Heterocyclic Carbene-Catalyzed Synthesis of Spirocyclic Oxindole-benzofuroazepinones, J. Org. Chem., 2018, 83, 15225 CrossRef CAS.
- D. Liu, Z. Hu, Y. Zhang, M. Gong, Z. Fu and W. Huang, Access to Enantioenriched Spiro-ε-Lactam Oxindoles by an N-Heterocyclic Carbene-Catalyzed [4 + 3] Annulation of Flexible Oxotryptamines with Enals, Chem. – Eur. J., 2019, 25, 11223 CAS.
- G. Zhan, M.-L. Shi, Q. He, W. Du and Y.-C. Chen, [4 + 3] Cycloadditions with Bromo-Substituted Morita-Baylis-Hillman Adducts of Isatins and N-(ortho-Chloromethyl)aryl Amides, Org. Lett., 2015, 17, 4750 CrossRef CAS.
- J. Y. Liu, H. Lu, C. G. Li, Y. M. Liang and P. F. Xu, Lewis Base and Brønsted Base Dual-Catalyzed Formal [4 + 3] Cyclo-addition Reaction: Synthesis of Aza-Spirocycloheptane Oxindole, Synlett, 2016, 27, 1287 CrossRef CAS.
- J.-Y. Du, Y.-H. Ma, F.-X. Meng, R.-R. Zhang, R.-N. Wang, H.-L. Shi, Q. Wang, Y.-X. Fan, H.-L. Huang and J.-C. Cui, et al., Lewis Base-Catalyzed [4 + 3] Annulation of ortho-Quinone Methides and MBH Carbonates: Synthesis of Functionalized Benzo[b]oxepines Bearing Oxindole Scaffolds, Org. Lett., 2019, 21, 465 CrossRef CAS.
- Z. Chen, Z. Chen, Z. Yang, L. Guo, W. Du and Y. Chen, Cooperative Tertiary Amine/Chiral Iridium Complex Catalyzed Asymmetric [4 + 3] and [3 + 3] Annulation Reactions, Angew. Chem., Int. Ed., 2019, 58, 15021 CrossRef CAS.
- F. Zhou, G. J. Cheng, W. Yang, Y. Long, S. Zhang, Y. D. Wu, X. Zhang and Q. Cai, Enantioselective Formation of Cyano-Bearing All-Carbon Quaternary Stereocenters: Desymmetrization by Copper-Catalyzed N-Arylation, Angew. Chem., Int. Ed., 2014, 53, 9555 CrossRef CAS.
- K. L. Ivanov, A. A. Kravtsova, E. A. Kirillova, M. Y. Melnikov and E. M. Budynina, Domino Michael/aza-Wittig reaction in the diastereoselective construction of spiro[azepane-4,3′-oxindoles], Tetrahedron Lett., 2019, 60, 1952 CrossRef CAS.
- W. Ren, Q.-M. Zuo, Y.-N. Niu and S.-D. Yang, Palladium-NHC-Catalyzed Allylic Alkylation of Pronucleophiles with Alkynes, Org. Lett., 2019, 21, 7956 CrossRef CAS.
- (a) B. Niu, X. Y. Wu, Y. Wei and M. Shi, Palladium-Catalyzed Diastereoselective Formal [5 + 3] Cycloaddition for the Construction of Spirooxindoles Fused with an Eight-Membered Ring, Org. Lett., 2019, 21, 4859 CrossRef CAS; (b) H. Zhao, L. Wang, J. Guo, W. Ding, X. Song, H. Wu, Z. Tang, X. Fan and X. Bi, Formal [5 + 3] Cycloaddition of Vinylethylene Carbonates with Isatin-Based α-(Trifluoromethyl)imines for Diastereoselective Synthesis of Medium-Heterocycle-Fused Spirooxindoles, Adv. Synth. Catal., 2019, 361, 4761–4771 CrossRef CAS; (c) H. Zhao, L. Wang, J. Guo, W. Ding, J. Du and N. Feng, Eight-membered nitrogen-oxygen heterocyclic spiro indolone compound and preparation method thereof, PCT Int. ApplCN108864147A, 2018 Search PubMed.
| This journal is © the Partner Organisations 2021 |