Facial synthesis of two-dimensional In2S3/Ti3C2Tx heterostructures with boosted photoactivity for the hydrogenation of nitroaromatic compounds†
Abstract
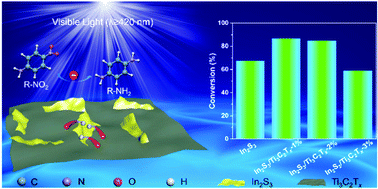
Two-dimensional (2D) heterostructures have gained increased interest in recent years due to the integrated functionalities of the individual building blocks and beyond. Herein, we have fabricated 2D In2S3/Ti3C2Tx heterostructures through a facile one-step low temperature refluxing method. While bare In2S3 tends to evolve as nanoparticles, it is found that Ti3C2Tx (MXene) acts as the platform for directing the growth of 2D In2S3 nanoflakes, thereby generating 2D In2S3/Ti3C2Tx heterostructures. For the probe reaction of nitroaromatic compound hydrogenation, In2S3/Ti3C2Tx composites exhibited boosted photocatalytic activities compared to bare In2S3, which can be ascribed to the favorable active site exposure in the 2D/2D heterostructures and the “electron sink” effect of the Ti3C2Tx nanosheets. This work distinctly indicates that Ti3C2Tx nanosheets are a promising platform for fabricating 2D/2D heterostructures for photoredox catalysis.
- This article is part of the themed collection: 2021 Materials Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...