A large-bandgap copolymer donor for efficient ternary organic solar cells†
Abstract
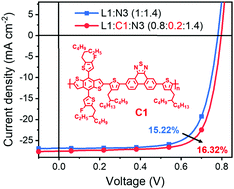
A 2.18 eV bandgap copolymer donor C1 based on a fused-ring unit phenanthro[9,10-c][1,2,5]thiadiazole (PT) was developed for 2D1A ternary organic solar cells. Incorporating a small amount of C1 into the L1:N3 blend deepened the HOMO of donor side, enhanced light absorption from N3, balanced hole/electron transport and optimized film mophogolgy, leading to an improved power conversion efficiency of 16.32%.
- This article is part of the themed collection: 2021 Materials Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...