Photoactive conjugated polymer/graphdiyne nanocatalyst for CO2 reduction to CO in living cells for hypoxia tumor treatment†
Abstract
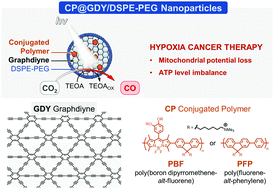
Carbon monoxide (CO) gas therapy has grown to be an emerging tumor therapy strategy to avoid the low treatment efficiency of photodynamic therapy (PDT) caused by the hypoxia tumor microenvironment. However, intracellular in situ generation of CO for hypoxia tumor treatment remains challenging. Herein, photoactive conjugated polymer/graphdiyne nanocatalyst (CP@GDY/DSPE-PEG) was constructed to in situ reduce endogenous carbon dioxide (CO2) into CO for hypoxia tumor therapy. Under light irradiation, the CO production rates of PFP@GDY/DSPE-PEG and PBF@GDY/DSPE-PEG reached 23 μmol h−1 gmat−1 and 31 μmol h−1 gmat−1 respectively, which can significantly affect mitochondrial respiration, leading to cancer cell apoptosis. Thus, the therapeutic effect of catalysis from metabolic CO2 into CO therapy under hypoxia conditions was achieved. This work provides a conjugated polymer/graphdiyne nanocatalyst to in situ generate CO for hypoxia tumor treatment.
- This article is part of the themed collections: 2021 Materials Chemistry Frontiers HOT articles and Graphyne


 Please wait while we load your content...
Please wait while we load your content...