Sulfonic acid functionalized graphitic carbon nitride as solid acid–base bifunctional catalyst for Knoevenagel condensation and multicomponent tandem reactions†
Abstract
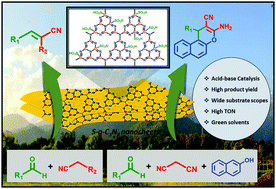
Rational design and development of acid–base bifunctional heterogeneous catalysts for organic synthesis is a tough process. A highly efficient, non-toxic, metal-free, low-cost, acid–base bifunctional sulfonated graphitic carbon nitride (S-g-C3N4) catalyst has been developed. The as-synthesized S-g-C3N4 catalyst exhibits high catalytic potential towards Knoevenagel condensation and sequential tandem reactions. The as-synthesized catalyst was developed by sulfonation of graphitic carbon nitride (g-C3N4). The sulfonation leads to surface functionalization of –SO3H groups onto the catalyst surface. These –SO3H groups impart acidic nature to the S-g-C3N4 catalyst along with the preexisting basic nature of the catalyst due to the presence of N-containing moieties. These dual acid–base functionalities behave as active sites for the sequential catalytic reactions to occur. The S-g-C3N4 catalyst exhibits high turnover numbers (TON) and high yields in shorter reaction time at optimum conditions of temperature which demonstrates the high catalytic activity of the S-g-C3N4 nanosheets. The corresponding green metrics parameters were also calculated, in addition to demonstrating the excellent catalyst recyclability and reusability. The as-synthesized S-g-C3N4 catalyst provides a metal-free, sustainable and green approach for utilizing acid–base bifunctional catalysts for sequential organic synthesis.
- This article is part of the themed collection: 2021 Materials Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...