Ordered mesoporous ZnGa2O4 for photocatalytic hydrogen evolution†
Abstract
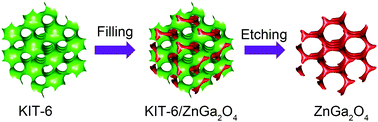
Zinc gallate (ZnGa2O4) materials as a mixed oxide of d10 metal zinc and gallium have a wide range of applications because of their intriguing properties. Considerable effort has been devoted to nanostructuring ZnGa2O4 to regulate or enhance its properties and performance, although further investigation would be still necessary. Herein, for the first time, we demonstrated the nanocasting synthesis of ordered mesoporous ZnGa2O4 with highly crystalline frameworks by using mesoporous silica as the hard template, which exhibits excellent photocatalytic hydrogen (H2) evolution performance. The key for fabricating ordered mesoporous ZnGa2O4 was its improved stability over individual zinc oxide or gallium oxide in alkaline etching solution during the removal of the silica template, which allows for the maintenance of the ordered mesoporous structure in a finely etching silica process. The resultant ordered mesoporous ZnGa2O4 possesses an ultrathin framework of 3–5 nm and a large pore size of 11 nm as well as a relatively high surface area of ∼157 m2 g−1. An enhanced photocatalytic H2 evolution performance of 2.72 mmol h−1 g−1 could be achieved under ultraviolet (UV) light irradiation, which is almost 1.8 and 3.9 times as high as those of ZnGa2O4 nanoflowers (1.5 mmol h−1 g−1) and bulk ZnGa2O4 (0.7 mmol h−1 g−1).
- This article is part of the themed collection: 2021 Materials Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...