Graphdiyne@NiOx(OH)y heterostructure for efficient overall water splitting†
Abstract
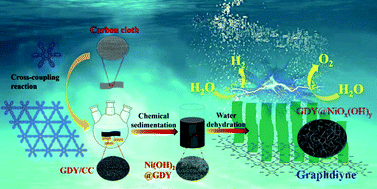
Graphdiyne (GDY), a rising star of two-dimensional (2D) carbon materials consisting of unique sp-/sp2-cohybridized carbon atoms, has been demonstrated to be an ideal platform for developing efficient catalysts with high activity and long-term durability. In this work, a new GDY-based heterostructure of GDY@NiOx(OH)y was successfully synthesized and used as an efficient electrocatalyst for water splitting. The creative incorporation of GDY with NiOx(OH)y endows the heterostructure with unique advantages; for example, the mixed valent Ni species, the strong interactions between GDY and NiOx(OH)y, and the greatly enhanced charge transfer ability, which are significantly beneficial for enhancing the catalytic activity and long-term durability. When applied in alkaline OWS electrolysis, GDY@NiOx(OH)y||GDY@NiOx(OH)y exhibited a voltage of only 1.54 V to achieve 10 mA cm−2, with robust stability over 100 h at 20 mA cm−2, much better than most of the reported benchmark catalysts, making them outstanding among water-splitting electrocatalysts.
- This article is part of the themed collections: 2021 Materials Chemistry Frontiers HOT articles and Graphyne


 Please wait while we load your content...
Please wait while we load your content...