Self-absorption-free excited-state intramolecular proton transfer (ESIPT) emitters for high brightness and luminous efficiency organic fluorescent electroluminescent devices†
Abstract
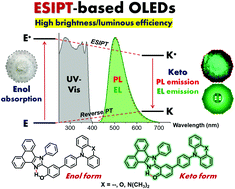
Excited-state intramolecular proton transfer (ESIPT) chromophores with attractive features such as a large Stokes shift have been widely considered prime candidates for various applications. However, as light-emissive materials for electroluminescent devices, device performance is still far behind traditional fluorophores. Herein, a series of ESIPT o-hydroxyphenyl phenanthroimidazole (o-HPI) derivatives (HPITPA, HPIPXZ, and HPIMAC) are designed and synthesized by functionalization with the N-electron donating moieties triphenylamine (TPA), N-(4-phenyl)phenoxazine (PXZ), and N-(4-phenyl)-9,9-dimethylacridine (MAC). The ESIPT and photophysical properties are thoroughly investigated using theoretical and experimental methods. All molecules show ESIPT properties with intense green color emissions from a pure keto form with high solid-state photoluminescence quantum yields (ΦPL) of 49–63%. They possess high thermal and electrochemical stabilities, as well as an improved hole-transporting capability. They are successfully fabricated as emitters in organic light-emitting diodes (OLEDs), and all devices exhibit green color emissions (λEL = 502–525 nm) with exceptionally high brightness (41 292–44 820 cd m−2), high luminous efficiency (LE) (8.41–10.30 cd A−1) and low turn-on voltages (3.0 V). In particular, the HPIPXZ-based OLED attains a high brightness of 44 820 cd m−2, an LE value of 10.30 cd A−1, and an external quantum efficiency (EQE) of 3.86% with a slight efficiency roll-off. Importantly, this represents an advance in the development of ESIPT molecules as emitters for fluorescent OLEDs.


 Please wait while we load your content...
Please wait while we load your content...