Research on metallic chalcogen-functionalized monolayer-puckered V2CX2 (X = S, Se, and Te) as promising Li-ion battery anode materials
Abstract
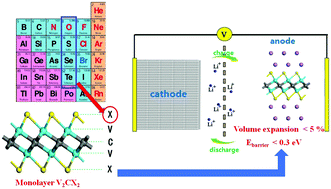
Two-dimensional MXene nanomaterials are promising anode materials for Li-ion batteries (LIBs) due to their excellent conductivity, large surface area, and high Li capability. The chalcogen-terminated monolayer-puckered V2CX2 (X = S, Se, and Te) structures are expected to embody remarkable properties for Li storage and diffusion in this paper. The metallic property enables the V2CX2 anodes to have a fast electron transport rate in the charge/discharge process. The phonon spectra prove their dynamical stabilities. The adsorption energy and energy diffusion barrier of the Li atom on the V2CX2 (X = S, Se, and Te) surface both decrease with increasing atomic number of the terminated element. The monolayer V2CSe2 shows higher Li capacity (394.41 mA h g−1), relatively low Ebarrier (0.21 eV) and a small volume expansion ratio (6.1%) when compared with those of V2CX2 (X = O, S, and Te) monolayers, indicating that they should be the most promising LIB anodes. These excellent properties indicate that monolayer chalcogen-terminated V2CX2 (X = S, Se, and Te) structures have promising applications as LIB anodes.
- This article is part of the themed collection: 2021 Materials Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...