A glutathione-activated carrier-free nanodrug of triptolide as a trackable drug delivery system for monitoring and improving tumor therapy†
Abstract
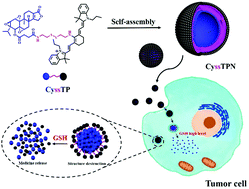
Triptolide (TP) is one of the most common systemic treatments for inflammatory and immune diseases in China for centuries. However, TP exhibits some disadvantages, such as poor solubility in water, poor bioavailability, liver toxicity, renal toxicity, and other side effects. In order to reduce the adverse effects of TP, researchers have developed numerous strategies to address the adverse properties of triptolide. Nano-carrier-based triptolide delivery systems represent an emerging technology and are one of the strategies of nanomedicine that combines diagnostic and therapeutic applications in a single agent. In this approach, we developed a glutathione-activated carrier-free nanodrug of triptolide (CyssTPN) as a trackable drug delivery system. In this system, CyssTP self-assemble to form a carrier-free nanodrug, which possesses a monodisperse spherical morphology with hydrodynamic average sizes of about 50 nm. In addition, CyssTPN had good stability under different physiological conditions (pH, high salt, etc.). Apart from cellular imaging and cell uptake, CyssTPN can be tracked by the activation of TP ability in real-time and applied for cancer cell treatment efficiently. The result showed that CyssTPN could improve solubility, reduce the side effects, and increase the bioavailability of triptolide. It could also track triptolide activation timely and tumor therapy successfully.
- This article is part of the themed collection: 2021 Materials Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...