Constructing a stable interface between the sulfide electrolyte and the Li metal anode via a Li+-conductive gel polymer interlayer†
Abstract
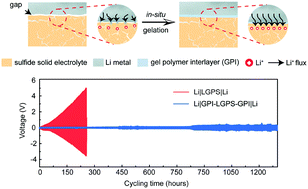
Due to high ionic conductivity, favorable mechanical plasticity, and non-flammable properties, inorganic sulfide solid electrolytes bring opportunities to the practical realization of rechargeable lithium–metal batteries with high energy, yet their use was impeded by an electrochemically unstable Li–electrolyte interface. Herein, we propose to address the issue via a Li+-conductive gel polymer interlayer, which is derived in situ from a conventional liquid ether electrolyte during the cell fabrication process. The gel polymer interlayer not only enables intimate solid–solid contact and uniform Li-ion flux at the heterointerface but also effectively inhibits interfacial reactions and Li dendrite growth. With improved interfacial stability, a Li–Li symmetric cell with the gel polymer interlayer demonstrates an ultra-stable Li plating/stripping performance of over 1300 hours at 0.1 mA cm−2 and 350 hours at 0.5 mA cm−2 at room temperature, and a high critical current density of >5 mA cm−2. This work offers general insights into a reasonable design of an anode/electrolyte interface for high-energy rechargeable Li–metal batteries.
- This article is part of the themed collections: Energy storage with rechargeable Li batteries and beyond and 2021 Materials Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...