Chlorine-doped Li1.3Al0.3Ti1.7(PO4)3 as an electrolyte for solid lithium metal batteries†
Abstract
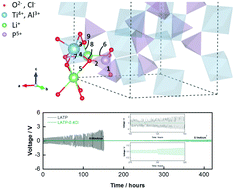
Solid-state electrolytes (SSEs) are expected to replace liquid electrolytes in lithium metal batteries (LMBs) with good safety and mechanical strength. However, the existing problems of the Li1.3Al0.3Ti1.7(PO4)3 (LATP) electrolyte, like its poor contact with electrodes, serious side reactions and low ionic conductivity, limit its practical application. In this work, a chlorine (Cl)-doped LATP electrolyte showing a high ionic conductivity of 0.423 mS cm−1 was successfully synthesized. Neutron powder diffraction (NPD) results reveal that the change of the Li+ coordination environment after Cl doping has an effect on the ion diffusion path in the bulk LATP. The fabricated Li symmetric battery with the Cl-doped LATP exhibits long-term compatibility and electrochemical stability against the lithium metal anode. The enhanced performance may be attributed to the in situ formed stable solid electrolyte interphase (SEI) layer. Meanwhile, compared to the LATP electrolyte, the Li|LiFePO4 (LFP) full battery with Cl-doped LATP as an electrolyte has an improved rate performance and cycling performance. This work demonstrates that Cl doping may be an effective approach for improving the performance of the LATP electrolyte.
- This article is part of the themed collection: Energy storage with rechargeable Li batteries and beyond


 Please wait while we load your content...
Please wait while we load your content...