The supercapacitor electrode properties and energy storage mechanism of binary transition metal sulfide MnCo2S4 compared with oxide MnCo2O4 studied using in situ quick X-ray absorption spectroscopy†
Abstract
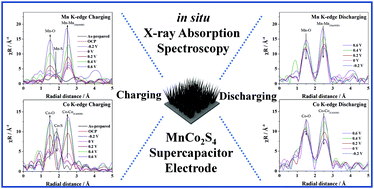
Binary transition-metal sulfide MnCo2S4 (MCS) and oxide MnCo2O4 (MCO) nanowires grown on nickel foam were prepared with facile hydrothermal method. The MCS electrode not only exhibits a large areal capacity, 1.17 mA h cm−2 at current density 3 mA cm−2 but also shows great rate capability and cycling stability in an alkaline electrolyte. In situ quick X-ray absorption spectroscopy of the Mn K-edge and Co K-edge of MCS demonstrate that both average valence states of elements Mn and Co show a notable change from the faradaic pseudocapacitance, inferring a synergistic effect of two elements. Based on a Co K-edge and Mn K-edge extended X-ray absorption fine structure analysis of MCS, some Mn–S/Co–S bonds on the surface of electrode are broken and then become active sites, which can react with hydroxide ions. Besides a synergistic effect of two elements, the active sites arising from the broken Mn–S/Co–S bonds hence enhance significantly the capacitive performance of the MCS electrode. Our work provides new insight into the electrochemical mechanism and the main difference for supercapacitor performance between MCS and the corresponding oxide MCO electrodes. These results demonstrate a different mechanism for binary transition metal sulfide and oxide in electrochemical reaction of energy storage.


 Please wait while we load your content...
Please wait while we load your content...