Sensitive and specific detection of peroxynitrite and in vivo imaging of inflammation by a “simple” AIE bioprobe†
Abstract
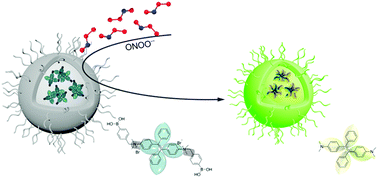
A luminogenic bioprobe TPE-DMAB for simple and specific detection of peroxynitrite (ONOO−) has been developed. TPE-DMAB exhibits aggregation-induced emission (AIE) characteristic and shows fluorescence enhancement (up to 100-fold) upon cleavage of phenylboronic moieties by ONOO−. TPE-DMAB can be facilely synthesized and displays excellent specificity and sensitivity towards ONOO− (detection limit of 54 nM). Based on these novel properties of TPE-DMAB, we further produced its encapsulated nanoparticles and successfully used them to detect endogenous ONOO− in macrophage cells and visualize the inflammation sites of mice, verifying the great potential of TPE-DMAB for biological applications.
- This article is part of the themed collections: FOCUS: Recent progress on bioimaging technologies, FOCUS: Recent progress on aggregation-induced emission, 2021 Materials Chemistry Frontiers HOT articles and Luminogenic bioprobes for personal health technologies


 Please wait while we load your content...
Please wait while we load your content...