Rational strategies toward efficient and stable lead-free tin halide perovskite solar cells
Abstract
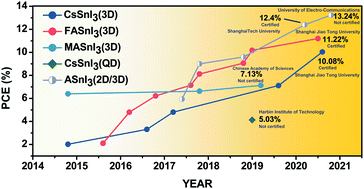
Despite the incredible progress of lead halide perovskite solar cells (LHPSCs), concerns remain regarding their potential impacts on the environment and human heath arising from their toxic and dissolvable lead content. Significant efforts have been devoted to searching lead-free halide perovskites over the past years. Among all these candidates, tin halide perovskites (THPs) have shown the most promise, where the efficiency of THP solar cells (THPSCs) has been progressing steadily. However, a considerable gap still exists between THPSCs and LHPSCs in terms of both efficiency and environmental stability. In this review, we first present a fundamental understanding of the origins of the inadequate efficiency and stability of THPSCs. Subsequently, a comprehensive and up-to-date overview focusing on the strategies addressing these issues facing THPSCs, including composition and dimension tuning, additive engineering and device engineering, is provided. Finally, we discuss the remaining challenges and give a perspective outlining the possible future directions for closing the gap between THPSCs and LHPSCs.
- This article is part of the themed collection: 2021 Materials Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...