An easily available ratiometric AIE probe for nitroxyl visualization in vitro and in vivo†
Abstract
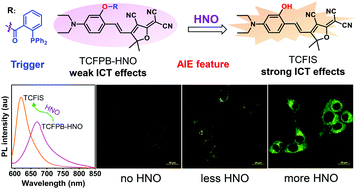
Nitroxyl (HNO), as a reactive nitrogen species (RNS), has a pivotal role in many physiological and pathological processes, but the generation mechanism of endogeneous HNO has not been elucidated, because of the lack of highly sensitive and selective probes to achieve real-time visualization and monitoring of HNO in biosystems. Ratiometric fluorescence probes have emerged as powerful platforms for bioimaging applications due to their high sensitivity and anti-interference ability. In this work, we developed firstly a ratiometric fluorescent probe (TCFPB-HNO) with typical aggregation-induced emission (AIE) characteristics for the detection and visualization of HNO with excellent photostability, high specificity and sensitivity. Importantly, TCFPB-HNO was facilely fabricated in high yields via a two-step process, as a serviceable AIE toolbox, which could be employed for real-time monitoring of the HNO level in live samples.
- This article is part of the themed collections: FOCUS: Recent progress on aggregation-induced emission, 2021 Materials Chemistry Frontiers HOT articles and Luminogenic bioprobes for personal health technologies


 Please wait while we load your content...
Please wait while we load your content...