Diagnosis of fatty liver disease by a multiphoton-active and lipid-droplet-specific AIEgen with nonaromatic rotors†
Abstract
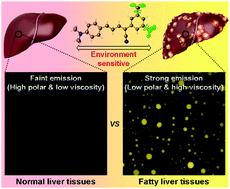
Fatty liver disease (FLD) has become an increasing global health risk. However, an accurate diagnosis of FLD at an early stage remains a great challenge due to the lack of suitable imaging tools. To this end, we developed the fluorescent two-photon aggregation-induced emission (AIE) luminogen ABCXF for high-contrast imaging of FLD tissue. ABCXF has a large Stokes shift, good two-photon absorption cross-section, bright red emission, and high fluorescence quantum yield in the solid-state, and excellent photostability. It shows a planar intramolecular charge transfer (PICT) effect instead of a twisted intramolecular charge transfer effect in polar solvents. Photophysical and crystal data demonstrated that the nonaromatic rotors – trifluoromethyl (–CF3) contribute to its AIE effect. Biocompatible lipid droplet-targeting ABCXF can selectively light up lesions in the FLD tissue with a high signal-to-noise ratio. It shows better imaging performance compared to Oil Red O. Thus, ABCXF can be a potential alternative to Oil Red O for the diagnosis and evaluation of FLD from a liver biopsy.
- This article is part of the themed collections: FOCUS: Recent progress on aggregation-induced emission and 2021 Materials Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...