More is better: aggregation induced luminescence and exceptional chirality and circularly polarized luminescence of chiral gold clusters†
Xiao-Yan
Wang‡
a,
Jing
Zhang‡
ab,
Jun
Yin
 a,
Sheng Hua
Liu
a,
Sheng Hua
Liu
 *a and
Ben Zhong
Tang
*a and
Ben Zhong
Tang
 *b
*b
aKey Laboratory of Pesticide and Chemical Biology, Ministry of Education, College of Chemistry, Central China Normal University, Wuhan, 430079, China. E-mail: chshliu@mail.ccnu.edu.cn
bDepartment of Chemistry, The Hong Kong Branch of Chinese National Engineering Research Center for Tissue Restoration and Reconstruction, Institute for Advanced Study, Department of Chemical and Biological Engineering and Institute of Molecular Functional Material, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China. E-mail: tangbenz@ust.hk
First published on 10th October 2020
Abstract
The influence of the aggregation-induced emission (AIE) effect on the luminescence and the chirality of two chiral Au clusters (R)-1 and (S)-1 was well demonstrated. Their solid states exhibited extremely bright phosphorescence with quantum yields above 70%. The two clusters were free of luminescence and chirality in diluted solution. Meanwhile, the formation of aggregate states could not only induce both the luminescence and the chirality, but also further facilitate their enhancements, which resulted in the output and the modulation of desirable circularly polarized luminescence.
Introduction
Luminescent materials have become one of the research hotspots, but traditional luminescent molecules suffer from the annoying aggregation-caused quenching (ACQ) effect and this greatly limits their applications in high concentrations. In 2001, an opposite aggregation-induced emission (AIE) phenomenon was proposed by Tang et al., which perfectly addressed the above ACQ problem. The discovery of AIE has played a milestone role in the development of luminescent materials and promoted the wide applications of AIE luminogens (AIEgens) in various areas including OLEDs, bio-imaging, biosensors and other fields.1 Research on pure organic AIEgens has been carried out for many years since the discovery of the AIE phenomenon, and large amounts of new AIEgens have been developed and relatively mature theoretical systems have been established associated with their luminescent mechanism.2 However, studies on the AIE-active transition metal complexes are relatively less reported, and metal clusters, as a unique kind of system among them, are rarely ventured into by researchers due to the complexity of their structures and their luminescent behaviors. To date, only very limited luminescent metal cluster systems including Pt, Au, Ag and Cu, have been reported, while the underlying luminescence mechanisms are still unclear.3–7During the exploration of novel metal cluster systems, chiral units have been tentatively introduced into the ligands coordinated with metal centers by researchers to construct distinctive luminescent materials with chirality and even circularly polarized luminescence (CPL).8,9 In parallel to chirality, CPL is another vital parameter of chiral luminescent materials that can reflect the excited-state chiral information. And CPL materials have drawn considerable attention due to their potential applications in chiroptical materials, photoelectric devices, information storage, and 3D displays.10 It has been well demonstrated that the chiral amplification or the generation of CPL could be realized by a self-assembly process.11–13 In the metal cluster systems, the AIE effect could be used to effectively trigger or regulate the CPL chiroptical activity. But such examples are quite limited. Recently, Zang and coworkers reported a pair of atomically precise enantiomeric copper(I) clusters containing chiral alkynyl ligands.14 The unique AIE effect of the two clusters could trigger their CPL with a high luminescence dissymmetry factor (glum) in the aggregate state. In addition, Tang’ group presented novel chiral Au clusters with chiral ligands (R)- or (S)-2,2′-bis(di-p-tolylphosphino)-1,1′-binaphthyl and investigated the influence of cluster assembly on the optical activity.15 The as-synthesized Au clusters showed strong circular dichroism but were free of luminescence and CPL. However, once ordered nanocubes were formed in the aggregate state, the CD signal was significantly enhanced and a remarkable CPL appeared due to the unique AIE effect and self-assembly.
Au atoms can form fascinating Au–Au interactions (aurophilicity). Gold clusters are regular molecular structures formed by aurophilicity. Compared with pure organic molecules or supramolecular systems, it is anticipated that gold clusters could more easily form a regular packing mode driven by the Au–Au interactions. The ordered molecular arrangement is quite conducive to enhancing the chiral optical properties, including CD, CPL and so on. Hence, the gold clusters could exhibit nice CPL signals and glum value. To the best of our knowledge, alkynyl–phosphine Au clusters with AIE effect or CPL properties have not been reported. Nevertheless, such type of Au clusters with simple synthesis and atomically precise structures have been reported previously by Chou et al.16 And their intriguing luminescence properties have attracted our great interest. Yet regrettably, Chou et al. were mainly concentrated on the presentation of their novel structures and only briefly demonstrated their emission behaviors without any detailed exploration. Herein, we focused on the investigations of the luminescent behaviors and the chirality in different aggregate states of the two chiral alkynyl–phosphine Au clusters ((R)-1 and (S)-1 ([Au6(C2C10H17O)4(PPh2C3H6PPh2)2](PF6)2) among them, as shown in Fig. 1. It could be anticipated that the luminescence, chirality and resulting chiroptical properties like CPL are highly sensitive to the surrounding solvent environment and spatial organization. And our results indicated that they were free of luminescence and chirality in diluted solution. Meanwhile, the formation of aggregate states could produce luminescence and induce chirality and further facilitate the enhancement of their luminescence and chirality, which further resulted in strong CPL signals. The present work will provide new insights into the exploration of metal clusters and the achievement of chirality and CPL.
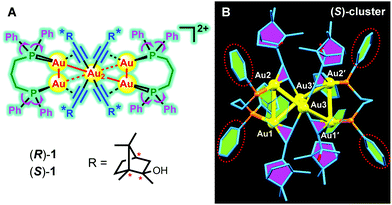 | ||
| Fig. 1 (A) Structures of (R)-1 and (S)-1 ([Au6(C2C10H17O)4(PPh2C3H6PPh2)2](PF6)2). (B) Crystal structures of (S)-1 (Au yellow; P orange; C blue; hydrogen atoms are omitted for clarity). | ||
Results and discussion
The synthetic route of all the intermediates and the products has been depicted in Scheme S1 (ESI†). Their structures have been well characterized by NMR and high-resolution mass spectroscopy and the results are presented in Fig. S1–S10 (ESI†). In particular, the alkynyl decanuclear gold(I) clusters [Au(C2C10H17O)]10(R)-1-b and (S)-1-b were obtained by the reactions of Au(tht)Cl (tht = tetrahydrothiphene) and alkynyl alcohol enantiomers (R)-1-a and (S)-1-a, respectively, both of which appeared as a yellow solid with poor solubility. In another aspect, the phosphine-bridged binuclear gold(I) complex [Au2(PPh2C3H6PPh2)2](PF6)2 (1-d) was synthesized by Au(tht)Cl and organic phosphine ligand. Ultimately, the hexanuclear gold clusters (R)-1 and (S)-1 were formed in high yields of above 70% by the reactions of gold–phosphine complex (1-d) and alkynyl–gold(I) enantiomers (R)-1-b and (S)-1-b, respectively. The resulting two chiral clusters were easy to crystallize and their solid states obtained from recrystallization in DCM/ether mixtures all existed in microcrystals. They all showed high stability under ambient conditions and were readily soluble in common organic solvents such as dichloromethane, acetone, tetrahydrofuran, chloroform, and dimethylformamide (DMF).Fortunately, we obtained the single crystal structures of (S)-1 by slow diffusion of poor ether into its dichloromethane solution. As shown in Fig. 1, the structure of (S)-1 is C2 symmetrical with a chiral space group of C2221. The whole crystal structure formed a propeller-like pattern, where the six Au atoms formed a solid shaft and four phenyl groups acted as the “propeller blades”. In addition, the surrounding four ethynyl also helped to stabilize the core shaft by constituting four triangles with four Au atoms, respectively. The Au–Au distances of Au(1)–Au(2), Au(1)–Au(3′), Au(2)–Au(3), and Au(3)–Au(3′) are 3.244 Å, 3.238 Å, 3.190 Å and 3.241 Å, respectively, similar to the reported values, indicative of multiple aurophilic interactions.16 From Fig. 2A, we can observe that there exist multiple C–H⋯F interactions between the PF6− anion and the aromatic rings of the surrounding three clusters. There are no intermolecular interactions between different clusters. And all clusters are inclined to pack in a layer-by-layer pattern and further formed complicated 2-D layered stacking (Fig. 2B and C).
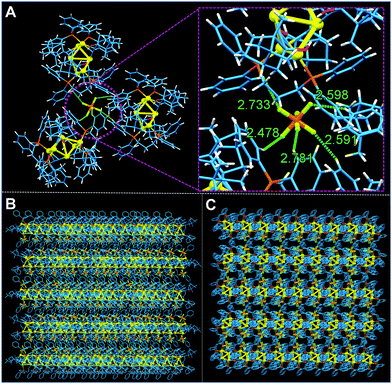 | ||
| Fig. 2 (A) Intermolecular C–H⋯F interactions formed in aromatic rings and PF6−. (B and C) Intermolecular stackings of (S)-1. | ||
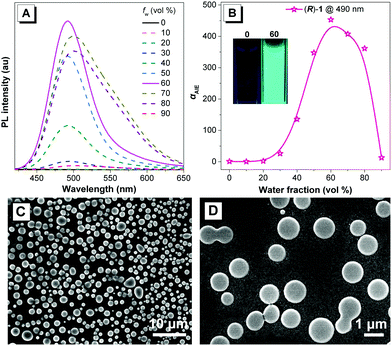
According to the “propeller-like” characteristic of the (S)-1 structure, we anticipated that the two clusters should be AIE-active. Accordingly, we measured their respective photoluminescence (PL) spectra in diluted DMF and DMF–water mixtures. As depicted in Fig. 3 and Fig. S11 (ESI†), their diluted DMF solutions were non-emissive. However, upon the addition of 30 vol% of water into their respective DMF solution, the resulting mixtures all emitted blue photoluminescence (PL) with the maximum emission wavelength at ∼490 nm. With the further increase of water fraction (fw), their emission intensities were enhanced unceasingly and reached their maximum when fw was 60%. These phenomena clearly indicated that the two clusters exhibited typical AIE behaviors.17 We further analyzed their aggregate states with 60% water fraction by employing dynamic light scattering (DLS, Fig. S12, ESI†) and scanning electron microscope (SEM) imaging (Fig. 3 and Fig. S13, ESI†). We found that the mixtures of 60% water fraction for the two clusters presented uniform nanoparticles with average diameters of ∼500 nm. The collected lifetime data indicated that the lifetimes of their aggregate states are ∼4.9 μs (Fig. S14, ESI†), suggestive of phosphorescence in nature. We also collected their respective quantum yield (QY) in different states (Table 1). It was amazing that their respective QY values were remarkably increased from the aggregate state (∼30%) to the film state (∼50%) and then to the solid state (∼73%). Of note, such high QY values in the solid state are relatively rare among the reported gold clusters. This change tendency further strengthens their AIE nature indicating that the more concentrated the molecules, the brighter the emission. Actually, we observed that the obtained solid of both clusters was microcrystals, revealing a real crystallization-induced emission enhancement (CIEE).18 The luminescence of their solid states is also phosphorescence according to the lifetime data of 2.6 μs (Fig. S15, ESI†).
| Sample | State | Abs. (nm) | λ ex (nm) | λ em (nm) | Lifetime (μs) | QY (%) | IglumI (10−3) |
|---|---|---|---|---|---|---|---|
| a Films are spin-coated from dichloroethane solution (25 mg mL−1). b Microcrystals. | |||||||
| (R)-1 | DMF solution | 267 | — | — | — | — | — |
| Aggregate (fw = 60%) | 267, 335, 388 | 330 | 492 | 4.85 | 31.0 | 6.2 | |
| Filma | 300, 382 | 386 | 500 | 5.63 | 52.7 | 3.3 | |
| Solidb | — | 274 | 489 | 2.60 | 72.8 | 0.8 | |
| (S)-1 | DMF solution | 267 | — | — | — | — | — |
| Aggregate (fw = 60%) | 267, 335, 388 | 330 | 492 | 4.85 | 33.4 | 6.2 | |
| Filma | 301, 382 | 386 | 500 | 5.32 | 52.9 | 3.5 | |
| Solidb | — | 278 | 488 | 2.60 | 73.9 | 2.1 | |
Then a question arises: what's the reason for their unique AIE or CIEE behaviors? We therefore attempted to find some clues from the crystal structure of (S)-1. We also mentioned that the central Au atoms are well fixed in the propeller-like structure and they are completely surrounded by the phosphine and alkynyl ligands, so it is very difficult for the Au atoms in different cluster units to get close to each other and further aggregate. However, the four phenyl groups in the two phosphine ligands can rotate freely. Thus, their rotations are considered to serve as a nonradiative relaxation pathway for excited electrons and cause the to be cluster non-luminescent in dilute solution. In the aggregate state or solid state, the intramolecular rotations of these phenyl rings are inhibited, causing the prohibition of the effective nonradiative relaxation of excited electrons, which results in strong luminescence of the clusters.19
Convincing evidence to support the restriction of intramolecular rotation (RIR) mechanism proposed above could be found from the crystal packing as illustrated in Fig. 2B. The phenyl rings from three cluster units could be perfectly fixed by the central PF6− through intermolecular C–H⋯F interactions. Furthermore, we can see that there are no direct intermolecular Au–Au interactions involved in the molecular stackings. It should be mentioned that the introduced phosphine ligands, especially the four phenyl rotors, play a critical role in the emission and following chirality induction for their aggregate states (vide infra).
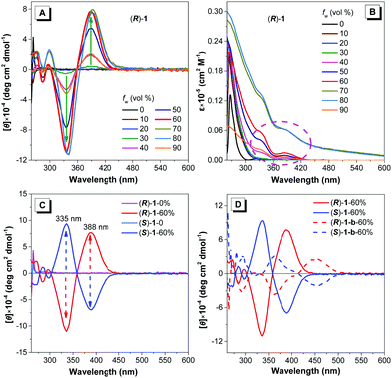
Considering that the two clusters are chiral, we then investigated their CD signals in different aggregate states. Amazingly, their CD signals of different aggregate states exhibited remarkable differences. Taking (R)-1 as a representative, as depicted in Fig. 4A, the CD signal of pure DMF solution was silent. However, with the addition of 10% water into the DMF solution, a weak negative CD peak located at 335 nm and a positive CD peak at 388 nm appeared. When increasing the water fraction, these two peaks were enhanced progressively and reached their respective maximum intensity when fw was 60%. This phenomenon indicated that the formation of aggregate states could induce chirality and further facilitate the amplification of chirality. When the water fraction exceeded 60%, the intensity of these two peaks all remained unchanged or even decreased. Correspondingly, in the UV/Vis spectra (Fig. 4B), two new absorption peaks at around 335 nm and 388 nm, respectively, gradually increased with the increase of water fraction, which was attributed to metal-to-ligand charge transfer (MLCT) according to the time-dependent density functional theory (TD-DFT) calculations (Table 2 and Fig. 5, for details see ESI†). The evolution of the CD profiles for (S)-1 in different aggregate states was completely mirrored compared with that of (R)-1 as well illustrated in Fig. 4C and Fig. S16 (ESI†). And the corresponding absorption change was also very similar to that of (R)-1. To confirm their CD signals induced in the aggregate state, we also detected their solid state.20 As illustrated in Fig. S17 (ESI†), the CD curves of (R)-1 and (S)-1 in the solid state are almost the same as their respective aggregate states.
| State | λ/nm | Osc. Str. (ƒ) | Major contributions | Assignment |
|---|---|---|---|---|
| a The computational method was wb97x/6-31G* (Au: Lanl2DZ). TD-DFT calculations of DMF solution were based on the optimized ground-state conformation of (S)-1 in the gas phase. The conductor polarizable continuum model (CPCM) in DMF was employed. The TD-DFT calculations of the solid state were based on the original crystal structure of (S)-1 without optimization. | ||||
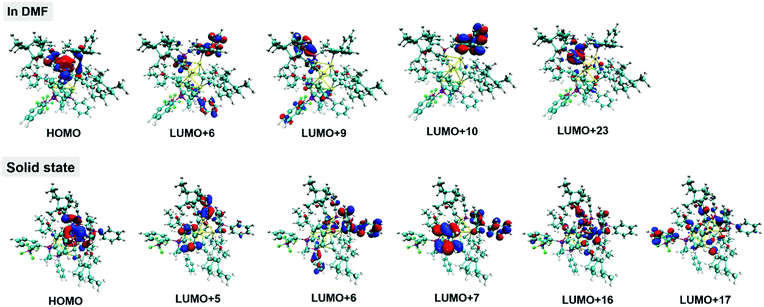
| Solution state (In DMF) | 421 | 0.1003 | HOMO → LUMO+6 (35%) | MLCT |
| HOMO → LUMO+9 (29%) | ||||
| HOMO → LUMO+23 (22%) | ||||
| 400 | 0.1076 | HOMO → LUMO+6 (65%) | MLCT | |
| HOMO → LUMO+10 (19%) | ||||
| Solid state | 596 | 0.0960 | HOMO → LUMO+5 (18%) | MLCT |
| HOMO → LUMO+16 (70%) | ||||
| 436 | 0.0613 | HOMO → LUMO+6 (25%) | MLCT | |
| HOMO → LUMO+7 (30%) | ||||
| HOMO → LUMO+17 (35%) | ||||
What is the reason for the generation and the amplification of the impressive CD signals in the aggregate state? We proposed that the introduced phosphine ligands especially the four phenyl rotors play a critical role in the chirality induction for their aggregate states. As presented in Fig. 4D and Fig. S18 (ESI†), we also detected the CD signals of the alkynyl Au(I) precursors (R)-1-b and (S)-1-b in the DMF/water (2/3, v/v) mixtures. Their respective CD signals were red-shifted and much weaker relative to the corresponding clusters. Moreover, according to the TD-DFT calculations in Fig. 5 and Fig. S19 (ESI†), the red-shifted absorption and the newly appeared CD signals of (S)-1 in the condensed state were well simulated and they are attributed to MLCT, where the LUMOs are predominantly distributed on the two phosphine ligands, thus indicating their significant contributions. Therefore, together with the unique AIE properties of the two clusters, we can conclude that the rotation restriction of the two flexible phosphine ligands not only determines the emission of the aggregate states but also facilitates the generation of remarkable CD signals in the aggregate state.
Inspired by the prominent emission and the remarkable CD signals of the two chiral clusters in the aggregate state, we then wondered whether we could detect CPL signals. Encouragingly, strong CPL signals centered at ∼490 nm were observed for both of them in different aggregate states with the luminescence dissymmetry factor (|glum|)21 values of 10−3 (Fig. 6 and Fig. S20, ESI†), comparable to those of the CPL-based gold cluster and organic assembly,11,22 revealing that the emitted lights from the excited electronic state of clusters (R)-1/(S)-1 are polarized in opposite directions. More impressively, the change tendency of the CPL intensity in different aggregate states is basically similar to those observed in their corresponding PL and CD experiments. And their mixtures with 60% water fraction exhibited the strongest and mirror CPL signals with |glum| of 6 × 10−3, revealing that both the prominent emission and the strong CD endowed by the optimal aggregate condition (fw = 60%) are determinants of desirable CPL. Here we showed an example of aggregation induced CPL. Therefore, the above results demonstrated that the elaborate manipulation of aggregate states is also an effective route to realize desirable CPL signals.
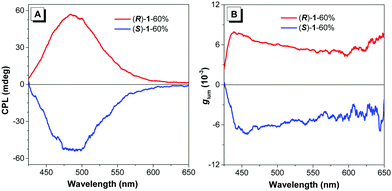 | ||
| Fig. 6 (A) CPL spectra and (B) corresponding dissymmetry factor glum of (R)-1 and (S)-1 in DMF/water (2/3, v/v) mixtures. Concentration: 5 × 10−5 M. λex = 340 nm. | ||
Conclusions
We have reported two chiral clusters with unique aggregation-induced emission (AIE) properties. Their solid states exhibited extremely bright phosphorescence with QY values above 70% due to the AIE characteristics and such high QY values in the solid state are relatively rare among the reported gold clusters. It has been demonstrated that their AIE properties are attributed to the rotation restriction of the deliberately introduced phosphine ligands based on the crystal structure analyses. In addition, the results indicated that the formation of aggregate states could induce chirality and further facilitate the enhancement of chirality, which further resulted in strong CPL signals. Therefore, the present results provide a facile and effective way to achieve desirable chirality and CPL through elaborate manipulation of aggregate states. This work may have great significance in providing insights into the research of new chiral clusters.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (21472059 and 21772054), the 111 Project (B17019), and the Huabo Project of CCNU.Notes and references
- (a) J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole, Chem. Commun., 2001, 1740–1741 RSC; (b) R. Hu, A. Qin and B. Z. Tang, AIE polymers: Synthesis and applications, Prog. Polym. Sci., 2019, 101176 Search PubMed; (c) R. Zhang, Y. Duan and B. Liu, Recent advances of AIE dots in NIR imaging and phototherapy, Nanoscale, 2019, 11, 19241 RSC; (d) H. Liu, L.-H. Xiong, R. T. K. Kwok, X. He, J. W. Y. Lam and B. Z. Tang, AIE Bioconjugates for Biomedical Applications, Adv. Opt. Mater., 2020, 2000162 CrossRef CAS; (e) Y. Hong, J. W. Y. Lam and B. Z. Tang, Aggregation-induced emission, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- (a) J. Guan, A. Prlj, R. Wei, J. Peng, K.-H. Lin, J. Liu, H. Han, Z. Yu, C. Corminboeuf, D. Zhao and J. Zheng, Direct Observation of Aggregation-Induced Emission Mechanism, Angew. Chem., Int. Ed., 2020, 59, 14903–14909 CrossRef CAS; (b) P. Zhou, P. Li, Y. Zhao and K. Han, Restriction of Flip-flop Motion as a Mechanism for Aggregation-Induced Emission, J. Phys. Chem. Lett., 2019, 10, 6929–6935 CrossRef CAS; (c) Y. Zhang, B. He, J. Liu, S. Hu, L. Pan, Z. Zhao and B. Z. Tang, Aggregation-induced emission and the working mechanism of 1-benzoyl and 1-benzyl pyrene derivatives, Phys. Chem. Chem. Phys., 2018, 20, 9922–9929 RSC; (d) Y. Cai, L. Du, K. Samedov, X. Gu, F. Qi, H. H. Y. Sung, B. O. Patrick, Z. Yan, X. Jiang, H. Zhang, J. W. Y. Lam, I. D. Williams, D. L. Phillips, A. Qin and B. Z. Tang, Deciphering the working mechanism of aggregation-induced emission of tetraphenylethylene derivatives by ultrafast spectroscopy, Chem. Sci., 2018, 9, 4662–4670 RSC.
- A. George, M. P. Maman, K. Bhattacharyya, S. D. Chakraborty, A. S. B. C. Das, D. Senapati, A. Datta and S. Mandal, Aggregation induced non-emissive-to-emissive switching of molecular platinum clusters., Nanoscale, 2019, 11, 5914 RSC.
- (a) Z. Wu, Q. Yao, O. Jin, H. Chai, N. Ding, W. Xu, S. Zang and J. Xie, Unraveling the Impact of Gold(I)–Thiolate Motifs on the Aggregation-Induced Emission of Gold Nanoclusters, Angew. Chem., Int. Ed., 2020, 59, 9934–9939 CrossRef CAS; (b) Z. Luo, X. Yuan, Y. Yu, Q. Zhang, D. T. Leong, J. Y. Lee and J. Xie, From Aggregation-Induced Emission of Au(I)–Thiolate Complexes to Ultrabright Au(0)@Au(I)-Thiolate Core–Shell Nanoclusters, J. Am. Chem. Soc., 2012, 134, 16662–16670 CrossRef CAS.
- M. Sugiuchi, J. Maeba, N. Okubo, M. Iwamura, K. Nozaki and K. Konishi, Aggregation-Induced Fluorescence-to-Phosphorescence Switching of Molecular Gold Clusters, J. Am. Chem. Soc., 2017, 139, 17731–17734 CrossRef CAS.
- T. Yang, S. Dai, S. Yang, L. Chen, P. Liu, K. Dong, J. Zhou, Y. Chen, H. Pan, S. Zhang, J. Chen, K. Zhang, P. Wu and J. Xu, Interfacial Clustering-Triggered Fluorescence-Phosphorescence Dual Solvoluminescence of Metal Nanoclusters, J. Phys. Chem. Lett., 2017, 8, 3980–3985 CrossRef CAS.
- (a) Y. J. Kong, Z. P. Yan, S. Li, H. F. Su, K. Li, Y. X. Zheng and S.-Q. Zang, Photoresponsive Propeller-like Chiral AIE Copper(I) Clusters, Angew. Chem., Int. Ed., 2020, 59, 5336 CrossRef CAS; (b) Y. Jin, S. Li, Z. Han, B.-J. Yan, H.-Y. Li, X.-Y. Dong and S.-Q. Zang, Cations Controlling the Chiral Assembly of Luminescent Atomically Precise Copper(I) Clusters, Angew. Chem., Int. Ed., 2019, 58, 12143–12148 CrossRef CAS; (c) M.-M. Zhang, K. Li and S.-Q. Zang, Progress in Atomically Precise Coinage Metal Clusters with Aggregation-Induced Emission and Circularly Polarized Luminescence, Adv. Opt. Mater., 2020, 1902152 CrossRef CAS; (d) M.-M. Xu, T.-T. Jia, B. Li, W. Ma, X. Chen, X. Zhao and S.-Q. Zang, Tuning the properties of atomically precise gold nanoclusters for biolabeling and drug delivery, Chem. Commun., 2020, 56, 8766–8769 RSC.
- L. Shi, L. Zhu, J. Guo, L. Zhang, Y. Shi, Y. Zhang, K. Hou, Y. Zheng, Y. Zhu, J. Lv, S. Liu and Z. Tang, Self-Assembly of Chiral Gold Clusters into Crystalline Nanocubes of Exceptional Optical Activity., Angew. Chem., Int. Ed., 2017, 129, 15599–15603 CrossRef.
- (a) L. Yao, G. Niu, J. Li, L. Gao, X. Luo, B. Xia, Y. Liu, P. Du, D. Li, C. Chen, Y.-X. Zheng, Z. Xiao and J. Tang, Circularly Polarized Luminescence from Chiral Tetranuclear Copper(I) Iodide Clusters, J. Phys. Chem. Lett., 2020, 11, 1255–1260 CrossRef CAS; (b) F. K.-W. Hau, T. K.-M. Lee, E. C.-C. Cheng, V. K.-M. Au and V. W.-W. Yam, Luminescence color switching of supramolecular assemblies of discrete molecular decanuclear gold(I) sulfido complexes, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 15900 CrossRef CAS; (c) L.-Y. Yao, T. K.-M. Lee and V. W.-W. Yam, Thermodynamic-Driven Self-Assembly: Heterochiral Self-Sorting and Structural Reconfiguration in Gold(I)–Sulfido Cluster System, J. Am. Chem. Soc., 2016, 138, 7260 CrossRef CAS.
- (a) X. Zhang, Y. Zhang, Y. Li, Y. Quan, Y. Cheng and Y. Li, High brightness circularly polarized blue emission from non-doped OLEDs based on chiral binaphthyl-pyrene emitters, Chem. Commun., 2019, 55, 9845–9848 RSC; (b) Y. Zhang, X. Zhang, H. Zhang, Y. Xiao, Y. Quan, S. Ye and Y. Cheng, High Green Brightness Circularly Polarized Electroluminescence Regulated by Rigid Chiral D-A Type Emitters, J. Phys. Chem. C, 2019, 123, 24746–24753 CrossRef CAS; (c) Y. Chen, X. Li, N. Li, Y. Quan, Y. Cheng and Y. Tang, Strong circularly polarized electroluminescence based on chiral salen-Zn(II) complex monomer chromophores, Mater. Chem. Front., 2019, 3, 867–873 RSC.
- J. Zhang, Q. Liu, W. Wu, J. Peng, H. Zhang, F. Song, B. He, X. Wang, H. H.-Y. Sung, M. Chen, B. S. Li, S. H. Liu, J. W. Y. Lam and B. Z. Tang, Real-Time Monitoring of Hierarchical Self-Assembly and Induction of Circularly Polarized Luminescence from Achiral Luminogens, ACS Nano, 2019, 13, 3618–3628 CrossRef CAS.
- X. Li, W. Hu, Y. Wang, Y. Quan and Y. Cheng, Strong CPL of achiral AIE-active dyes induced by supramolecular self-assembly in chiral nematic liquid crystals (AIE-N*-LCs), Chem. Commun., 2019, 55, 5179–5182 RSC.
- D. Liu, Y. Zhou, Y. Zhang, H. Li, P. Chen, W. Sun, T. Gao and P. Yan, Chiral BINAPO-Controlled Diastereoselective Self-Assembly and Circularly Polarized Luminescence in Triple-Stranded Europium(III) Podates, Inorg. Chem., 2018, 57, 8332–8337 CrossRef CAS.
- M.-M. Zhang, X.-Y. Dong, Z.-Y. Wang, H.-Y. Li, S.-J. Li, X. Zhao and S.-Q. Zang, AIE Triggers the Circularly Polarized Luminescence of Atomically Precise Enantiomeric Copper(I) Alkynyl Clusters, Angew. Chem., Int. Ed., 2020, 59, 10052–10058 CrossRef CAS.
- L. Shi, L. Zhu, J. Guo, L. Zhang, Y. Shi, Y. Zhang, K. Hou, Y. Zheng, Y. Zhu, J. Lv, S. Liu and Z. Tang, Self-Assembly of Chiral Gold Clusters into Crystalline Nanocubes of Exceptional Optical Activity., Angew. Chem., Int. Ed., 2017, 56, 15397–15401 CrossRef CAS.
- I. O. Koshevoy, Y.-C. Chang, Y.-A. Chen, A. J. Karttunen, E. V. Grachova, S. P. Tunik, J. Janis, T. A. Pakkanen and P.-T. Chou, Luminescent Gold(I) Alkynyl Clusters Stabilized by Flexible Diphosphine Ligands, Organometallics, 2014, 33, 2363–2371 CrossRef CAS.
- Y. N. Hong, J. W. Y. Lam and B. Z. Tang, Aggregation-induced emission: phenomenon, mechanism and applications, Chem. Commun., 2009, 4332–4353 RSC.
- Y. Dong, J. W. Y. Lam, A. Qin, Z. Li, J. Sun, H. H. Y. Sung, I. D. Williams and B. Z. Tang, Switching the light emission of (4-biphenylyl)phenyldibenzofulvene by morphological modulation: crystallization-induced emission enhancement, Chem. Commun., 2007, 40–42 RSC.
- N. Goswami, Q. Yao, Z. Luo, J. Li, T. Chen and J. Xie, Luminescent Metal Nanoclusters with Aggregation-Induced Emission, J. Phys. Chem. Lett., 2016, 7, 962–975 CrossRef CAS.
- L. Ding, L. Lin, C. Liu, H. Li, A. Qin, Y. Liu, L. Song, H. Zhang, B. Z. Tang and Y. Zhao, Concentration effects in solid-state CD spectra of chiral atropisomeric compounds, New J. Chem., 2011, 35, 1781–1786 RSC.
- N. Berova, K. Nakanishi and R. W. Woody, Circular Dichroism: Principles and Applications, John Wiley & Sons, 2000 Search PubMed.
- (a) S. Huo, P. Duan, T. Jiao, Q. Peng and M. Liu, Self-Assembled Luminescent Quantum Dots To Generate Full-Color and White Circularly Polarized Light, Angew. Chem., Int. Ed., 2017, 56, 12174–12178 CrossRef CAS; (b) S. Huo, P. Duan, T. Jiao, Q. Peng and M. Liu, Self-Assembled Luminescent Quantum Dots To Generate Full-Color and White Circularly Polarized Light, Angew. Chem., 2017, 129, 12342–12346 CrossRef; (c) J. P. Riehl and F. S. Richardson, Circularly polarized luminescence spectroscopy, Chem. Rev., 1986, 86, 1–16 CrossRef CAS; (d) J. Kumar, T. Kawai and T. Nakashima, Circularly polarized luminescence in chiral silver nanoclusters, Chem. Commun., 2017, 53, 1269–1272 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed Experimental section, NMR spectra, details of the crystal data collection, and selected bond lengths and angles. CCDC 1978728 for (S)-1 and characterization data mentioned in the paper. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0qm00552e |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2021 |