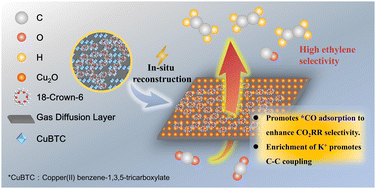
The electroconversion of CO2 into ethylene (C2H4) offers a promising solution to environmental and energy challenges. Crown ether (CE) modification significantly enhances the C2H4 selectivity of copper-based MOFs, improving C2H4 faradaic efficiency (FE) in CuBTC, CuBDC, and CuBDC-NH2 by 3.1, 1.7, and 2.4 times, respectively. Among these, CuBTC achieves the highest FE for C2H4, reaching ca. 52% at 120 mA cm−2. Control experiments and in situ Fourier transform infrared spectroscopy (FTIR) reveal that CE stabilizes Cu+ during the catalyst's in situ reconstruction, promoting the formation of Cu2O, which is more favorable for C2H4 production. Furthermore, CE increases the local concentration of K+ at the catalyst–electrolyte interface, enhancing *CO adsorption and facilitating C–C coupling reactions. This process promotes the formation of key intermediates, such as *CO*CO, *CO*COH and *COCHO, ultimately boosting C2H4 production.