Synthesis of polyethers from epoxides via a binary organocatalyst system†
Abstract
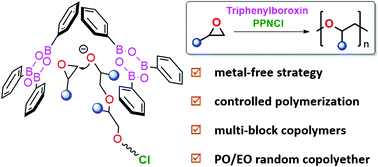
The development of “metal-free” catalyst systems with high efficiency for the ring-opening polymerization (ROP) of epoxides, providing polyethers with controlled molecular weights and dispersity as well as the desired main-chain sequence, is crucially required. In this paper, we present a binary catalyst system, in which triphenylboroxin (TPBX) is employed as a catalyst in conjunction with bis-(triphenylphosphine)iminium chloride (PPNCl) as the initiator, for the ROP of epoxides to precisely synthesize polyethers. The binary TPBX/PPNCl system exhibits good activity under mild conditions, yielding polyethers with predictable molecular weights and low dispersity (Đ < 1.25). Of importance is that the ROP of epoxides mediated by the binary system shows a living polymerization character, allowing for the synthesis of multi-block polyethers. In addition, the synthesis of propylene oxide/ethylene oxide copolyethers with a random structure is achieved. Furthermore, on the basis of the kinetics study, a bimolecular TPBX cooperatively catalyzed mechanism is proposed and verified by density functional theory (DFT) calculations.


 Please wait while we load your content...
Please wait while we load your content...