Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polymers from sugars and CS2: ring opening copolymerisation of a D-xylose anhydrosugar oxetane†
Thomas M.
McGuire
 and
Antoine
Buchard
and
Antoine
Buchard
 *
*
Centre for Sustainable and Circular Technologies, Department of Chemistry, University of Bath, Claverton Down, Bath BA2 7AY, UK. E-mail: a.buchard@bath.ac.uk
First published on 6th July 2021
Abstract
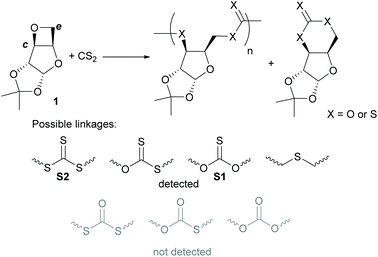
A D-xylose anhydro sugar derivative (1) has been applied in the ring-opening copolymerisation (ROCOP) with CS2 to form a polythiocarbonate (poly(CS2-co-1)) with high head-head/tail-tail regioselectivity towards alternating thiono- and trithiocarbonate linkages (up to 95%). Through variation of the reaction parameters (e.g. temperature and CS2 stoichiometry), some control over the regioselectivity (head-head/tail-tail linkages 57–95%) and the nature of the polymer linkages is possible. Conditions can also be tailored to enable the facile isolation of a polymerisable cyclic xanthate, 2. Kinetic experiments suggest that across the range of temperatures studied, the formation of poly(CS2-co-1) proceeds at least partially by direct copolymerisation of 1 and CS2, without necessarily going through the ring-opening polymerisation (ROP) of 2. Poly(CS2-co-1) exhibits partial chemical recyclability into cyclic monomer 2 (up to 45% after 20 h at 110 °C with [poly(CS2-co-1)]0 = 1.34 mol L−L). Finally, rapid degradation (<1 h) of poly(CS2-co-1) is possible under UV radiation (λ = 365 nm) and is accelerated in the presence of tris(trimethylsilyl)silane (TTMSS).
Introduction
Owing to the environmental issues surrounding plastic use, a societal shift away from traditional petroleum-derived feedstocks is driving innovation in the field of sustainable polymer chemistry. Polymers derived from sugars have huge potential as sustainable alternatives to current commodity plastics.1–5 For instance, polymers which incorporate pyranose or furanose motifs typically exhibit remarkably high glass transition temperatures (Tg) and the availability of hydroxyl groups of sugar derivatives significantly broadens the scope for prospective material functionalisation.6–9It is known that the replacement of oxygen atoms with sulfur ones in oxygenated polymers can give the resulting polythioethers, polythiocarbonates and polythioesters enhanced physical and thermal properties,10–16 as well as additional advanced optical17 and electrical characteristics.18 Moreover, sulfur-containing polymers exhibit biocompatibility and have shown potential in metal and bacterial adhesion.19,20 The presence of sulfur atoms in the backbone of polymers may also accelerate degradation under appropriate conditions (e.g. under UV radiation).19,21
The synthesis of polythiocarbonates is possible through polycondensation,22–25 the ring-opening polymerisation (ROP) of cyclic thiocarbonates,26,27 polyalkylation of trithiocarbonates28,29 or the ring-opening copolymerisation (ROCOP) of CS215,30–33 and COS11,34–38 with cyclic ethers (epoxides and oxetane). ROCOP methods are particularly interesting given the array of synthetic possibilities provided by the pool of cyclic ethers usable, coupled with the polymerisation control brought by existing ROCOP catalysts.39,40 To date, both heterogeneous (e.g. Zn–Co(III) double-metal cyanide complexes)41 and homogeneous catalysts/initiators (e.g. metal salen/onium catalysts30,34,42 and LiOtBu31) have been reported for CS2/cyclic ether ROCOP. However, reports of ROCOP between an oxetane and CS2 remain rare.32
CS2 is manufactured by reaction of charcoal or natural gas with sulfur, and despite its known toxicity, incorporating it into polymers has some benefits in terms of waste valorisation. Indeed, sulfur is an abundant by-product of the oil and chemical industry.43–45 Our group has previously used CS2 to synthesise a series of cyclic thiocarbonates for ROP towards sugar-derived polythiocarbonates.27 However, the synthesis of these monomers was challenging, necessitating extensive purification (e.g. successive column chromatography) to be suitable for ROP techniques. For xylose-derived xanthate monomer, 2, ROP towards poly(2) formed a regioregular polymer with up to 87% head-head/tail-tail (HH/TT), trithiocarbonate/thionocarbonate linkages, although no control over the polymer microstructure was demonstrated.
It was envisioned that the ROCOP of CS2 with oxetane-functionalised xylofuranose derivative, 1, may expediate the polymer synthesis given its ease of preparation in purities suitable for ROP46 and ROCOP.47 Moreover, we hypothesised that alternative polymer sequences may be accessible through ROCOP. Lastly, through judicious choice of conditions, it was hoped that the reaction between CS2 and 1 could be tailored towards cycloaddition over copolymerisation, to access 2 with fewer purification steps.
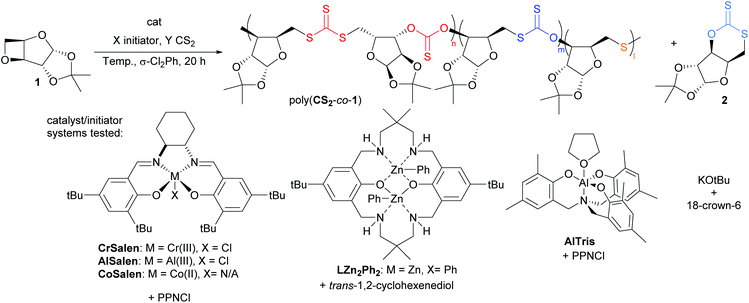
We have previously used 1,2-cyclohexanediamino-N,N′-bis(3,5-di-t-butylsalicyilidene)-chromium(III), CrSalen and bis(triphenylphosphine)iminium chloride (PPNCl) to catalyse the ROCOP of cyclic anhydrides and 1 for the preparation of polyesters.47 CrSalen/onium salts (PPNN3) binary catalytic system has also been applied in the ROCOP of oxetane with CS2. Detailed study revealed the presence of multiple polymer linkages indicative of sulfur/oxygen scrambling which became more prevalent at higher temperatures.32 Werner and co-workers have reported on the ROCOP of terminal epoxides with CS2 initiated by LitOBu.31 The polythiocarbonates were found to be highly regioregular with up to 94% head-head/tail-tail alternating thionocarbonate/trithiocarbonate linkages.
Herein we describe the ROCOP of 1 with CS2 to form sugar-based sulfur-containing polymers with very high regioselectivity towards HH/TT, trithiocarbonate/thionocarbonate linkages (up to 95%), marking an improvement on the similar polymer synthesised through the ROP of 2. The head/tail configuration of poly(CS2-co-1) can be varied through modulation of the reaction temperature and CS2 stoichiometry. Mechanistic investigations show that ROCOP proceeds at least partially directly. Lastly, poly(CS2-co-1) exhibits partial chemical recyclability into 2, as well as full degradation under UV light.
Results and discussion
Poly(CS2-co-1) made by ROCOP: identification of polymer linkages
Oxetane 1 was synthesised in three steps from D-xylose in accordance with previous reports (51% overall yield).46,47 ROCOP was initially trialed with CrSalen and PPNCl with [1]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CS2]0
[CS2]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CrSalen]0
[CrSalen]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PPNCl]0 loadings of 100
[PPNCl]0 loadings of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
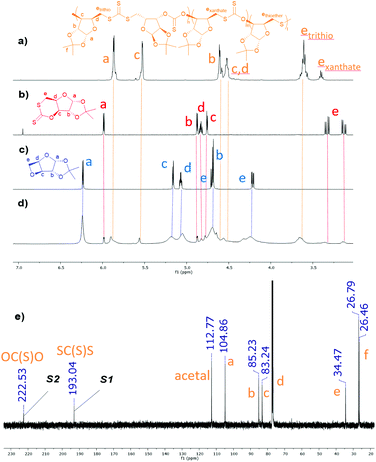
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 80 °C. In contrast with other polymerisations developed with 1 so far,46–49 the reaction proceeded readily at relatively mild temperatures, with 33% conversion of 1 after 4.5 h at 80 °C to yield poly(CS2-co-1) of 2800 g mol−1 (ĐM = 1.54; measured by size-exclusion chromatography (SEC)). The formation of resonances at 192.9 ppm (S1) and 222.4 ppm (S2) in the 13C{1H} NMR spectrum and 5.52 ppm and 5.80–6.03 ppm in the 1H NMR spectrum suggested the incorporation of CS2 within the polymer backbone, later confirmed by 1H–13C{1H} HSQC and HMBC experiments (Scheme 1, Fig. 1 and 2). 1H and 1H–13C{1H} HMBC NMR spectra indicated that the ROCOP of CS2 and 1 was ring-selective, with CS2 incorporation across the oxetane moiety only. Furthermore, the 1H–13C{1H} HMBC NMR spectrum of isolated and crude poly(CS2-co-1) revealed correlations between carbon resonance S1 and proton environment c (i.e. the CH in position 3 on the xylofuranose core), and between carbon resonance S2 and proton environment e (i.e. the CH2 in position 6 on the xylofuranose core), consistent with HH/TT configuration and alternating thiono- and trithiocarbonate linkages. Overall, the NMR spectroscopic data for poly(CS2-co-1) was similar to that of the regioregular polymer obtained previously by ROP of 2 (Table 2, entry 1).27
1 at 80 °C. In contrast with other polymerisations developed with 1 so far,46–49 the reaction proceeded readily at relatively mild temperatures, with 33% conversion of 1 after 4.5 h at 80 °C to yield poly(CS2-co-1) of 2800 g mol−1 (ĐM = 1.54; measured by size-exclusion chromatography (SEC)). The formation of resonances at 192.9 ppm (S1) and 222.4 ppm (S2) in the 13C{1H} NMR spectrum and 5.52 ppm and 5.80–6.03 ppm in the 1H NMR spectrum suggested the incorporation of CS2 within the polymer backbone, later confirmed by 1H–13C{1H} HSQC and HMBC experiments (Scheme 1, Fig. 1 and 2). 1H and 1H–13C{1H} HMBC NMR spectra indicated that the ROCOP of CS2 and 1 was ring-selective, with CS2 incorporation across the oxetane moiety only. Furthermore, the 1H–13C{1H} HMBC NMR spectrum of isolated and crude poly(CS2-co-1) revealed correlations between carbon resonance S1 and proton environment c (i.e. the CH in position 3 on the xylofuranose core), and between carbon resonance S2 and proton environment e (i.e. the CH2 in position 6 on the xylofuranose core), consistent with HH/TT configuration and alternating thiono- and trithiocarbonate linkages. Overall, the NMR spectroscopic data for poly(CS2-co-1) was similar to that of the regioregular polymer obtained previously by ROP of 2 (Table 2, entry 1).27
| Entry | T (°C) | [1]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CS2]0 [CS2]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : [cat]0 : [cat]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [co-cat]0 [co-cat]0 |
Cat. | Co-catalyst | % conv. of 1a | % 2b | % poly(CS2-co-1) b | Polymer linkages ratio n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lc lc |
M n,SEC [ĐM] (g mol−1)d |
|---|---|---|---|---|---|---|---|---|---|
| Reactions carried out in σ-dichlorobenzene at [1]0 = 1.34 mol L−1 over 20 h unless stated otherwise.a Conversion of 1 determined by 1H NMR spectroscopy in CDCl3 using relative integration of anomeric protons in 1 (δ = 6.26 ppm (d, J = 3.7 Hz, 1H)), poly(CS2-co-1) (δ = 5.88–5.99 ppm (1H)),and 2 (CDCl3, δ = 6.03 ppm (d, J = 3.7 Hz, 1H)).b Calculated by 1H NMR spectroscopy using relative integration of anomeric protons in poly(CS2-co-1) and 2.c Calculated by 1H NMR spectroscopy in CDCl3 using relative integration of e environments (CH2) assigned to HH/TT trithiocarbonate linkages n (δ = 3.68 ppm (h, J = 6.9 Hz, 4H)), HT xanthate linkages m (δ = 3.51 ppm (t, J = 6.6 Hz, 2H).and thioether linkages l (δ = 3.04–2.74 ppm (m, 2H)).d Calculated by SEC relative to polystyrene standards in THF eluent; ĐM = Mw/Mn..e Time = 309 h.f Bracketed values taken at 119 h.g [1]0 = 0.67 mol L−1.h [1]0 = 0.335 mol L−1.i 0.08 mol L−1. | |||||||||
| 1e | 25 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CrSalen | PPNCl | 61 | 4 | 96 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
8700 [1.70] |
| 2f | 60 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CrSalen | PPNCl | 88 | 15 {10} | 85 {90} | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 {59 6 {59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31} 31} |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000[1.77] {5300 [2.02] } 000[1.77] {5300 [2.02] } |
| 3 | 80 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CrSalen | PPNCl | 100 | 13 | 77 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 [2.13] 000 [2.13] |
| 4 | 100 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CrSalen | PPNCl | 100 | 14 | 76 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
7700 [1.63] |
| 5 | 140 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CrSalen | PPNCl | 100 | 38 | 62 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
6300 [1.93] |
| 6 | 80 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CrSalen | PPNCl | 58 | 34 | 66 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 36 |
2700 [1.68] |
| 7 | 80 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CrSalen | PPNCl | 100 | 22 | 78 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
9100 [1.76] |
| 8 | 80 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CrSalen | PPNCl | 100 | 10 | 90 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000[1.78] 000[1.78] |
| 9 | 80 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CoSalen | PPNCl | 0 | — | — | — | — |
| 10 | 80 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
AlSalen | PPNCl | 1 | — | — | — | — |
| 11 | 25 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
AlTris | PPNCl | 12 | 8 | 92 | — | — |
| 12 | 80 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
AlTris | PPNCl | 100 | 19 | 81 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
6300 [1.69] |
| 13 | 100 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
AlTris | PPNCl | 100 | 52 | 48 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
2200 [1.53] |
| 14 | 80 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
LZn 2 Ph 2 | CHD | 1 | — | — | — | — |
| 15 | 80 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOtBu | 18-Crown-6 | 2 | — | — | — | — |
| 16 | 80 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
CrSalen | — | 8 | 43 | 57 | — | — |
| 17 | 80 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | PPNCl | 0 | — | — | — | — |
| 18 | 80 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CrSalen | NBu4Cl | 91 | 16 | 84 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
6600 [2.00] |
| 19 | 80 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CrSalen | NBu4Br | 4 | — | — | — | — |
| 20 | 80 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CrSalen | NBu4I | 51 | 31 | 69 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
4700 [1.77] |
| 21 | 110 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CrSalen | PPNCl | 100 | 35 | 65 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
4400 [1.58] |
| 22g | 110 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CrSalen | PPNCl | 100 | 58 | 42 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
1700 [1.33] |
| 23h | 110 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CrSalen | PPNCl | 93 | 65 | 35 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51 51 |
1400 [1.28] |
| 24i | 110 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CrSalen | PPNCl | 33 | 52 | 48 | — | — |
| Entry | T (°C) | Reagent | % a Conv | % b Polym |
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lc lc |
M n,SEC [ĐM]d |
|---|---|---|---|---|---|---|
Reactions carried out for 20 h in σ-dichlorobenzene with [2/1monomerunit]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CrSalen]0 [CrSalen]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PPNCl]0 loadings of 200 [PPNCl]0 loadings of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and [2/1monomerunit]0 = 1.34 mol L−1 unless otherwise stated.a Conversion of substrate determined by 1H NMR spectroscopy in CDCl3 using relative integration of anomeric protons (1H) in substrates and products (1: δ = 6.26 ppm (d, J = 3.7 Hz)); poly(CS2-co-1): δ = 5.88–5.99 ppm; 2: δ = 6.03 ppm (d, J = 3.7 Hz).b Calculated by 1H NMR spectroscopy using relative integration of anomeric protons in poly(CS2-co-1) and 2.c Calculated by 1H NMR spectroscopy in CDCl3 using relative integration of e environments (CH2) assigned to HH/TT trithiocarbonate linkages n (δ = 3.68 ppm (h, J = 6.9 Hz, 4H)), HT xanthate linkages m (δ = 3.51 ppm (t, J = 6.6 Hz, 2H).and thioether linkages l (δ = 3.04–2.74 ppm (m, 2H)).d Calculated by SEC relative to polystyrene standards in THF eluent; ĐM = Mw/Mn.e Reaction performed with [2]0 1, and [2/1monomerunit]0 = 1.34 mol L−1 unless otherwise stated.a Conversion of substrate determined by 1H NMR spectroscopy in CDCl3 using relative integration of anomeric protons (1H) in substrates and products (1: δ = 6.26 ppm (d, J = 3.7 Hz)); poly(CS2-co-1): δ = 5.88–5.99 ppm; 2: δ = 6.03 ppm (d, J = 3.7 Hz).b Calculated by 1H NMR spectroscopy using relative integration of anomeric protons in poly(CS2-co-1) and 2.c Calculated by 1H NMR spectroscopy in CDCl3 using relative integration of e environments (CH2) assigned to HH/TT trithiocarbonate linkages n (δ = 3.68 ppm (h, J = 6.9 Hz, 4H)), HT xanthate linkages m (δ = 3.51 ppm (t, J = 6.6 Hz, 2H).and thioether linkages l (δ = 3.04–2.74 ppm (m, 2H)).d Calculated by SEC relative to polystyrene standards in THF eluent; ĐM = Mw/Mn.e Reaction performed with [2]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TBD]0 [TBD]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [4-MeBnOH]0 loadings of 100 [4-MeBnOH]0 loadings of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in DCM with [2]0 = 1.0 mol L−1, time = 15 minutes.f Substrate polymer data: n 1 in DCM with [2]0 = 1.0 mol L−1, time = 15 minutes.f Substrate polymer data: n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) l ratio = 82 l ratio = 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12; Mn,SEC = 8500 g mol−1.g Insoluble black residue formed during 20 h reaction. 12; Mn,SEC = 8500 g mol−1.g Insoluble black residue formed during 20 h reaction. |
||||||
| 1e (ref. 27) | 25 | 2 | 86 | 86 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 [1.5] 600 [1.5] |
| 2 | 80 | 2 | 75 | 75 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 [2.05] 700 [2.05] |
| 3f | 80 | Poly(CS2-co-1) | 0 | 100 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
8200 [1.70] |
| 4f | 110 | Poly(CS2-co-1) | 45 | 55 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
3600 [1.62] |
| 5f | 140 | Poly(CS2-co-1) | —g | — | — | — |
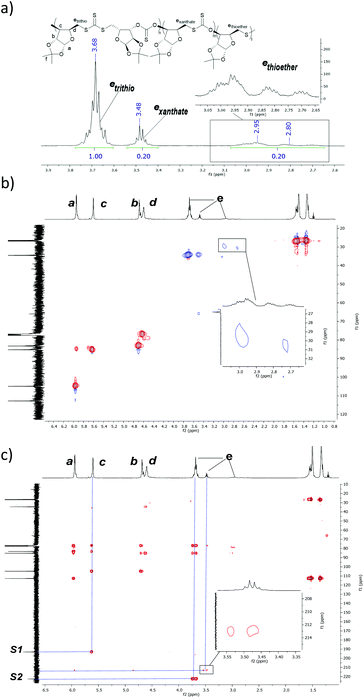
Minor resonances associated with the e environment were also observed between δ = 2.65–3.13 ppm and δ = 3.42–3.56 ppm, suggesting the occurrence of other polymer linkages (Fig. 2). While only resonances S1 and S2 were observed in the thiocarbonyl region of the 13C{1H} NMR spectrum of isolated poly(CS2-co-1) (Fig. 1e), a weak correlation between a xanthate-like (δ = 212.8 ppm) and an e (δ = 3.42–3.56 ppm) proton environment was detected by 1H–13C{1H} NMR HMBC spectroscopy (Fig. 2c), revealing the presence of xanthate polymer linkages (exanthate). Conversely, no 1H–13C{1H} NMR HMBC correlation was observed for the e proton environment detected at δ = 2.65–3.13 ppm. Comparison with literature data32 revealed that the 1H NMR and 13C{1H} NMR shift values of this resonance was comparable to known polythioethers (Fig. 2b),36 corroborating the absence of a nearby quaternary carbon. This resonance was therefore attributed to a thioether polymer linkage (ethioether). The complexity of the signals seen may stem from random position of the thioether linkages in the polymer sequence. Regardless of the origins of those various linkages, the e environments in the 1H NMR spectra were thus identified as an easy way to assess the selectivity of the ROCOP process.
Encouraged by this preliminary data, further catalytic ROCOP experiments were performed to study the effect of concentration, temperature, CS2 stoichiometry and nature of the catalyst and of co-catalyst on the products of the ROCOP reaction (Table 1).
Impact of temperature on polymer linkages
Polymerisations were carried out at 25 °C, 60 °C, 80 °C, 100 °C and 140 °C at [1]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CS2]0
[CS2]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CrSalen]0
[CrSalen]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PPNCl]0 loadings of 200
[PPNCl]0 loadings of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400
400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 1, entries 1–5). CrSalen was found to be active for 1/CS2 ROCOP at all temperatures tested, including at 25 °C (Table 1, entry 1). The room temperature reactivity of the CS2/1 ROCOP contrasts strongly with analogous reactions with cyclic anhydrides which necessitate high temperatures and long reaction times (100 °C, 2–6 days) to give similar oxetane conversions. Overall, the reactivity of 1 with CS2 seems comparable with that of cyclohexene oxide,30 cyclopentene oxide42 and oxetane,32 although direct comparisons of TOFs are not appropriate due to substantial differences in the reaction conditions used. The enhanced activity of CrSalen in the ROCOP of CS2/1 as compared with cyclic anhydrides may be a result of two effects. Firstly, CS2 is a stronger electrophile than cyclic anhydrides owing to the lower enthalpy of the C
1 (Table 1, entries 1–5). CrSalen was found to be active for 1/CS2 ROCOP at all temperatures tested, including at 25 °C (Table 1, entry 1). The room temperature reactivity of the CS2/1 ROCOP contrasts strongly with analogous reactions with cyclic anhydrides which necessitate high temperatures and long reaction times (100 °C, 2–6 days) to give similar oxetane conversions. Overall, the reactivity of 1 with CS2 seems comparable with that of cyclohexene oxide,30 cyclopentene oxide42 and oxetane,32 although direct comparisons of TOFs are not appropriate due to substantial differences in the reaction conditions used. The enhanced activity of CrSalen in the ROCOP of CS2/1 as compared with cyclic anhydrides may be a result of two effects. Firstly, CS2 is a stronger electrophile than cyclic anhydrides owing to the lower enthalpy of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond vs. the C
S bond vs. the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (573 and 799 kJ mol−1, respectively).50,51 Secondly, following CS2 insertion, the resulting thiocarbonate species are more nucleophilic than a carboxylate and the Cr–S bond is likely less strong than a Cr–O bond, which promotes propagation.
O bond (573 and 799 kJ mol−1, respectively).50,51 Secondly, following CS2 insertion, the resulting thiocarbonate species are more nucleophilic than a carboxylate and the Cr–S bond is likely less strong than a Cr–O bond, which promotes propagation.
Within the limits of 1H NMR spectroscopy, in all the experiments performed, all resonances seen could be assigned to poly(CS2-co-1) (as described above), cyclic xanthate 2 or oxetane monomer 1 (Fig. 1). However, varying the reaction temperature caused significant divergences in product distributions. Increasing the temperature resulted in a loss of regioselectivity (Table 1, entries 1–5 and Fig. S6†). At 25 °C, 60 °C and 80 °C, high HH/TT regioselectivity was observed (92, 85 and 89%, respectively, Table 1, entries 1–3). Conversely, for reactions performed at 100 °C and 140 °C, lower percentages of trithiocarbonates linkages were observed (73 and 57%, respectively, Table 1, entries 4 and 5) with an increase in thioether linkages detected (16% and 31%, respectively). The increased occurrence of thioether linkages at elevated temperatures likely arises from the elimination of COS from propagation intermediates, favoured by entropy (Scheme S2†) and as noted in previous studies.30,32,42 The ratio of xanthate links also increased with temperature, but to a lesser extent than for the thioethers (6% at 25 °C up to 12% at 140 °C, Table 1 entries 1–5).The formation of xanthate linkages may be a result of several mechanisms: the ‘expected’ CS2/1 ROCOP, but also various S/O rearrangements occurring during ROCOP,27 including transthiocarbonation between polymer chains, as well as the regioregular ROP of 2 (Schemes S1 and S2†).
At 80 °C, quantitative conversion of 1 was observed after 20 h, yielding the maximum molar mass of polymer achieved under the conditions tested (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, ĐM = 2.13, Table 1, entry 3). More generally, obtained Mn,SEC values were far from Mn,theo, likely due to residual protic impurities (e.g. 1,2-O-isopropylidene-D-xylofuranose) in the monomer samples which may act as chain transfer agents to decrease the molar mass of the polymers. This is typical within the field of cyclic ether/CS2 ROCOP.30,32,42 Further purification of 1, including via successive or reactive distillation with NaH/MeI,52 unfortunately did not increase the polymer molar masses. While analysis of the polymer by MALDI ToF mass spectrometry proved unsuccessful, end-group titration by 31P NMR spectroscopy indicated that some of the polymer chains were linear and terminated by secondary alcohol end groups (Fig. S24 and 25†).
000 g mol−1, ĐM = 2.13, Table 1, entry 3). More generally, obtained Mn,SEC values were far from Mn,theo, likely due to residual protic impurities (e.g. 1,2-O-isopropylidene-D-xylofuranose) in the monomer samples which may act as chain transfer agents to decrease the molar mass of the polymers. This is typical within the field of cyclic ether/CS2 ROCOP.30,32,42 Further purification of 1, including via successive or reactive distillation with NaH/MeI,52 unfortunately did not increase the polymer molar masses. While analysis of the polymer by MALDI ToF mass spectrometry proved unsuccessful, end-group titration by 31P NMR spectroscopy indicated that some of the polymer chains were linear and terminated by secondary alcohol end groups (Fig. S24 and 25†).
Increasing the temperature to 100 °C and 140 °C, also resulted in quantitative conversion of 1, although the polymer molar masses were lower (7700 g mol−1, ĐM = 1.63 and 6300, ĐM = 1.93, Table 1 entries 4 and 5, respectively). At these temperatures, several factors may be responsible for a decrease in molar mass, including the increased occurrence of thioether linkages (concurrent with loss of COS) in the polymer, and the decreased reaction selectivity towards polymer (in favour of cyclic monomer 2), in line with thermodynamics principles.
The reaction at 60 °C was monitored for up to 5 days to assess the impact of longer reaction times on the polymer linkages and molar mass (Table 1, entry 2). A decrease in molar mass and increasing thioether linkages were observed, suggesting decarbonylsulfonation of the polymer chains upon extended heating.
Impact of CS2 stoichiometry and co-catalyst
The impact of CS2 loadings on product distributions and ROCOP regioselectivity was next investigated (Table 1, entries 1 and 6–8). The reactions were performed at 80 °C with a [1]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CrSalen]0
[CrSalen]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PPNCl] feed ratio of 200
[PPNCl] feed ratio of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. At [1]0
1. At [1]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CS2]0 ratio of 2
[CS2]0 ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, conversion of 1 was limited to 58% after 20 h (Table 1, entry 6). Selectivity for alternating linkages also decreased significantly with a concurrent increase in thioether links (56% and 36%, respectively), as compared with the analogous reaction performed with a [1]0
1, conversion of 1 was limited to 58% after 20 h (Table 1, entry 6). Selectivity for alternating linkages also decreased significantly with a concurrent increase in thioether links (56% and 36%, respectively), as compared with the analogous reaction performed with a [1]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CS2]0 ratio of 1
[CS2]0 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Given that the homopolymerisation of 1 is kinetically inaccessible at 80 °C with a CrSalen/PPNCl catalyst, this clearly suggests some S–O exchange reactions, which either progressively liberate COS/CS2 so that ROCOP can continue beyond 50% conversion, or which form a thietane prone to ROP under these conditions. Reactions performed with [1]0
2. Given that the homopolymerisation of 1 is kinetically inaccessible at 80 °C with a CrSalen/PPNCl catalyst, this clearly suggests some S–O exchange reactions, which either progressively liberate COS/CS2 so that ROCOP can continue beyond 50% conversion, or which form a thietane prone to ROP under these conditions. Reactions performed with [1]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CS2]0 ratio of 1
[CS2]0 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 gave quantitative conversion of 1 and an increased percentage of alternating links (87% and 95%, respectively, Table 1, entries 7 and 8). Similar observations have been made previously in the oxetane/CS2 ROCOP, with higher loadings of CS2 shown to inhibit S/O exchange reactions.32 Moreover, the amount of 2 decreased with increasing [CS2]0 (Table 1, entries 6–8): high concentrations of CS2 would likely favour insertion into propagating chains over backbiting reactions.
4 gave quantitative conversion of 1 and an increased percentage of alternating links (87% and 95%, respectively, Table 1, entries 7 and 8). Similar observations have been made previously in the oxetane/CS2 ROCOP, with higher loadings of CS2 shown to inhibit S/O exchange reactions.32 Moreover, the amount of 2 decreased with increasing [CS2]0 (Table 1, entries 6–8): high concentrations of CS2 would likely favour insertion into propagating chains over backbiting reactions.
Impact of the nature of ROCOP catalyst and co-catalyst
The use of AlSalen (Al(III)) and CoSalen (Co(II)) complexes failed to give any conversion of 1 under the screened conditions (Table 1, entries 9 and 10). Conversely, the trisphenolate complex, AlTris (Al(III), was found to be active at 25 °C, 80 °C and 100 °C, although, as compared with the analogous CrSalen reactions, the product microstructure was less regioregular and the molar masses of the polymer were lower (Table 1, entries 11–13). Dizinc complex, LZn2Ph2 (with 1,2-cyclohexandiol (CHD) chain transfer agent) and alkoxide/crown-ether initiator gave no conversion of 1 (Table 1, entries 14 and 15). Lastly, no conversion of 1 was noted in when using only the PPNCl co-catalyst, whilst when performing the reaction with just CrSalen, poor conversion of 1 was obtained as compared with the standard conditions (Table 1, entries 16 and 17).The effect of the co-catalyst nature was next studied (Table 1, entries 18–20). A reaction performed with NBu4Cl at 80 °C with [1]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CS2]
[CS2]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CrSalen]0
[CrSalen]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [NBu4Cl]0 loadings of 200
[NBu4Cl]0 loadings of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400
400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gave 91% conversion of 1 with comparable selectivity ratios to those observed with PPNCl (Table 1, entry 18), albeit with lower molar mass polymer obtained (6600 vs. 15
1 gave 91% conversion of 1 with comparable selectivity ratios to those observed with PPNCl (Table 1, entry 18), albeit with lower molar mass polymer obtained (6600 vs. 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 in entry 3). Conversely, a copolymerisation carried out with NBu4Br gave only 4% conversion, indicating the bromide salt is unsuitable for 1/CS2 ROCOP (Table 1, entry 19). Under similar conditions, NBu4I gave improved conversion of 1 although with lower activity than observed with PPNCl and NBu4Cl (Table 1, entry 20). An increased amount of 2 was also observed suggesting that iodide may promote backbiting.
000 g mol−1 in entry 3). Conversely, a copolymerisation carried out with NBu4Br gave only 4% conversion, indicating the bromide salt is unsuitable for 1/CS2 ROCOP (Table 1, entry 19). Under similar conditions, NBu4I gave improved conversion of 1 although with lower activity than observed with PPNCl and NBu4Cl (Table 1, entry 20). An increased amount of 2 was also observed suggesting that iodide may promote backbiting.
Collectively, these data thus identified CrSalen/PPNCl as the optimal binary catalytic system for 1/CS2 ROCOP so far, in terms of activity, polymer molar mass, and regioselectivity of the polymer linkages.
Optimisation of reaction conditions towards formation of 2
Although the reaction selectivity could not be tailored towards exclusive formation of 2, 1/CS2 cycloaddition could be promoted over ROCOP at high temperatures and low [1]0. At 110 °C and [1]0 = 0.335 mol L−1, formation of up to 65% of 2 was observed (Table 1 entries 21–23). Monomer grade xanthate 2 could then be easily isolated using a simple filtration of the reaction mixture on silica, leading to isolated yields of up to 60%, a considerable improvement on the previous report (15% yield).27 Unfortunately, a further decrease in [1]0 to 0.08 mol L−1 led to a significant drop in catalyst activity and poor conversions of 1 (33%; Table 1, entry 24).Kinetic and mechanistic studies
Considering the presence of 2 in all ROCOP reactions performed, it can be envisaged that the formation of poly(CS2-co-1) does not proceed directly but stepwise, first by the cycloaddition of CS2 and 1 into 2, then ROP of 2 (Fig. 3). This would be analogous to what has been reported in the coupling of CO2 and oxetanes by Darensbourg,53–56 and by Dove and Coulembier.57 A reaction performed at [2]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CrSalen]0
[CrSalen]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PPNCl]0 loadings of 200
[PPNCl]0 loadings of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gave 75% conversion of 2 into a polymer with similar microstructure, molar mass and ĐM values to those obtained previously via reaction of 1 with CS2 (Table 2, entry 1 vs.Table 1, entry 3). In addition, the final product distribution matched well that observed in 1/CS2 ROCOP (around 75
1 gave 75% conversion of 2 into a polymer with similar microstructure, molar mass and ĐM values to those obtained previously via reaction of 1 with CS2 (Table 2, entry 1 vs.Table 1, entry 3). In addition, the final product distribution matched well that observed in 1/CS2 ROCOP (around 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 polymer
25 polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclic monomer ratio). This demonstrates that ROP of xanthate 2 is accessible at 80 °C with the CrSalen/PPNCl catalyst system.
cyclic monomer ratio). This demonstrates that ROP of xanthate 2 is accessible at 80 °C with the CrSalen/PPNCl catalyst system.
In order to probe whether the copolymerisation of CS2/1 occurred via ROP of 2, reaction monitoring by 1H NMR spectroscopy was performed at 25 °C, 80 °C and 110 °C with [1]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CS2]0
[CS2]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CrSalen]0
[CrSalen]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PPNCl]0 loadings of 200
[PPNCl]0 loadings of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400
400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
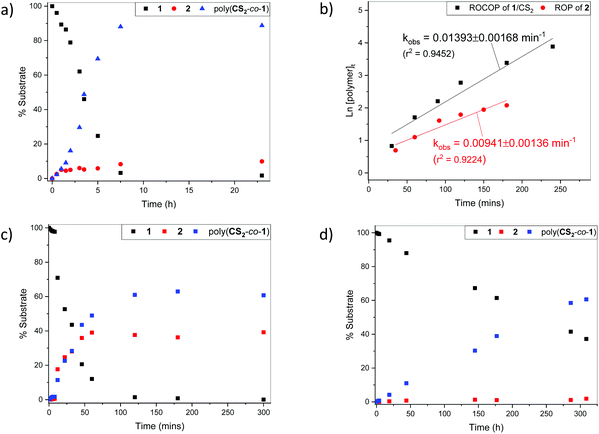
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 4). At 80 °C, inspection of the early stages (<1 h) of the reaction revealed that polymer and 2 formed at approximately the same rate (Fig. 4a). After 1–2 h, the concentration of 2 plateaued as the polymer concentration increased.
1 (Fig. 4). At 80 °C, inspection of the early stages (<1 h) of the reaction revealed that polymer and 2 formed at approximately the same rate (Fig. 4a). After 1–2 h, the concentration of 2 plateaued as the polymer concentration increased.
At 110 °C, simultaneous rapid formation of both the polymer and cyclic xanthate was also observed (Fig. 4c), whereas at 25 °C, minimal xanthate was detected across several days (Fig. 4d). An experiment to monitor the ROP of 2 was also performed at 80 °C, with [2]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CS2]0
[CS2]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CrSalen]0
[CrSalen]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PPNCl]0 loadings of 200
[PPNCl]0 loadings of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the presence of a alcohol initiator, 4-MeBnOH. CS2 and 4-MeBnOH were added to the reaction mixture in attempt to simulate ROCOP conditions, in which excess CS2 and polymer growing chains are present in solution. Assuming first order kinetics in monomer, the rate of polymer formation was slower in the ROP of 2 than in the 1/CS2 ROCOP (Fig. 4b). With no build-up of 2 seen during ROCOP, this data therefore suggest that polymer is formed at least partially via direct ROCOP.
1 in the presence of a alcohol initiator, 4-MeBnOH. CS2 and 4-MeBnOH were added to the reaction mixture in attempt to simulate ROCOP conditions, in which excess CS2 and polymer growing chains are present in solution. Assuming first order kinetics in monomer, the rate of polymer formation was slower in the ROP of 2 than in the 1/CS2 ROCOP (Fig. 4b). With no build-up of 2 seen during ROCOP, this data therefore suggest that polymer is formed at least partially via direct ROCOP.
2 could also be formed via back-biting of the polymer chains (Fig. 3). A reaction performed at 80 °C with isolated polymer as substrate, at [poly(CS2-co-1)]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CrSalen]0
[CrSalen]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PPNCl]0 loadings of 200
[PPNCl]0 loadings of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, gave no formation of cyclic xanthate after 20 h (Table 2, entry 3). This suggests that at 80 °C, cycloaddition of 1 and CS2 is the dominant mechanism for formation 2. However, at 110 °C, backbiting was observed, with approximately 45% xanthate formed after 20 h. No other cyclic species were detected in solution (Table 2, entry 4). The polymer molar mass also decreased from 8500 g mol−1 to 3600 g mol−1, corroborating the loss of 2 from polymer chains. Increasing the temperature further to 140 °C led to formation of a black, insoluble residue (Table 2, entry 5). SEC analysis of the THF-soluble fraction indicated the presence of oligomers only. These data suggest that at elevated temperatures, alternative depolymerisation mechanisms are operative leading to significant, uncontrolled, polymer degradation.
1, gave no formation of cyclic xanthate after 20 h (Table 2, entry 3). This suggests that at 80 °C, cycloaddition of 1 and CS2 is the dominant mechanism for formation 2. However, at 110 °C, backbiting was observed, with approximately 45% xanthate formed after 20 h. No other cyclic species were detected in solution (Table 2, entry 4). The polymer molar mass also decreased from 8500 g mol−1 to 3600 g mol−1, corroborating the loss of 2 from polymer chains. Increasing the temperature further to 140 °C led to formation of a black, insoluble residue (Table 2, entry 5). SEC analysis of the THF-soluble fraction indicated the presence of oligomers only. These data suggest that at elevated temperatures, alternative depolymerisation mechanisms are operative leading to significant, uncontrolled, polymer degradation.
Thermal properties of the polymers
Thermal analysis of the polymers with a range of n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m
m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) l ratios was undertaken to understand the impact of varying polymer linkage on properties (Table 3, Fig. S7–15†). All polymers analysed were found to have temperatures of onset of degradation (Td,onset) of between 167–208 °C. Glass transition temperatures (Tg) were between 108–137 °C, higher than those reported previously,27 but mostly lower than those reported for the analogous, fully oxygenated polycarbonate developed by Gross and co-workers (Tg = 128 °C).58 Contrasting with earlier studies which showed a clear impact of sulfur content on polymer properties,30,42 no general trend between the polymer microstructure and thermal properties was observed. However, as samples of similar molar masses could not be obtained and compared, any existing relationship may be overshadowed by the effect of chain length variations. No crystallinity was detected by differential scanning calorimetry (DSC) or wide-angle X-ray scattering (WAXS) analysis (Fig. S16†).
l ratios was undertaken to understand the impact of varying polymer linkage on properties (Table 3, Fig. S7–15†). All polymers analysed were found to have temperatures of onset of degradation (Td,onset) of between 167–208 °C. Glass transition temperatures (Tg) were between 108–137 °C, higher than those reported previously,27 but mostly lower than those reported for the analogous, fully oxygenated polycarbonate developed by Gross and co-workers (Tg = 128 °C).58 Contrasting with earlier studies which showed a clear impact of sulfur content on polymer properties,30,42 no general trend between the polymer microstructure and thermal properties was observed. However, as samples of similar molar masses could not be obtained and compared, any existing relationship may be overshadowed by the effect of chain length variations. No crystallinity was detected by differential scanning calorimetry (DSC) or wide-angle X-ray scattering (WAXS) analysis (Fig. S16†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m
m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) l) on polymer thermal properties
l) on polymer thermal properties
| Entry |
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) la la |
M n,SEC [ĐM] (g mol−1)b | T d,onset (°C) | T d5 (°C) | T g (°C) |
|---|---|---|---|---|---|
| a Calculated by 1H NMR spectroscopy in CDCl3 using relative integration of e environments (CH2) assigned to HH/TT trithiocarbonate linkages n (δ = 3.68 ppm (h, J = 6.9 Hz, 4H)), HT xanthate linkages m (δ = 3.51 ppm (t, J = 6.6 Hz, 2H).and thioether linkages l (δ = 3.04–2.74 ppm (m, 2H)). b Calculated by SEC relative to polystyrene standards in THF eluent; ĐM = Mw/Mn. | |||||
| 1 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 [1.78] 000 [1.78] |
188 | 216 | 114 |
| 2 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 [2.13] 400 [2.13] |
167 | 190 | 110 |
| 3 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
9100 [1.78] | 191 | 216 | 108 |
| 4 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 [1.27] 400 [1.27] |
208 | 257 | 137 |
| 5 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 [1.19] 000 [1.19] |
172 | 186 | 117 |
| 6 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
8400 [1.67] | 178 | 209 | 120 |
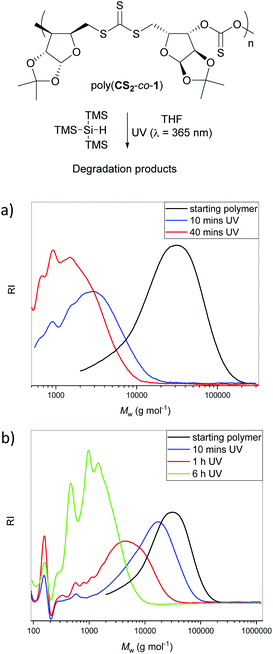
Degradability of the polymers
A potential advantage of sulfur-containing analogues of polycarbonates is that their degradation into small molecules may be possible under UV light. For example, a catalyst-free method for removal of trithiocarbonate RAFT chain transfer agents from poly(vinylpyridine)s has recently been developed by Kennemur and co-workers.21 It was envisioned that such method may also affect the trithiocarbonate linkages in poly(CS2-co-1). A reaction performed in THF with 7.5 monomer equivalents of tris(trimethylsilyl)silane (TTMSS) led to oligomeric products after approximately 10 min of UV irradiation (λ = 365 nm; Fig. 5a). Further analysis of the degradation products by 1H NMR spectroscopy proved challenging (Fig. S20†). A control experiment performed in the absence of TTMSS resulted in degradation of poly(CS2-co-1) too, albeit at a slower rate, as inferred from SEC traces (Fig. 5b). Under these conditions, up to 12% formation of xanthate 2 was also observed by 1H NMR spectroscopy after 1 hour, before degrading progressively along with poly(CS2-co-1).Conclusions
The ROCOP of CS2 with an anhydro-functionalised xylofuranose derivative has been reported. Through variation of the reaction parameters (e.g. temperature and CS2 stoichiometry), some control over the regioselectivity and the nature of the polymer linkages is possible. Conditions can also be tailored to enable the isolation of a polymerisable cyclic xanthate with good yields. Chemical recycling and degradation of the polymers have also been demonstrated. Further investigations are ongoing to develop a deeper understanding of the polymerisation mechanism, of the sulfur/oxygen exchange reactions, and of the impact of the polymer sequence on physical properties.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Analytical facilities were provided through the Material and Chemical Characterisation Facility at the University of Bath. We thank the UK EPSRC (EP/N022793/1, DTP studentship for T.M.G.), as well as the Royal Society (UF/160021 fellowship to A.B.) for research funding.Notes and references
- G. L. Gregory, E. M. López-Vidal and A. Buchard, Chem. Commun., 2017, 53, 2198–2217 RSC.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC.
- F. Fenouillot, A. Rousseau, G. Colomines, R. Saint-Loup and J. P. Pascault, Prog. Polym. Sci., 2010, 35, 578–622 CrossRef CAS.
- J. A. Galbis, M. d. G. García-Martín, M. V. de Paz and E. Galbis, Chem. Rev., 2016, 116, 1600–1636 CrossRef CAS PubMed.
- R. Xiao and M. W. Grinstaff, Prog. Polym. Sci., 2017, 74, 78–116 CrossRef CAS.
- S. E. Felder, M. J. Redding, A. Noel, S. M. Grayson and K. L. Wooley, Macromolecules, 2018, 51, 1787–1797 CrossRef CAS.
- A. T. Lonnecker, Y. H. Lim and K. L. Wooley, ACS Macro Lett., 2017, 6, 748–753 CrossRef CAS.
- K. Mikami, A. T. Lonnecker, T. P. Gustafson, N. F. Zinnel, P.-J. Pai, D. H. Russell and K. L. Wooley, J. Am. Chem. Soc., 2013, 135, 6826–6829 CrossRef CAS PubMed.
- Y. Shen, X. Chen and R. A. Gross, Macromolecules, 1999, 32, 2799–2802 CrossRef CAS.
- J. Kawada, T. Lütke-Eversloh, A. Steinbüchel and R. H. Marchessault, Biomacromolecules, 2003, 4, 1698–1702 CrossRef CAS PubMed.
- M. Luo, X.-H. Zhang and D. J. Darensbourg, Acc. Chem. Res., 2016, 49, 2209–2219 CrossRef CAS PubMed.
- B. Ochiai and T. Endo, Prog. Polym. Sci., 2005, 30, 183–215 CrossRef CAS.
- H.-L. Wu, J.-L. Yang, M. Luo, R.-Y. Wang, J.-T. Xu, B.-Y. Du, X.-H. Zhang and D. J. Darensbourg, Macromolecules, 2016, 49, 8863–8868 CrossRef CAS.
- M. Kato, K. Toshima and S. Matsumura, Biomacromolecules, 2007, 8, 3590–3596 CrossRef CAS.
- J.-L. Yang, Y. Wang, X.-H. Cao, C.-J. Zhang, Z. Chen and X.-H. Zhang, Macromol. Rapid Commun., 2021, 42, 2000472 CrossRef CAS.
- H. R. Kricheldorf and G. Schwarz, J. Macromol. Sci., Part A: Pure Appl.Chem., 2007, 44, 625–649 CrossRef CAS.
- E. Marianucci, C. Berti, F. Pilati, P. Manaresi, M. Guaita and O. Chiantore, Polymer, 1994, 35, 1564–1566 CrossRef CAS.
- R. K. Sadhir and K. F. Schoch, Chem. Mater., 1996, 8, 1281–1286 CrossRef CAS.
- A. Kultys, in Encyclopedia of Polymer Science and Technology, 2010, DOI:10.1002/0471440264.pst355.pub2.
- S. Wu, M. Luo, D. J. Darensbourg and X. Zuo, Macromolecules, 2019, 52, 8596–8603 CrossRef CAS.
- B. A. Fultz, D. Beery, B. M. Coia, K. Hanson and J. G. Kennemur, Polym. Chem., 2020, 11, 5962–5968 RSC.
- L. Tagle, F. Diaz and P. Riveros, Polym. J., 1986, 18, 501–504 CrossRef CAS.
- L. Tagle, F. Diaz and P. Salas, J. Macromol. Sci., Part A: Pure Appl.Chem., 1989, 26, 1321–1334 CrossRef.
- L. H. Tagle, F. R. Diaz, J. C. Vega and P. F. Alquinta, Makromol. Chem., 1985, 186, 915–921 CrossRef CAS.
- C. Berti, E. Marianucci and F. Pilati, Makromol. Chem., 1988, 189, 1323–1330 CrossRef CAS.
- W. Choi, F. Sanda and T. Endo, Macromolecules, 1998, 31, 9093–9095 CrossRef CAS.
- E. M. López-Vidal, G. L. Gregory, G. Kociok-Köhn and A. Buchard, Polym. Chem., 2018, 9, 1577–1582 RSC.
- Y.-Z. You, C.-Y. Hong and C.-Y. Pan, Macromol. Rapid Commun., 2002, 23, 776–780 CrossRef CAS.
- A. W. M. Lee, W. H. Chan and H. C. Wong, Synth. Commun., 1988, 18, 1531–1536 CrossRef CAS.
- D. J. Darensbourg, J. R. Andreatta, M. J. Jungman and J. H. Reibenspies, Dalton Trans., 2009, 8891–8899 RSC.
- J. Diebler, H. Komber, L. Häußler, A. Lederer and T. Werner, Macromolecules, 2016, 49, 4723–4731 CrossRef CAS.
- M. Luo, X.-H. Zhang and D. J. Darensbourg, Macromolecules, 2015, 48, 5526–5532 CrossRef CAS.
- J.-L. Yang, L.-F. Hu, X.-H. Cao, Y. Wang and X.-H. Zhang, Chin. J. Chem., 2020, 38, 269–274 CrossRef CAS.
- Y. Li, H.-Y. Duan, M. Luo, Y.-Y. Zhang, X.-H. Zhang and D. J. Darensbourg, Macromolecules, 2017, 50, 8426–8437 CrossRef CAS.
- T.-J. Yue, W.-M. Ren, Y. Liu, Z.-Q. Wan and X.-B. Lu, Macromolecules, 2016, 49, 2971–2976 CrossRef CAS.
- C.-J. Zhang, T.-C. Zhu, X.-H. Cao, X. Hong and X.-H. Zhang, J. Am. Chem. Soc., 2019, 141, 5490–5496 CrossRef CAS PubMed.
- T.-J. Yue, W.-M. Ren, L. Chen, G.-G. Gu, Y. Liu and X.-B. Lu, Angew. Chem., Int. Ed., 2018, 57, 12670–12674 CrossRef CAS PubMed.
- J.-L. Yang, H.-L. Wu, Y. Li, X.-H. Zhang and D. J. Darensbourg, Angew. Chem., Int. Ed., 2017, 56, 5774–5779 CrossRef CAS PubMed.
- S. Paul, Y. Zhu, C. Romain, R. Brooks, P. K. Saini and C. K. Williams, Chem. Commun., 2015, 51, 6459–6479 RSC.
- A. Plajer and C. K. Williams, Angew. Chem., Int. Ed, 2021 DOI:10.1002/anie.202104495.
- X.-H. Zhang, F. Liu, X.-K. Sun, S. Chen, B.-Y. Du, G.-R. Qi and K. M. Wan, Macromolecules, 2008, 41, 1587–1590 CrossRef CAS.
- D. J. Darensbourg, S. J. Wilson and A. D. Yeung, Macromolecules, 2013, 46, 8102–8110 CrossRef CAS.
- M. J. H. Worthington, R. L. Kucera and J. M. Chalker, Green Chem., 2017, 19, 2748–2761 RSC.
- J. M. Chalker, M. J. H. Worthington, N. A. Lundquist and L. J. Esdaile, Top. Curr. Chem., 2019, 377, 16 CrossRef PubMed.
- X. Wu, J. A. Smith, S. Petcher, B. Zhang, D. J. Parker, J. M. Griffin and T. Hasell, Nat. Commun., 2019, 10, 647 CrossRef PubMed.
- T. M. McGuire, J. Bowles, E. Deane, E. H. E. Farrar, M. N. Grayson and A. Buchard, Angew. Chem., Int. Ed., 2021, 60, 4524–4528 CrossRef CAS PubMed.
- T. M. McGuire, E. F. Clark and A. Buchard, Macromolecules, 2021, 54, 5094–5105 CrossRef CAS.
- T. Uryu, Y. Koyama and K. Matsuzaki, Makromol. Chem., 1984, 185, 2099–2107 CrossRef CAS.
- T. Uryu, Y. Koyama and K. Matsuzaki, J. Polym. Sci., Polym. Lett. Ed., 1979, 17, 673–678 CrossRef CAS.
- D. Stevenson, J. Am. Chem. Soc., 1955, 77, 2350–2350 CrossRef.
- B. d. Darwent, in National Bureau of Standards, 1970, vol. 31, pp. 15–22 Search PubMed.
- O. Hauenstein, M. Reiter, S. Agarwal, B. Rieger and A. Greiner, Green Chem., 2016, 18, 760–770 RSC.
- D. J. Darensbourg and A. I. Moncada, Macromolecules, 2010, 43, 5996–6003 CrossRef CAS.
- D. J. Darensbourg, A. I. Moncada and S.-H. Wei, Macromolecules, 2011, 44, 2568–2576 CrossRef CAS.
- G. A. Bhat, M. Luo and D. J. Darensbourg, Green Chem., 2020, 22, 7707–7724 RSC.
- D. J. Darensbourg, A. I. Moncada, W. Choi and J. H. Reibenspies, J. Am. Chem. Soc., 2008, 130, 6523–6533 CrossRef CAS PubMed.
- J. Huang, J. De Winter, A. P. Dove and O. Coulembier, Green Chem., 2019, 21, 472–477 RSC.
- Y. Shen, X. Chen and R. A. Gross, Macromolecules, 1999, 32, 2799–2802 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR spectra of polymers and degradation products. SEC, TGA-MS, WAXS and DSC traces. Mn plots and mechanistic considerations. See DOI: 10.1039/d1py00753j |
| This journal is © The Royal Society of Chemistry 2021 |