Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and anionic polymerization of isosorbide mono-epoxides for linear biobased polyethers†
Livia
Matt
 a,
Ilme
Liblikas
a,
Olivier
Bonjour
a,
Ilme
Liblikas
a,
Olivier
Bonjour
 b,
Patric
Jannasch
b,
Patric
Jannasch
 *ab and
Lauri
Vares
*ab and
Lauri
Vares
 *a
*a
aInstitute of Technology, University of Tartu, Nooruse 1, Tartu 50411, Estonia. E-mail: lauri.vares@ut.ee; patric.jannasch@chem.lu.se
bDepartment of Chemistry, Lund University, Box 124, Lund 221 00, Sweden
First published on 12th October 2021
Abstract
A series of regioisomeric isosorbide mono-epoxides, as well as diastereomerically pure mono-epoxy derivatives, have been prepared and studied. Anionic ring-opening polymerization of methoxy-capped monomers produced linear polyethers tethered with isosorbide units. These reasonably high molecular weight polymers exhibited glass transition temperatures at around 10–15 °C and thermal stability up to ∼300 °C, which indicated that the mono-epoxides are promising building blocks for well-defined biobased polymers.
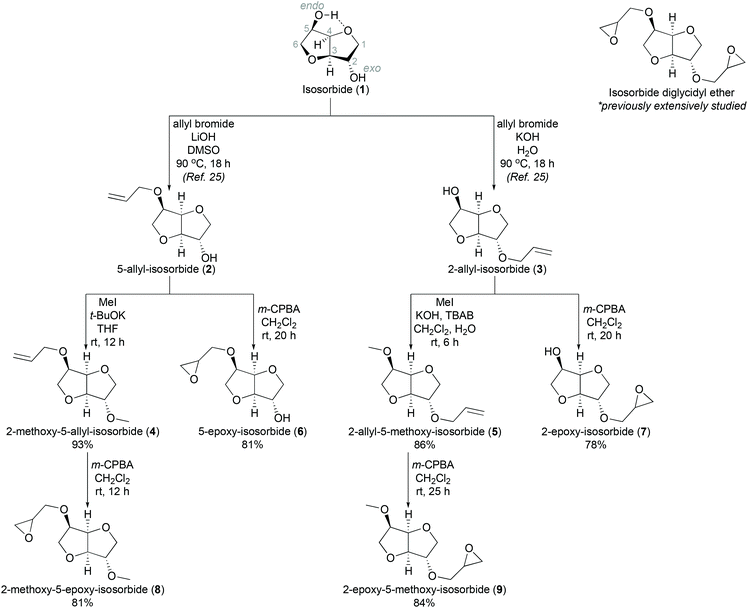
Due to the growing environmental concerns, the motivation to find green alternatives for different chemicals and materials has increased enormously. One compound that has received considerable attention in recent years is isosorbide (1, Scheme 1),1 a platform chemical produced from sorbitol on a commercial scale.2 Sorbitol, in turn, is produced by reduction of glucose. Structurally, isosorbide is a chiral, rigid, V-shaped bicyclic compound3 bearing two non-equivalent secondary hydroxyl groups in endo and exo configurations, respectively. Due to the structural characteristics and availablility,4 isosorbide has been studied and found use in, e.g., the medical5 and cosmetics6 industries, as well as in asymmetric synthesis.7 In the recent decades, however, the number of studies of isosorbide as a building block for new materials has grown sharply.8 In particular, it has been widely considered as a potential bio-derived alternative in various high-performance condensation polymers such as polycarbonates, polyesters and polyurethanes.9,10 In addition, the synthesis and polymerization of isosorbide diglycidyl ether (see Scheme 1) has been extensively studied.11 This compound, also known as isosorbide bisepoxide, has attracted significant interest due to its structural similarities to bisphenol A diglycidyl ether, a commonly used component in plastic materials.12 Regrettably, bisphenol A has proven to have endocrine disruptive effect,13 and bio-derived isosorbide is considered to be a potentially safer substitute.14 Isosorbide diglycidyl ether can be obtained directly by reacting isosorbide with epichlorohydrin (ECH) in aqueous sodium hydroxide, or by a two-step process of sequential allylation and oxidation.15 Such isosorbide bisepoxides have been extensively used in the formation of different epoxy networks,15,16 including hydrogels17 and resins with isosorbide-based amines.18 Moreover, isosorbide diglycidyl ether has been used as a precursor for other bifunctional monomers. For instance, corresponding dimethacrylic monomers have been introduced into different polymer structures,19 and isosorbide dicyclocarbonates have been used in polyurethane synthesis.20
In most cases, the exo and endo hydroxyl groups in isosorbide have not been differentiated and, in the case of polymers, the isosorbide unit has been fully incorporated into the polymer backbone. Regioselective functionalization is less investigated. It has been acknowledged that the endo OH-group at C5 forms intramolecular hydrogen bond and has therefore a higher nucleophilicity.21 The exo-OH group, on the other hand, is sterically less hindered compared to endo-OH.22 Hence, this non-equality opens possibility to regioselectively functionalize either the C2 or the C5 position. For instance, an acetylation of isosorbide with acetic anhydride has been reported with moderate selectively: exo-acetate can be preferentially obtained using KOH at 120–140 °C, whereas using PbO as an additive at room temperature gives a preference towards the endo-acetate formation.23 These mono-acetylated isosorbide derivatives can be used as intermediates for several other mono-functionalized isosorbide compounds, because the deacetylation under basic conditions can readily be performed in high yields.24,25 Similarly, benzylation26 and tosylation27 of isosorbide also give modest preference towards the C5 derivative. Additionally, a majority of biocatalytic procedures have shown selectivity towards the endo hydroxyl group in isosorbide.28 Recently, a highly regioselective Lipozyme RM IM-catalyzed 5-OH methacrylation of isosorbide was reported by our group.29
Such regioselective functionalization enables the preparation of polymers with the rigid isosorbide units as pendant groups. Usually, these polymers are obtained by chain-growth polymerization methods. Conversely, most polymers of isosorbide derivatives have so far been synthesized via step-growth polymerization, which are generally very energy extensive processes where often severely discoloured products are formed.30 Investigations on chain-growth polymerizations of isosorbide derivatives under milder conditions have been rather scarce. In particular, ring-opening polymerizations have not been given much attention.9,31 For example, isosorbide mono-epoxides may be polymerized via cationic or anionic ring-opening mechanism to obtain polyethers with pendant isosorbide groups. Polyethers prepared from mono-epoxides such as ethylene oxide and propylene oxide are used in a wide variety of areas, ranging from surfactants, additives, coatings and surface modifying agents.32 Moreover, the heteroatoms in the polyether backbone increase the potential for biodegradability of the polymer.33
To our surprise, the synthesis of isosorbide mono-epoxides (i.e., isosorbide monoglycidyl ethers) has hardly been studied.34 Thus, no polymerization of isosorbide mono-epoxy derivatives has been reported yet. In the present communication, we report on a straightforward method to prepare both C2 and C5 epoxy-derivatives of isosorbide. In addition to the racemic epoxide units, we demonstrate the synthesis of pure isosorbide diastereomers bearing epoxides with either the (R)- or (S)-configuration. The utility of new monomers has been demonstrated in anionic ring-opening polymerization of two of the isosorbide mono-epoxides.
The synthesis of the isosorbide mono-epoxides is outlined in Scheme 1. We speculated that the free hydroxyl groups in the isosorbide epoxides might hinder the subsequent polymerizations to linear polymers. Therefore, we decided to methylate the free hydroxyl groups in the final monomer structure.
We started our work by the alkylation of isosorbide with allyl bromide according to a previously reported procedure.25 Next, the mono-allylated derivatives 2 and 3 were alkylated with methyl iodide (MeI) in basic conditions to obtain the methylated intermediate compounds 4 and 5. 2-Methoxy-5-allyl-isosorbide 4 was obtained in 93% yield using potassium tert-butoxide (t-BuOK) in THF/H2O. The synthesis of the regioisomeric 5-methoxy allyl-derivative 5 was also achieved in a high yield (86%), but by using KOH and a phase transfer catalyst tetrabutylammonium bromide (TBAB) in CH2Cl2/H2O. The subsequent oxidation of all the mono-allylated isosorbide derivatives (2, 3, 4, and 5) was carried out with meta-chloroperoxybenzoic acid (m-CPBA). In this way, monomers 6 and 7 with free hydroxyl groups were obtained in good yields as expected (81% and 78%, respectively). The synthesis of the methylated isosorbide mono-epoxides 8 and 9 was carried out in a similar fashion, resulting 81% and 84% in yield, respectively. The structures of the monomers were confirmed by NMR and HRMS analysis (see ESI†). In the case of epoxides 6 and 7, the free OH groups can be easily distinguished in the 1H NMR spectrum.29 The 2-exo OH in monomer 6 shows a broad singlet, whereas the 5-endo OH group in compound 7, is intramolecularly hydrogen-bonded and therefore shows a clear doublet with a coupling constant of around 7 Hz (see Fig. S4 and S5 in ESI†).
The epoxidation of allylic compounds using m-CPBA affords derivatives with racemic epoxide units. Hence, another approach must be employed in order to obtain isosorbide derivatives with enantiomerically pure epoxy groups. One possibility is to introduce the chirally pure epoxy-moiety directly into the molecular structure using a corresponding chiral synthon such as (R)- and (S)-epichlorohydrin (ECH). Thus, to obtain (S)- and (R)-epoxy derivatives ((S)-8, (R)-8, (S)-9, and (R)-9, Scheme 2), the corresponding methyl-protected isosorbide derivatives 10 and 11 had to be synthesized first. As there is no direct regioselective method for the mono-methylation of isosorbide,27,28 these intermediates (10 and 11) were prepared from isosorbide using either selective 5-benzylation or 2-tetrahydropyranylation followed by methylation and the subsequent removal of benzyl and tetrahydropyranyl groups, respectively, as described in detail in ESI† (Schemes S1 and S2).
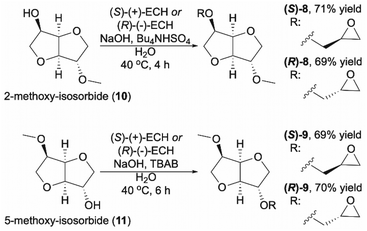 | ||
| Scheme 2 Synthesis of single diastereomers of isosorbide 5-epoxies (S)-8 and (R)-8, and isosorbide 2-epoxies (S)-9 and (R)-9. | ||
Finally, the 2-methoxy-isosorbide 10 was treated with (S)-(+)-ECH or (R)-(−)-ECH and Bu4NHSO4 in aqueous NaOH solution at 40 °C to afford (S)-8 and (R)-8 in 71% and 69% yield, respectively (Scheme 2).35 Monomers (S)-9 and (R)-9 were obtained from 5-methoxy-isosorbide 11 in a similar way, only TBAB was used instead of Bu4NHSO4 (Scheme 2).36 Both methods turned out to be applicable for the synthesis of pure (S)- and (R)-monomers as the yields reached up to 70%.
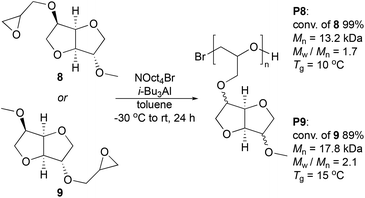
The ring-opening polymerization of mono-epoxy isosorbide derivates 8 and 9 was evaluated by anionic mechanism. We selected a method with tetraoctylammonium bromide (NOct4Br) as initiator and triisobutylaluminium (i-Bu3Al) as catalyst, as this is a widely utilized procedure to prepare polyethers with relatively high molecular masses.37 Epoxides 8 and 9 were both polymerized following the same procedure using NOct4Br and i-Bu3Al during 24 h in toluene to obtain polymers P8 and P9, respectively (Scheme 3). Initiation was performed at −30 °C and then the system was allowed to reach to room temperature. The conditions employed provided polyethers P8 and P9 with high monomer conversions, >99% and 89%, respectively. Analysis by size-exclusion chromatography (SEC) in THF revealed number average molecular weights of Mn = 13.2 and 17.8 kg mol−1 and polydispersity indexes of Mw/Mn = 1.7 and 2.1 for P8 and P9, correspondingly (ESI, Fig. S1†). We also initially attempted cationic ring-opening polymerization using the Sc(OTf)3/propylene oxide system in acetonitrile,31 as well as organocatalytic polymerization conditions38 (Et3B and phosphazene bases). However, in both cases no polymerization occurred and the unreacted monomer was fully recovered.
Thermogravimetric analysis (TGA) under N2 indicated the onset of thermal decomposition of polyethers P8 and P9 at around 300 °C, as revealed by the derivative thermogravimetric (DTG) curves. The highest mass-loss rates were reached at Tp = 390 and 394 °C for P8 and P9, respectively (ESI, Fig. S2†). Interestingly, the glass transition temperature (Tg) determined by differential scanning calorimetry (DSC) was 10 and 15 °C for P8 and P9, correspondingly (ESI, Fig. S3†). Compared to Tg's of other common polyethers like poly(ethylene glycol) (−67 °C), poly(propylene glycol) (−74 °C), poly(epichlorohydrin) (−22 °C),39 the Tg values of the current linear isosorbide polyethers P8 and P9 are rather high because of the rigid isosorbide groups. The reasons for the slightly different properties of P8 and P9 may be caused by the differences in stereochemistry and will be further studied in the future. The solubility of polymers P8 and P9 was evaluated in multiple solvents, arranged according to their hydrogen-bonding capacity and solubility parameter δ (ESI, Table S1†). Both polyethers were soluble in methanol, DMSO, THF, chloroform, and toluene, but insoluble in H2O, n-butanol, Et2O, and acetonitrile at room temperature.
These initial results indicate that the polymerization of isosorbide mono-epoxides can afford very interesting polymers. Thus, this motivates the further investigation of these bioderived monomers, including copolymerizations to obtain new materials and the possibility for biodegradability of corresponding isosorbide polyethers which would be particularly enticing to study.
In conclusion, we have prepared and characterized a series of novel isosorbide mono-epoxides, where the remaining free hydroxyl group in isosorbide was capped as a methyl ether. Additionally, a method to synthesize diastereomerically pure isosorbide mono-epoxides was developed. The usefulness of the novel isosorbide mono-epoxides was demonstrated in anionic ring-opening polymerization leading to polyethers with comparatively high molecular weight and Tg. This clearly demonstrates that isosorbide-based mono-epoxides are promising new building blocks in the field of biobased polymers tailored to replace conventional fossil-based materials in various applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support by the European Regional Development Fund, the Estonian Research Council via MOBTT21 and ResTA7 projects, and the Swedish Research Council Formas (2016-00468). We thank Thanh Huong Pham for initial DSC and TGA analyses.Notes and references
- R. C. Hockett, H. G. Fletcher, E. L. Sheffield and R. M. Goepp, J. Am. Chem. Soc., 1946, 68, 927–930 CrossRef CAS PubMed.
- C. Dussenne, T. Delaunay, V. Wiatz, H. Wyart, I. Suisse and M. Sauthier, Green Chem., 2017, 19, 5332–5344 RSC.
- G. Flèche and M. Huchette, Starch/Staerke, 1986, 38, 26–30 CrossRef.
- Addit. Polym., 2015, 6, 6, 8–9, DOI:10.1016/s0306-3747(15)30073-7.
- DrugBank: isosorbide mononitrate, https://go.drugbank.com/drugs/DB01020, (accessed May 10, 2021).
- SpecialChem: dimethyl isosorbide, https://cosmetics.specialchem.com/inci/dimethyl-isosorbide#, (accessed May 10, 2021).
- M. Kadraoui, T. Maunoury, Z. Derriche, S. Guillarme and C. Saluzzo, Eur. J. Org. Chem., 2015, 441–457 CrossRef CAS.
- M. Al–Naji, H. Schlaad and M. Antonietti, Macromol. Rapid Commun., 2021, 42, 2000485 CrossRef PubMed.
- (a) F. Fenouillot, A. Rousseau, G. Colomines, R. Saint-Loup and J. P. Pascault, Prog. Polym. Sci., 2010, 35, 578–622 CrossRef CAS; (b) M. Rose and R. Palkovits, ChemSusChem, 2012, 5, 167–176 CrossRef CAS PubMed; (c) D. J. Saxon, A. M. Luke, H. Sajjad, W. B. Tolman and T. M. Reineke, Prog. Polym. Sci., 2020, 101, 101196 CrossRef CAS.
- DURABIO, https://www.mcpp-global.com/en/asia/products/brand/durabiotm/, (accessed May 10, 2021).
- J. D. Zech and J. W. Le Maistre, 3272845A, 1966.
- H. Q. Pham and M. J. Marks, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, vol. 13, pp. 155–244 Search PubMed.
- R. Steinmetz, N. A. Mitchner, A. Grant, D. L. Allen, R. M. Bigsby and N. Ben-Jonathan, Endocrinology, 1998, 139, 2741–2747 CrossRef CAS PubMed.
- F. Liguori, C. Moreno-Marrodan and P. Barbaro, Chem. Soc. Rev., 2020, 49, 6329–6363 RSC.
- X. Feng, A. J. East, W. B. Hammond, Y. Zhang and M. Jaffe, Polym. Adv. Technol., 2011, 22, 139–150 CrossRef CAS.
- (a) C. Lorenzini, D. L. Versace, E. Renard and V. Langlois, React. Funct. Polym., 2015, 93, 95–100 CrossRef CAS; (b) S. Zheng, D. A. Bellido-Aguilar, J. Hu, Y. Huang, X. Zhao, Z. Wang, X. Zeng, Q. Zhang and Z. Chen, Prog. Org. Coat., 2019, 136, 105265 CrossRef CAS; (c) S. Zheng, D. A. Bellido-Aguilar, X. Wu, X. Zhan, Y. Huang, X. Zeng, Q. Zhang and Z. Chen, ACS Sustainable Chem. Eng., 2019, 7, 641–649 CrossRef CAS; (d) Q. Li, S. Ma, J. Wei, S. Wang, X. Xu, K. Huang, B. Wang, W. Yuan and J. Zhu, Macromol. Res., 2020, 28, 480–493 CrossRef CAS; (e) C. Musa, A. Kervoëlen, P.-E. Danjou, A. Bourmaud and F. Delattre, Mater. Lett., 2020, 258, 126818 CrossRef; (f) C. Lemesle, S. Bellayer, S. Duquesne, A.-S. Schuller, L. Thomas, M. Casetta and M. Jimenez, Appl. Surf. Sci., 2021, 536, 147687 CrossRef CAS.
- J. Cheng, P. Zhang, T. Liu and J. Zhang, Polymer, 2015, 78, 212–218 CrossRef CAS.
- (a) J. Hong, D. Radojčić, M. Ionescu, Z. S. Petrović and E. Eastwood, Polym. Chem., 2014, 5, 5360–5368 RSC; (b) C. Li, J. Dai, X. Liu, Y. Jiang, S. Ma and J. Zhu, Macromol. Chem. Phys., 2016, 217, 1439–1447 CrossRef CAS.
- (a) J. Łukaszczyk, B. Janicki and A. Frick, J. Mater. Sci.: Mater. Med., 2012, 23, 1149–1155 CrossRef PubMed; (b) S. Shin, Y.-J. Kim, M. Toan, J.-G. Kim, T. Nguyen and J. K. Cho, Eur. Polym. J., 2017, 92, 338–345 CrossRef CAS; (c) T. Nguyen, B. Kim, Y.-J. Kim, S.-H. Lee, S. Shin and J. K. Cho, Int. J. Adhes. Adhes., 2018, 80, 60–65 CrossRef CAS.
- V. Besse, R. Auvergne, S. Carlotti, G. Boutevin, B. Otazaghine, S. Caillol, J.-P. Pascault and B. Boutevin, React. Funct. Polym., 2013, 73, 588–594 CrossRef CAS.
- J. S. Brimacombe, A. B. Foster, M. Stacey and D. H. Whiffen, Tetrahedron, 1958, 4, 351–360 CrossRef CAS.
- A. C. Cope and T. Y. Shen, J. Am. Chem. Soc., 1956, 78, 3177–3182 CrossRef CAS.
- P. Stoss, P. Merrath and G. Schlüter, Synthesis, 1987, 174–176 CrossRef CAS.
- R. Tamion, F. Marsais, P. Ribereau, G. Queguiner, D. Abenhaim, A. Loupy and L. Munnier, Tetrahedron: Asymmetry, 1993, 4, 1879–1890 CrossRef CAS.
- D. Abenhaïm, A. Loupy, L. Munnier, R. Tamion, F. Marsais and G. Quéguiner, Carbohydr. Res., 1994, 261, 255–266 CrossRef.
- A. Loupy and D. A. Monteux, Tetrahedron, 2002, 58, 1541–1549 CrossRef CAS.
- R. U. Lemieux and A. G. McInnes, Can. J. Chem., 1960, 38, 136–140 CrossRef CAS.
- (a) R. Seemayer, N. Bar and M. P. Schneider, Tetrahedron: Asymmetry, 1992, 3, 1123–1126 CrossRef CAS; (b) D. Mukesh, D. Sheth, A. Mokashi, J. Wagh, J. M. Tilak, A. A. Banerji and K. R. Thakkar, Biotechnol. Lett., 1993, 15, 1243–1246 CrossRef CAS; (c) Z. Chalecki and E. Guibé-Jampel, Synth. Commun., 1997, 27, 3847–3852 CrossRef CAS.
- L. Matt, J. Parve, O. Parve, T. Pehk, T. H. Pham, I. Liblikas, L. Vares and P. Jannasch, ACS Sustainable Chem. Eng., 2018, 6, 17382–17390 CrossRef CAS.
- J. Wu, P. Eduard, L. Jasinska-Walc, A. Rozanski, B. A. J. Noordover, D. S. van Es and C. E. Koning, Macromolecules, 2013, 46, 384–394 CrossRef CAS.
- D. J. Saxon, M. Nasiri, M. Mandal, S. Maduskar, P. J. Dauenhauer, C. J. Cramer, A. M. LaPointe and T. M. Reineke, J. Am. Chem. Soc., 2019, 141, 5107–5111 CrossRef CAS PubMed.
- J. Herzberger, K. Niederer, H. Pohlit, J. Seiwert, M. Worm, F. R. Wurm and H. Frey, Chem. Rev., 2016, 116, 2170–2243 CrossRef CAS PubMed.
- M. Okada, Prog. Polym. Sci., 2002, 27, 87–133 CrossRef CAS.
- (a) P. Stoss, G. Schlüter and R. Axmann, Arznem.-Forsch./Drug Res., 1990, 40, 13–18 CAS; (b) P. Stoss and M. Leitold, DE3421072A1, 1984; (c) A. Lavergne, Y. Zhu, V. Molinier and J.-M. Aubry, Colloids Surf., A, 2012, 404, 56–62 CrossRef CAS.
- N. G. Luk'yanenko, A. V. Lobach and O. N. Leus, Russ. J. Org. Chem., 2003, 39, 1042–1047 CrossRef.
- Y. Le Huérou, J. Doyon and R. L. Grée, J. Org. Chem., 1999, 64, 6782–6790 CrossRef PubMed.
- (a) V. Rejsek, D. Sauvanier, C. Billouard, P. Desbois, A. Deffieux and S. Carlotti, Macromolecules, 2007, 40, 6510–6514 CrossRef CAS; (b) S. Carlotti, A. Labbe, V. Rejsek, S. Doutaz, M. Gervais and A. Deffieux, Macromolecules, 2008, 41, 7058–7062 CrossRef CAS.
- Y. Chen, J. Shen, S. Liu, J. Zhao, Y. Wang and G. Zhang, Macromolecules, 2018, 51, 8286–8297 CrossRef CAS.
- Polymer Properties Database: Glass Transition Temperatures, http://polymerdatabase.com/polymer%20physics/Polymer%20Tg%20C.html, (accessed April 27, 2021).
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, 1H and 13C NMR spectra, polymers' solubility table, TGA profiles, SEC and DSC curves. See DOI: 10.1039/d1py00687h |
| This journal is © The Royal Society of Chemistry 2021 |