Synthesis and free radical photopolymerization of triphenylamine-based oxime ester photoinitiators†
Abstract
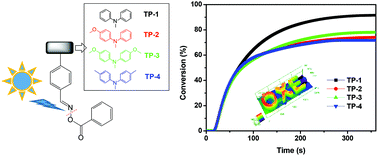
Four visible light triphenylamine-based oxime ester photoinitiators (TP-1–4) were synthesized and confirmed successfully through 1H NMR, 13C NMR, and electron impact mass spectrometry (EI-MS) measurements as well as elementary analyses (EA). The design strategy for triphenylamine substituted photoinitiators involved the use of hydrogen atoms only for TP-1, one methoxy group for TP-2, two methoxy groups for TP-3 and two methyl groups for TP-4. The incorporation of triphenylamine derivatives in oxime esters gives near UV and visible light absorption characters with maximum absorption peaks at around 360 nm. Upon using a UV lamp as an irradiation source, all the oxime esters showed good double bond conversion efficiency in a range of 72–92% in the free radical polymerization of trimethylolpropane triacrylate (TMPTA) through photo-DSC experiments. Among all, TP-1 displayed the highest final conversion efficiency. Accordingly, the photochemical reaction behavior was studied using photolysis, cyclic voltammograms (CV), electron spin resonance (ESR) and theoretical calculations. Additionally, selected photoinitiating systems exhibited outstanding 3D patterns with high resolution and good edge definition through direct laser write (DLW) experiments @405 nm. This work thus demonstrates a judicious chemical design to fine-tune the photopolymerization character in triphenylamine oxime ester analogues.


 Please wait while we load your content...
Please wait while we load your content...