 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Supramolecular hydrogels prepared from fluorescent alkyl pyridinium acrylamide monomers and CB[8]†
Daniel J.
Whitaker
,
Zehuan
Huang
,
Brooke W.
Longbottom
 ,
Renata L.
Sala
,
Guanglu
Wu
,
Renata L.
Sala
,
Guanglu
Wu
 and
Oren A.
Scherman
and
Oren A.
Scherman
 *
*
Melville Laboratory for Polymer Synthesis, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW, UK. E-mail: oas23@cam.ac.uk
First published on 18th December 2020
Abstract
We introduce a new facile synthetic methodology for the preparation of alkyl pyridinium-containing acrylamide monomers. Inclusion complexes of these new monomers with CB[8] facilitate supramolecular crosslinking in a series of hydrogels formed through in situ polymerisation. The resulting hydrogels not only show greatly enhanced mechanical properties over a purely acrylamide-based gel, but also by selecting fluorescent pyridine moieties, the materials are endowed with the fluorescence properties desirable for several sensing applications.
Introduction
Owing to their unique mechanical properties, dynamic nature and diverse functionality, supramolecular hydrogels have been widely studied over the past few decades.1–10 Specifically, in the field of host–guest mediated supramolecular hydrogels there are two main approaches to prepare the final material. In the first approach, a polymer with pendent guest or host molecules is synthesised; a subsequent mixing step (with the addition of a free guest if required) then induces host–guest interactions that lead to gelation.11–15 In the second approach, pioneered by Harada et al., an inclusion complex is first formed between the host and guest molecule(s). The resulting complex is then co-polymerised with a water-soluble monomer yielding a supramolecular hydrogel in situ.16One molecular host, which has been utilised to introduce supramolecular crosslinks into polymer-based hydrogels, is cucurbit[8]uril (CB[8]), a barrel shaped molecule with a hydrophobic inner cavity.17–20 In recent reports from our group we prepared a range of hydrogels with high CB[8] loadings (2.5 mol%) and mass fractions (20 wt%) by performing in situ polymerisation of aqueous solutions of various monomers with 5 mol% 1-benzyl-3-vinylimidazolium, a polymerisable monomer exhibiting 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation with CB[8].21–23 We also demonstrated that this benzyl-imidazolium motif could be simultaneously bound with a perfluorophenyl moiety forming strong polar-π interactions within CB[8] mediated hydrogels.24 Similarly, Zhang et al. demonstrated that an inclusion complex formed from an ester derivative of the tripeptide Phe–Gly–Gly and CB[8] could be polymerised to form both supramolecular hydrogels and microgels.25,26 They noted that supramolecular hydrogels formed using this approach were significantly more mechanically robust than their counterparts produced by first synthesising the polymers and then forming the CB[8] complex (a phenomenon which was suggested to be a result of the viscous polymer solution limiting CB[8] diffusion).26 These examples demonstrate that performing in situ polymerisation of CB[8] inclusion complexes is a promising method to prepare supramolecular hydrogels; however, only two monomer architectures have been identified to date, which can be harnessed for this approach.
1 complexation with CB[8].21–23 We also demonstrated that this benzyl-imidazolium motif could be simultaneously bound with a perfluorophenyl moiety forming strong polar-π interactions within CB[8] mediated hydrogels.24 Similarly, Zhang et al. demonstrated that an inclusion complex formed from an ester derivative of the tripeptide Phe–Gly–Gly and CB[8] could be polymerised to form both supramolecular hydrogels and microgels.25,26 They noted that supramolecular hydrogels formed using this approach were significantly more mechanically robust than their counterparts produced by first synthesising the polymers and then forming the CB[8] complex (a phenomenon which was suggested to be a result of the viscous polymer solution limiting CB[8] diffusion).26 These examples demonstrate that performing in situ polymerisation of CB[8] inclusion complexes is a promising method to prepare supramolecular hydrogels; however, only two monomer architectures have been identified to date, which can be harnessed for this approach.
Side-chain functionalised polymers bearing fluorescent moieties, fluorescent gels and, specifically, fluorescent supramolecular hydrogels make up a wide field of interest across chemistry, biology and physics, with potential applications in energy conversion, biolabeling, tissue engineering and sensors.27–29 In most polymer-based preparations of fluorescent hydrogels three components are required: a backbone polymer, a dye and a gelator/crosslinker. By selecting pyridine moieties with known fluorescence properties for a generic monomer synthesis, we envisioned that we could reduce the number of components required for hydrogel formation as the crosslink-forming moiety and fluorescent dye could be combined as illustrated in Fig. 1. We propose that incorporation of new properties through the physical crosslink-forming unit enhances the functionality of the gel without significantly altering its overall structure.
 | ||
| Fig. 1 Schematic representation of a supramolecular hydrogel prepared via in situ polymerisation of a pre-formed inclusion complex. | ||
Herein a general monomer architecture was designed containing both an acrylamide and a fluorescent alkyl pyridinium subunit connected via a short carbon chain. The complexation of these molecules with CB[8] is then investigated by isothermal calorimetry (ITC) and 1H NMR spectroscopy. A series of hydrogels are then prepared, and their mechanical properties characterised by strain and frequency dependent oscillatory rheology. Finally, the fluorescence properties of the monomers and hydrogels are explored using UV/Vis and fluorescence spectroscopy.
Results and discussion
Alkyl pyridinium moieties were selected for this study as it has been demonstrated that these compounds often make excellent guests for both cucurbit[7]uril (CB[7]) and CB[8].30–35 The strong binding constant (K1 = 105–107 M−1) between CB[7] or CB[8] and the alkyl pyridinium moiety is attributed both to an electrostatic interaction of the electron deficient aromatic system with the electron rich carbonyl groups of the CB portal and the complementary size of the relatively bulky aromatic ring within the CB cavity.36 The alkyl pyridinium monomers were synthesised via a generic, straightforward, column-free procedure shown in Fig. 2.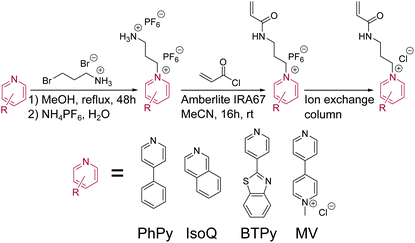 | ||
| Fig. 2 General synthetic route towards alkyl pyridinium monomers. PhPy = 4-phenylpyridine, IsoQ = isoquinoline, BTPy 2-(pyridin-4-yl)benzo[d]thiazole and MV = 1-methyl-[4,4′-bipyridin]-1-ium. | ||
An alkyl linker three carbon atoms in length was selected as it was postulated that a shorter chain might hinder the accessibility of the acrylamide once the inclusion complex is formed. On the other hand, a longer alkyl linker might lead to the formation of a U-shaped 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complex with CB[8] rather than a 2
1 inclusion complex with CB[8] rather than a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 homodimer.37 Though N-heterocyclic structures linked to acrylate moieties are known,38,39 they were not selected for this study as acrylates are susceptible to hydrolysis and their synthetic strategies (formation of the bromoalkyl acrylate followed by nucleophilic substitution to form the quaternary amine) do not yield pure monomers evidenced by baseline impurities in 1H NMR.38 It was found that alkyl-pyridinium acrylamides could not be formed in high purity or good yield from a bromoalkyl acrylamide as the conditions of the nucleophillic substitution (reflux for several days) led to degradation of both the starting material and product. As such, a different synthetic approach was sought.
1 homodimer.37 Though N-heterocyclic structures linked to acrylate moieties are known,38,39 they were not selected for this study as acrylates are susceptible to hydrolysis and their synthetic strategies (formation of the bromoalkyl acrylate followed by nucleophilic substitution to form the quaternary amine) do not yield pure monomers evidenced by baseline impurities in 1H NMR.38 It was found that alkyl-pyridinium acrylamides could not be formed in high purity or good yield from a bromoalkyl acrylamide as the conditions of the nucleophillic substitution (reflux for several days) led to degradation of both the starting material and product. As such, a different synthetic approach was sought.
After initial nucleophilic substitution with 3-bromopropyl-amine hydrobromide, cooling the reaction mixture readily allowed the crystallisation of the pure alkyl pyridinium intermediate. A salt metathesis with ammonium hexafluorophosphate followed to allow solubility in aprotic acetonitrile. Activation of the primary amine with a weakly basic resin and a reaction with acryloyl chloride formed the acrylamide monomer. It is noted that a similar tactic was used by Nordhaus et al.40 who employed trifluoroacetate anions to afford alkyl pyridinium solubility in DMF. A related synthesis was also reported by Katz et al.41 who added N-(acryloxy)succinimide in DMSO to a HEPES buffered solution of propylamine substituted pyridinium, but in our hands this was not found to yield the desired product.
As a resin immobilised base was employed in the previous step, the product was obtained by simple filtering and precipitation, negating the need to separate the cationic product from a cationic conjugate acid. Finally, the counter ion was exchanged back to the chloride via an ion exchange column for greater water solubility. To the best of our knowledge, this provides the first generic strategy for forming alkyl pyridinium acrylamide type monomers.
To test the generality of this synthetic approach, a range of pyridine derivatives were selected (Fig. 2 bottom). 4-Phenylpyridine (PhPy) was selected as it has been shown to form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes with CB[7] and homoternary 2
1 complexes with CB[7] and homoternary 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes with CB[8] after methylation of the pyridyl nitrogen.35,42 Furthermore, PhPy derivatives in the absence of CB exhibit strong fluorescence.43 Isoquinoline (IsoQ) has some structural similarity to 4-phenylpyridine and so it was predicted that, once quaternerised, it would also form a 2
1 complexes with CB[8] after methylation of the pyridyl nitrogen.35,42 Furthermore, PhPy derivatives in the absence of CB exhibit strong fluorescence.43 Isoquinoline (IsoQ) has some structural similarity to 4-phenylpyridine and so it was predicted that, once quaternerised, it would also form a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 homodimer with CB[8]. Previous work44,45 has shown that alkyl-pyridinium derivatives of isoquinoline bind in a 1
1 homodimer with CB[8]. Previous work44,45 has shown that alkyl-pyridinium derivatives of isoquinoline bind in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 manner with CB[7]. 2-(Pyridin-4-yl)benzo[d]thiazole (BTPy) was selected for the interesting photophysical behaviour of benzothiazole derivatives, which depends strongly on the local environment, enabling their use in the monitoring of pH and the sensing of metal cations.46,47 Additionally, benzothiazole derivatives have been demonstrated to form 1
1 manner with CB[7]. 2-(Pyridin-4-yl)benzo[d]thiazole (BTPy) was selected for the interesting photophysical behaviour of benzothiazole derivatives, which depends strongly on the local environment, enabling their use in the monitoring of pH and the sensing of metal cations.46,47 Additionally, benzothiazole derivatives have been demonstrated to form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes with CB[7].48–51 The final pyridine explored, 1-methyl-[4,4′-bipyridin]-1-ium (MV), forms a viologen derivative upon alkylation of the second nitrogen. It was predicted that this molecule will form a 1
1 complexes with CB[7].48–51 The final pyridine explored, 1-methyl-[4,4′-bipyridin]-1-ium (MV), forms a viologen derivative upon alkylation of the second nitrogen. It was predicted that this molecule will form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with CB[8] similar to other viologen derivatives.52–55 As crosslinking in these CB[8]-based gels is mediated by two guests on different chains forming a homoternary 2
1 complex with CB[8] similar to other viologen derivatives.52–55 As crosslinking in these CB[8]-based gels is mediated by two guests on different chains forming a homoternary 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with CB[8], forming a 1
1 complex with CB[8], forming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex cannot facilitate a crosslink. Thus, MV was therefore selected as an ideal control.
1 complex cannot facilitate a crosslink. Thus, MV was therefore selected as an ideal control.
In total, four novel alkyl pyridinium monomers each with a propylacrylamide (PrAm) moiety were synthesised: 1-(3-acrylamidopropyl)-4-phenylpyridin-1-ium chloride (PhPyPrAm), 2-(3-acrylamidopropyl)isoquinolin-2-ium chloride (IsoQPrAm), 1-(3-acrylamidopropyl)-4-(benzo[d]thiazol-2-yl)pyridin-1-ium chloride (BTPyPrAm) and 1-(3-acrylamido-propyl)-1′-methyl-[4,4′-bipyridine]-1,1′-diium chloride (MVPrAm).
Isothermal titration calorimetry (ITC) was employed to explore the binding of the alkyl pyridinium guests with CB[8] (Fig. 3). Typical titration profiles for a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry were observed for PhPyPrAm, IsoQPrAm and BTPyPrAm, while 1
1 binding stoichiometry were observed for PhPyPrAm, IsoQPrAm and BTPyPrAm, while 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding was observed with MVPrAm.37 The thermodynamic parameters shown in Table S2† were in good agreement with previously described homoternary CB[8] complexes.56 Furthermore, the calculated binding constants (K1 ≈ 107 M−1, K2 ≈ 105 M−1) observed for PhPyPrAm, IsoQPrAm and BTPyPrAm match closely with the values for 1-benzyl-3-vinylimidazolium (K1 = 4.21 × 107 M−1, K2 = 4.25 × 105 M−1), indicating that the three monomers are good candidates to form CB[8] mediated dynamic crosslinks in a hydrogel network.
1 binding was observed with MVPrAm.37 The thermodynamic parameters shown in Table S2† were in good agreement with previously described homoternary CB[8] complexes.56 Furthermore, the calculated binding constants (K1 ≈ 107 M−1, K2 ≈ 105 M−1) observed for PhPyPrAm, IsoQPrAm and BTPyPrAm match closely with the values for 1-benzyl-3-vinylimidazolium (K1 = 4.21 × 107 M−1, K2 = 4.25 × 105 M−1), indicating that the three monomers are good candidates to form CB[8] mediated dynamic crosslinks in a hydrogel network.
To understand the homoternary complex formation in greater depth, a series of 1H NMR titrations were performed in which aqueous solutions of guest compound (2.0 mM) were titrated into an aqueous solution of CB[8] (0.1 mM) (Fig. S6–8†). Analysis of the NMR spectra further confirmed the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry, as the peaks from the unbound alkyl pyridinium only begin to appear once a guest
1 binding stoichiometry, as the peaks from the unbound alkyl pyridinium only begin to appear once a guest![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[8] ratio of 2
CB[8] ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 has been surpassed. In all cases, a 2
1 has been surpassed. In all cases, a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode is observed by 1H NMR, indicating that any 1
1 binding mode is observed by 1H NMR, indicating that any 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding dynamics were faster than the timescale of the NMR measurement. For IsoQPrAm the signals are broadened throughout, hindering the ability to fully assign the spectra and indicating that all binding dynamics were fast compared to the timescale of the measurement. Additionally, for BTPyPrAm some splitting of the pyridinium protons is observed. This is attributed to desymmetrisation upon complexation presumably arrising from the loss of free rotation.
1 binding dynamics were faster than the timescale of the NMR measurement. For IsoQPrAm the signals are broadened throughout, hindering the ability to fully assign the spectra and indicating that all binding dynamics were fast compared to the timescale of the measurement. Additionally, for BTPyPrAm some splitting of the pyridinium protons is observed. This is attributed to desymmetrisation upon complexation presumably arrising from the loss of free rotation.
For each of the three guest monomers, at all CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest ratios a large upfield shift was observed for the aromatic protons, especially those far from the cyclic nitrogen, implying that the aromatic region is included within the cavity. Conversely, only a slight downfield shift was observed for the vinyl and alkyl protons indicating that they are close to the CB[8] portal but not in the cavity. As a result, the vinyl and alkyl protons are accessible and able to undergo polymerisation. Together with the ITC results these data confirm that the alkyl pyridinium monomers in 2
guest ratios a large upfield shift was observed for the aromatic protons, especially those far from the cyclic nitrogen, implying that the aromatic region is included within the cavity. Conversely, only a slight downfield shift was observed for the vinyl and alkyl protons indicating that they are close to the CB[8] portal but not in the cavity. As a result, the vinyl and alkyl protons are accessible and able to undergo polymerisation. Together with the ITC results these data confirm that the alkyl pyridinium monomers in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes with CB[8] form excellent candidates for the introduction of supramolecular bonds into hydrogel networks via an in situ polymerisation approach.
1 complexes with CB[8] form excellent candidates for the introduction of supramolecular bonds into hydrogel networks via an in situ polymerisation approach.
To investigate the monomers’ ability to crosslink supramolecular gels each monomer was first pre-complexed with CB[8] and copolymerised with acrylamide (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 mol%) in water (2 M) via a thermoinitiated free radical polymerisation at 50 °C. To ensure good reproducibility each hydrogel was prepared three times and for each rheological measurement three samples of the same gel were used (giving a total of 9 datasets per averaged result). A guest
95 mol%) in water (2 M) via a thermoinitiated free radical polymerisation at 50 °C. To ensure good reproducibility each hydrogel was prepared three times and for each rheological measurement three samples of the same gel were used (giving a total of 9 datasets per averaged result). A guest![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[8] ratio of 2
CB[8] ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used in all guest containing hydrogels.
1 was used in all guest containing hydrogels.
Small-amplitude oscillatory rheological analysis was employed to explore the mechanical properties of the hydrogels (Fig. 4). In an amplitude sweep experiment (Fig. 4a) each of the materials prepared displayed a broad linear viscoelastic region with the storage modulus (G′) higher than the loss modulus (G′′), indicating that they are gels under the conditions analysed. Statistical analysis (Fig. S9†) demonstrated that within the viscoelastic region the storage moduli (G′) of the three gels containing 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding monomers were statistically similar (p = 0.01–0.37), demonstrating that they presented similar elastic like solid behaviour. The storage modulus of the two control samples (100% AAm and 95% AAm with 5% of the 1
1 binding monomers were statistically similar (p = 0.01–0.37), demonstrating that they presented similar elastic like solid behaviour. The storage modulus of the two control samples (100% AAm and 95% AAm with 5% of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding MVPrAm guest) showed no significant change between them (p = 0.68), indicating that the 1
1 binding MVPrAm guest) showed no significant change between them (p = 0.68), indicating that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding MVPrAm guest did not form supramolecular crosslinks. Additionally, the hydrogels with 2
1 binding MVPrAm guest did not form supramolecular crosslinks. Additionally, the hydrogels with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding guests had (G′) and (G′′) more than one order of magnitude higher than those of the control samples, indicating the formation of robust hydrogels by the introduction of supramolecular crosslinks.
1 binding guests had (G′) and (G′′) more than one order of magnitude higher than those of the control samples, indicating the formation of robust hydrogels by the introduction of supramolecular crosslinks.
Towards the end of the linear viscoelastic regime, G′′ of the BTPyPrAm hydrogel shows a slight increase with strain before hydrogel deformation: a weak strain overshoot. This is a known phenomenon for fluids with extended polymer chains with a complex inter-chain structure, which resists deformation up to a certain strain (observed by increasing G′′). As this structure breaks down, chains can align with the direction of flow and G′′ decreases.57 The AAm control hydrogel presented a less pronounced strain overshoot, which has been observed by others.58,59 This behaviour, observed in AAm-based hydrogels, may be related to the presence of an additional structure associated by hydrogen bonding that imposes some resistance to deformation.57 In all other gels a strain thinning behaviour is observed, in which as strain is increased polymer chains disentangle to align with the direction of flow. This implies that the supramolecular interactions in the BTPyPrAm hydrogel induced the formation of additional weak structural complexes throughout the polymer networks that were not observed in PhPyPrAm and ISOQPrAm hydrogels. We postulate that as BTPyPram has the largest area of π-conjugation it may be able to induce some additional weak stacking interactions between chains.
Tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ (G′′/G′) taken from the linear viscoelastic region provides a good metric for the tough and elastic-like behaviour of hydrogels; Fig. 4b shows tan
δ (G′′/G′) taken from the linear viscoelastic region provides a good metric for the tough and elastic-like behaviour of hydrogels; Fig. 4b shows tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ for all of the gels tested. BTPyPrAm hydrogels have a significantly lower tan
δ for all of the gels tested. BTPyPrAm hydrogels have a significantly lower tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ than the others containing supramolecular crosslinks suggesting that it is more highly elastic and has properties closer to those of a tough material. It is also apparent that the pure AAm gel has more tough material-like properties than the control formed from the 1
δ than the others containing supramolecular crosslinks suggesting that it is more highly elastic and has properties closer to those of a tough material. It is also apparent that the pure AAm gel has more tough material-like properties than the control formed from the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding MVPrAm. We believe that this is due to an increase in free volume (and a related decrease in hydrogen bonding) in the MVPrAm containing gels. In the pure AAm gel, polymer chains can readily hydrogen bond with one another, while in the MVPrAm gels pendant MV molecules increase the inter-chain distance through both repulsive interactions between the dications and the steric bulk of the pendant viologens (especially when complexed with CB[8]) leading to less hydrogen bonding.
1 binding MVPrAm. We believe that this is due to an increase in free volume (and a related decrease in hydrogen bonding) in the MVPrAm containing gels. In the pure AAm gel, polymer chains can readily hydrogen bond with one another, while in the MVPrAm gels pendant MV molecules increase the inter-chain distance through both repulsive interactions between the dications and the steric bulk of the pendant viologens (especially when complexed with CB[8]) leading to less hydrogen bonding.
Frequency dependent rheological analysis performed within the linear viscoelastic region is shown in Fig. 4c. G′ of the AAm hydrogel slightly increased in the frequency range investigated, presenting a slope of 0.24 when the power law is applied, while PhPyPrAm and MVPrAm showed similar behaviour but with a larger value for the slope 0.35 and 0.40 respectively. The rheological behaviour observed for the AAm sample can only be a result of the polymer entanglements since no crosslinker moieties were added; these have finite relaxation time and so the elastic contribution would increase with frequency.60 The behaviour was instead influenced by the associative network formed by hydrogen bonding between acrylamide groups.61,62 Again, this was partially disrupted in the presence of MVPrAm pendant groups complexed with CB[8], reducing the G′ of the MVPrAm hydrogel in Fig. 4c. Though PhPyPrAm showed higher moduli than the control samples its G′ showed a similar frequency dependence in the linear viscoelastic regime, indicating that the storage modulus was mainly dominated by polymer entanglement. Additionally, the G′′ profile of the AAm, MVPrAm and PhPyPrAm hydrogels exhibits the same power law as their G′ profiles, meaning that their tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ does not vary significantly with frequency (Fig. S10†). Tan
δ does not vary significantly with frequency (Fig. S10†). Tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ values that are independent of frequency in this way are characteristic of a critical gel and has been observed in weakly crosslinked polyacrylamide gels previously.60
δ values that are independent of frequency in this way are characteristic of a critical gel and has been observed in weakly crosslinked polyacrylamide gels previously.60
Conversely, BTPyPrAm and IsoQPrAm exhibited a distinct profile of G′, G′′ and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ (Fig. 4c and S10†). At lower frequency, G′ and G′′ have similar values and at higher frequencies G′ reaches a plateau, followed by a drastic decrease of tan
δ (Fig. 4c and S10†). At lower frequency, G′ and G′′ have similar values and at higher frequencies G′ reaches a plateau, followed by a drastic decrease of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ due to the reduction of G′′, which is typical of solid-like behaviour. Additionally, the loss moduli reach their maximum value at 1.01 rad s−1 (or 6.20 s) and 8.05 rad s−1 (or 0.80 s) for BTPyPrAm and IsoQPrAm, respectively, which relates to the relaxation time of the supramolecular crosslinkers and the rate of dissociation of the host–guest interactions. A longer time, as observed for the BTPyPrAm hydrogels, implies that the 2
δ due to the reduction of G′′, which is typical of solid-like behaviour. Additionally, the loss moduli reach their maximum value at 1.01 rad s−1 (or 6.20 s) and 8.05 rad s−1 (or 0.80 s) for BTPyPrAm and IsoQPrAm, respectively, which relates to the relaxation time of the supramolecular crosslinkers and the rate of dissociation of the host–guest interactions. A longer time, as observed for the BTPyPrAm hydrogels, implies that the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 guest
1 guest![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[8] complex is less dynamic.63,64 Although the three hydrogels have the same crosslinking density and CB[8] guests of a similar binding constant, different rheological properties were observed, possibly related to distinct microstructural homogeneity arising from their varied chemical structure. While polymer entanglements control the hydrogel network relaxation in PhPyPrAm, supramolecular crosslinks dominate the relaxation profile of BTPyPrAm and IsoQPrAm.64
CB[8] complex is less dynamic.63,64 Although the three hydrogels have the same crosslinking density and CB[8] guests of a similar binding constant, different rheological properties were observed, possibly related to distinct microstructural homogeneity arising from their varied chemical structure. While polymer entanglements control the hydrogel network relaxation in PhPyPrAm, supramolecular crosslinks dominate the relaxation profile of BTPyPrAm and IsoQPrAm.64
Overall, these results demonstrate that supramolecular crosslinks enabled by the inclusion of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding CB[8] guests endow the hydrogels studied with superior mechanical properties; by selecting appropriate guest molecules the elastic properties of the hydrogel can be tuned independent of their storage modulus. This additional degree of tunability of the loss modulus may be of particular relevance to model biological systems where viscoelasticity can uniquely affect how cells interact with a surface.65
1 binding CB[8] guests endow the hydrogels studied with superior mechanical properties; by selecting appropriate guest molecules the elastic properties of the hydrogel can be tuned independent of their storage modulus. This additional degree of tunability of the loss modulus may be of particular relevance to model biological systems where viscoelasticity can uniquely affect how cells interact with a surface.65
UV-Vis and fluorescence spectroscopy were used to verify the absorption and emission properties of the alkyl pyridinium monomers (Fig. 5a). The guests were found to have a good variation in absorption profiles spanning from the UVC to UVA range (250–400 nm). The emission profiles also varied, covering nearly the entire visible light spectrum and having maxima in the violet and green regions. Fluorescence quantum yields (ϕF) were also calculated for the monomers and these were found to be 0.0007, 0.017 and 0.34 for BTPyPrAm, PhPyPrAm and IsoQPrAm, respectively. To verify whether fluorescence properties might be retained upon complexation with CB[8], emission spectra were recorded for 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 guest
1 guest![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[8] ratios (Fig. S16–18†). For IsoQPrAm and BTPyPram a slight decrease in integrated emission was observed (68% and 66% of their uncomplexed form, respectively). We suggest that this could be due to the formation of a charge-transfer complex between molecules bound by CB[8], which is consistent with previous reports.66 Conversely, for PhPyPrAm a tremendous 341% increase in integrated emission was observed upon binding, which we postulate is the result of the free rotation around the bond connecting the phenyl and pyridine subunits becoming restricted, leading to more efficient, planar π-conjugation than in the unbound state.67 X-ray crystallography analysis of PhPyPrAm as a PF6 salt indicated a 12.62° torsion angle around this bond in its unbound state, providing some qualitative evidence to support this hypothesis (Fig. S19 and 20†). Furthermore, encapsulation of PhPyPrAm inside both CB[7] and CB[8] results in a bathochromic shift in emission. This change can be attributed to the stabilization of the excited state, thus deactivating non-radiative pathways and decreasing the HOMO–LUMO band gap upon inclusion of the phenyl moiety in the cucurbituril cavity.68–70 We suggest that the difference in fluorescence emission for bound and unbound PhPyPrAm may also enable its use as a fluorescent reporter in a self-reporting hydrogel preparation. To determine if fluorescence is indeed retained upon hydrogel formation, samples were illuminated using a longwave (365 nm) UV lamp. For all three 2
CB[8] ratios (Fig. S16–18†). For IsoQPrAm and BTPyPram a slight decrease in integrated emission was observed (68% and 66% of their uncomplexed form, respectively). We suggest that this could be due to the formation of a charge-transfer complex between molecules bound by CB[8], which is consistent with previous reports.66 Conversely, for PhPyPrAm a tremendous 341% increase in integrated emission was observed upon binding, which we postulate is the result of the free rotation around the bond connecting the phenyl and pyridine subunits becoming restricted, leading to more efficient, planar π-conjugation than in the unbound state.67 X-ray crystallography analysis of PhPyPrAm as a PF6 salt indicated a 12.62° torsion angle around this bond in its unbound state, providing some qualitative evidence to support this hypothesis (Fig. S19 and 20†). Furthermore, encapsulation of PhPyPrAm inside both CB[7] and CB[8] results in a bathochromic shift in emission. This change can be attributed to the stabilization of the excited state, thus deactivating non-radiative pathways and decreasing the HOMO–LUMO band gap upon inclusion of the phenyl moiety in the cucurbituril cavity.68–70 We suggest that the difference in fluorescence emission for bound and unbound PhPyPrAm may also enable its use as a fluorescent reporter in a self-reporting hydrogel preparation. To determine if fluorescence is indeed retained upon hydrogel formation, samples were illuminated using a longwave (365 nm) UV lamp. For all three 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding monomers fluorescence could be visibly observed in the gels (Fig. 5b).
1 binding monomers fluorescence could be visibly observed in the gels (Fig. 5b).
Together these results demonstrate that alkyl pyridinium monomers prepared via our general synthetic route were able to endow the resultant hydrogels with fluorescence properties. Furthermore, combining the crosslink-forming unit with the dye results in a simplified approach for the formation of supramolecular fluorescent hydrogels. To the best of our knowledge, this is the first example of fluorescence being introduced to CB[8]-mediated hydrogels directly through the crosslink-forming unit. We envision that supramolecular hydrogels prepared by this method could find widespread use in both physical and biomedical applications.
Conclusions
A facile, generic, column-free synthetic pathway is reported for the preparation of alkyl pyridinium containing acrylamide monomers on a gram scale. ITC and NMR spectroscopy analysis confirmed that these monomers have a high affinity to form complexes with CB[8] and, when complexed, the acrylamide moieties are still readily available for polymerisation. A series of hydrogels with a broad viscoelastic region were prepared via in situ polymerisation of an inclusion complex of these monomers with CB[8]. It was demonstrated that the presence of supramolecular crosslinks greatly enhances the mechanical properties of the gel, increasing the storage and loss moduli by more than an order of magnitude. Moreover, by varying the guest, the elastic and solid-like properties of the hydrogel can be tuned independent of the storage modulus. Finally, it was demonstrated that by selecting pyridine moieties with known fluorescence properties the generic synthesis yielded fluorescent monomers, which could then be polymerised into fluorescent hydrogels. This approach reduces the number of components necessary to create fluorescent supramolecular hydrogels by combining the dye and crosslink forming monomer into a single unit.The preparation of fluorescent supramolecular hydrogels, which may find use in biolabeling, as sensors or in tissue engineering, is just one example of this generic synthetic route. By varying the pyridine used for monomer synthesis any number of potential new uses could be imagined from corrosion inhibition, to use as an electrolyte in batteries, or even in the preparation of antimicrobial materials.71 Furthermore, by selecting an acrylamide as the polymerisable unit, these 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding alkyl pyridinium moieties have a very similar polymerisation activity to the acrylamide monomer used to form the backbone. This will enable the use of controlled radical polymerisation in future material preparation, a topic which will be the subject of further papers from our group.
1 binding alkyl pyridinium moieties have a very similar polymerisation activity to the acrylamide monomer used to form the backbone. This will enable the use of controlled radical polymerisation in future material preparation, a topic which will be the subject of further papers from our group.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
D. J. W. thanks the EPSRC for a PhD studentship (EP/R512461/1). The authors also thank the Leverhulme Trust (Natural Material Innovation, RP2013-SL-008) and in particular the EPSRC (NOtCH, EP/L027151/1) for financial support. Finally, the authors thank Dr Stefan Mommer for several fruitful discussions and Anton Graf for the initial preparation of 2-(pyridin-4-yl)benzo[d]thiazole used in this study.References
- W. E. Hennink and C. F. van Nostrum, Adv. Drug Delivery Rev., 2002, 54, 13–36 CrossRef CAS.
- N. M. Sangeetha and U. Maitra, Chem. Soc. Rev., 2005, 34, 821–836 RSC.
- L. Yu and J. Ding, Chem. Soc. Rev., 2008, 37, 1473–1481 RSC.
- F. Huang and O. A. Scherman, Chem. Soc. Rev., 2012, 41, 5879–5880 RSC.
- X. Liao, G. Chen and M. Jiang, Polym. Chem., 2013, 4, 1733–1745 RSC.
- A. Harada, Y. Takashima and M. Nakahata, Acc. Chem. Res., 2014, 47, 2128–2140 CrossRef CAS PubMed.
- H. Wang and S. C. Heilshorn, Adv. Mater., 2015, 27, 3717–3736 CrossRef CAS PubMed.
- Y. Shao, H. Jia, T. Cao and D. Liu, Acc. Chem. Res., 2017, 50, 659–668 CrossRef CAS PubMed.
- G. Sinawang, M. Osaki, Y. Takashima, H. Yamaguchi and A. Harada, Chem. Commun., 2020, 56, 4381–4395 RSC.
- K. Wang, K. Amin, Z. An, Z. Cai, H. Chen, H. Chen, Y. Dong, X. Feng, W. Fu, J. Gu, Y. Han, D. Hu, R. Hu, D. Huang, F. Huang, F. Huang, Y. Huang, J. Jin, X. Jin, Q. Li, T. Li, Z. Li, Z. Li, J. Liu, J. Liu, S. Liu, H. Peng, A. Qin, X. Qing, Y. Shen, J. Shi, X. Sun, B. Tong, B. Wang, H. Wang, L. Wang, S. Wang, Z. Wei, T. Xie, C. Xu, H. Xu, Z.-K. Xu, B. Yang, Y. Yu, X. Zeng, X. Zhan, G. Zhang, J. Zhang, M. Q. Zhang, X.-Z. Zhang, X. Zhang, Y. Zhang, Y. Zhang, C. Zhao, W. Zhao, Y. Zhou, Z. Zhou, J. Zhu, X. Zhu and B. Z. Tang, Mater. Chem. Front., 2020, 4, 1803–1915 RSC.
- Y. Takashima, T. Nakayama, M. Miyauchi, Y. Kawaguchi, H. Yamaguchi and A. Harada, Chem. Lett., 2004, 33, 890–891 CrossRef CAS.
- C. T. S. Wong Po Foo, J. S. Lee, W. Mulyasasmita, A. Parisi-Amon and S. C. Heilshorn, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 22067–22072 CrossRef PubMed.
- P. Mi, X.-J. Ju, R. Xie, H.-G. Wu, J. Ma and L.-Y. Chu, Polymer, 2010, 51, 1648–1653 CrossRef CAS.
- E. A. Appel, F. Biedermann, U. Rauwald, S. T. Jones, J. M. Zayed and O. A. Scherman, J. Am. Chem. Soc., 2010, 132, 14251–14260 CrossRef CAS PubMed.
- M. Nakahata, Y. Takashima, H. Yamaguchi and A. Harada, Nat. Commun., 2011, 2, 511 CrossRef PubMed.
- T. Kakuta, Y. Takashima, M. Nakahata, M. Otsubo, H. Yamaguchi and A. Harada, Adv. Mater., 2013, 25, 2849–2853 CrossRef CAS PubMed.
- E. A. Appel, X. J. Loh, S. T. Jones, F. Biedermann, C. A. Dreiss and O. A. Scherman, J. Am. Chem. Soc., 2012, 134, 11767–11773 CrossRef CAS PubMed.
- M. J. Rowland, M. Atgie, D. Hoogland and O. A. Scherman, Biomacromolecules, 2015, 16, 2436–2443 CrossRef CAS PubMed.
- M. J. Rowland, C. C. Parkins, J. H. McAbee, A. K. Kolb, R. Hein, X. J. Loh, C. Watts and O. A. Scherman, Biomaterials, 2018, 179, 199–208 CrossRef CAS PubMed.
- A. Tabet, R. A. Forster, C. C. Parkins, G. Wu and O. A. Scherman, Polym. Chem., 2019, 10, 467–472 RSC.
- J. Liu, C. S. Y. Tan, Z. Yu, N. Li, C. Abell and O. A. Scherman, Adv. Mater., 2017, 29, 1605325 CrossRef PubMed.
- J. Liu, C. Soo Yun Tan, Y. Lan and O. A. Scherman, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 3105–3109 CrossRef CAS PubMed.
- J. Liu, C. S. Y. Tan, Z. Yu, Y. Lan, C. Abell and O. A. Scherman, Adv. Mater., 2017, 29, 1604951 CrossRef PubMed.
- Z. Huang, X. Chen, G. Wu, P. Metrangolo, D. Whitaker, J. A. McCune and O. A. Scherman, J. Am. Chem. Soc., 2020, 142, 7356–7361 CrossRef CAS PubMed.
- Q. Song, Y. Gao, J.-F. Xu, B. Qin, M. J. Serpe and X. Zhang, ACS Macro Lett., 2016, 5, 1084–1088 CrossRef CAS.
- W. Xu, Q. Song, J.-F. Xu, M. J. Serpe and X. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 11368–11372 CrossRef CAS PubMed.
- A. M. Breul, M. D. Hager and U. S. Schubert, Chem. Soc. Rev., 2013, 42, 5366–5407 RSC.
- Y. Li, D. J. Young and X. J. Loh, Mater. Chem. Front., 2019, 3, 1489–1502 RSC.
- N. Mehwish, X. Dou, Y. Zhao and C.-L. Feng, Mater. Horiz., 2019, 6, 14–44 RSC.
- J. Mohanty, A. C. Bhasikuttan, S. D. Choudhury and H. Pal, J. Phys. Chem. B, 2008, 112, 10782–10785 CrossRef CAS PubMed.
- Z. Miskolczy, L. Biczók, M. Megyesi and I. Jablonkai, J. Phys. Chem. B, 2009, 113, 1645–1651 CrossRef CAS PubMed.
- Z. Miskolczy, J. G. Harangozó, L. Biczók, V. Wintgens, C. Lorthioir and C. Amiel, Photochem. Photobiol. Sci., 2014, 13, 499–508 CrossRef CAS PubMed.
- X. Xiao, Q. Wang, Y.-H. Yu, Z.-Y. Xiao, Z. Tao, S.-F. Xue, Q.-J. Zhu, J.-X. Liu and X.-H. Liu, Eur. J. Org. Chem., 2011, 2366–2371 CrossRef CAS.
- S. Senler, L. Cui, A. M. Broomes, E. L. Smith, J. N. Wilson and A. E. Kaifer, J. Phys. Org. Chem., 2012, 25, 592–596 CrossRef CAS.
- Y. Zhang, T.-Y. Zhou, K.-D. Zhang, J.-L. Dai, Y.-Y. Zhu and X. Zhao, Chem. – Asian J., 2014, 9, 1530–1534 CrossRef CAS PubMed.
- S. J. Barrow, S. Kasera, M. J. Rowland, J. del Barrio and O. A. Scherman, Chem. Rev., 2015, 115, 12320–12406 CrossRef CAS PubMed.
- Z. Huang, K. Qin, G. Deng, G. Wu, Y. Bai, J.-F. Xu, Z. Wang, Z. Yu, O. A. Scherman and X. Zhang, Langmuir, 2016, 32, 12352–12360 CrossRef CAS PubMed.
- B. Zhang, X. Yan, P. Alcouffe, A. Charlot, E. Fleury and J. Bernard, ACS Macro Lett., 2015, 4, 1008–1011 CrossRef CAS.
- X. Li, H. Zhang, P. Zhang and Y. Yu, Chem. Mater., 2018, 30, 3752–3758 CrossRef CAS.
- T. T. H. Mark, A. Nordhaus and V. V. Krongauz, J. Appl. Polym. Sci., 2017, 44451 Search PubMed.
- I. Willner, N. Lapidot, A. Riklin, R. Kasher, E. Zahavy and E. Katz, J. Am. Chem. Soc., 1994, 116, 1428–1441 CrossRef CAS.
- S. Li, H. Chen, X. Yang, D. Bardelang, I. W. Wyman, J. Wan, S. M. Y. Lee and R. Wang, ACS Med. Chem. Lett., 2015, 6, 1174–1178 CrossRef CAS PubMed.
- E. Solis Jr., I. Zdravkovic, I. D. Tomlinson, S. Y. Noskov, S. J. Rosenthal and L. J. De Felice, J. Biol. Chem., 2012, 287, 8852–8863 CrossRef PubMed.
- Y. Gong, H. Chen, X. Ma and H. Tian, ChemPhysChem, 2016, 17, 1934–1938 CrossRef CAS PubMed.
- Z. Miskolczy, L. Biczók and G. Lendvay, Phys. Chem. Chem. Phys., 2018, 20, 15986–15994 RSC.
- K. Tanaka, T. Kumagai, H. Aoki, M. Deguchi and S. Iwata, J. Org. Chem., 2001, 66, 7328–7333 CrossRef CAS PubMed.
- Y. Li, Y. Wang, S. Yang, Y. Zhao, L. Yuan, J. Zheng and R. Yang, Anal. Chem., 2015, 87, 2495–2503 CrossRef CAS PubMed.
- R. M. Batista, S. P. Costa and M. M. Raposo, Tetrahedron Lett., 2004, 45, 2825–2828 CrossRef CAS.
- X.-F. Yang, Q. Huang, Y. Zhong, Z. Li, H. Li, M. Lowry, J. O. Escobedo and R. M. Strongin, Chem. Sci., 2014, 5, 2177–2183 RSC.
- M. N. Shinde, S. Dutta Choudhury, N. Barooah, H. Pal, A. C. Bhasikuttan and J. Mohanty, J. Phys. Chem. B, 2015, 119, 3815–3823 CrossRef CAS PubMed.
- M. N. Shewale, D. N. Lande and S. P. Gejji, J. Mol. Liq., 2016, 216, 309–317 CrossRef CAS.
- W. S. Jeon, H.-J. Kim, C. Lee and K. Kim, Chem. Commun., 2002, 1828–1829 RSC.
- C. S. Y. Tan, G. Agmon, J. Liu, D. Hoogland, E.-R. Janeček, E. A. Appel and O. A. Scherman, Polym. Chem., 2017, 8, 5336–5343 RSC.
- E. A. Appel, F. Biedermann, D. Hoogland, J. del Barrio, M. D. Driscoll, S. Hay, D. J. Wales and O. A. Scherman, J. Am. Chem. Soc., 2017, 139, 12985–12993 CrossRef CAS PubMed.
- V. K. Rana, A. Tabet, J. A. Vigil, C. J. Balzer, A. Narkevicius, J. Finlay, C. Hallou, D. H. Rowitch, H. Bulstrode and O. A. Scherman, ACS Macro Lett., 2019, 8, 1629–1634 CrossRef CAS.
- G. Wu, M. Olesińska, Y. Wu, D. Matak-Vinkovic and O. A. Scherman, J. Am. Chem. Soc., 2017, 139, 3202–3208 CrossRef CAS PubMed.
- K. Hyun, S. H. Kim, K. H. Ahn and S. J. Lee, J. Non-Newtonian Fluid Mech., 2002, 107, 51–65 CrossRef CAS.
- S. Gu, G. Cheng, T. Yang, X. Ren and G. Gao, Macromol. Mater. Eng., 2017, 302, 1700402 CrossRef.
- J. Xu, Z. Fan, L. Duan and G. Gao, Polym. Chem., 2018, 9, 2617–2624 RSC.
- C. Du and R. J. Hill, J. Rheol., 2018, 63, 109–124 CrossRef.
- C. Osterwinter, C. Schubert, C. Tonhauser, D. Wilms, H. Frey and C. Friedrich, Macromolecules, 2015, 48, 119–130 CrossRef CAS.
- E. Patyukova, T. Rottreau, R. Evans, P. D. Topham and M. J. Greenall, Macromolecules, 2018, 51, 7032–7043 CrossRef CAS.
- S. C. Grindy, R. Learsch, D. Mozhdehi, J. Cheng, D. G. Barrett, Z. Guan, P. B. Messersmith and N. Holten-Andersen, Nat. Mater., 2015, 14, 1210–1216 CrossRef CAS PubMed.
- F. Vidal, J. Gomezcoello, R. A. Lalancette and F. Jäkle, J. Am. Chem. Soc., 2019, 141, 15963–15971 CrossRef CAS PubMed.
- E. E. Charrier, K. Pogoda, R. G. Wells and P. A. Janmey, Nat. Commun., 2018, 9, 449 CrossRef PubMed.
- M. Olesińska, G. Wu, S. Gómez-Coca, D. Antón-García, I. Szabó, E. Rosta and O. A. Scherman, Chem. Sci., 2019, 10, 8806–8811 RSC.
- R. N. Dsouza, U. Pischel and W. M. Nau, Chem. Rev., 2011, 111, 7941–7980 CrossRef CAS PubMed.
- A. L. Koner and W. M. Nau, Supramol. Chem., 2007, 19, 55–66 CrossRef CAS.
- S. Schoder, H. V. Schröder, L. Cera, R. Puttreddy, A. Güttler, U. Resch-Genger, K. Rissanen and C. A. Schalley, Chem. – Eur. J., 2019, 25, 3257–3261 CAS.
- G. Wu, Y. J. Bae, M. Olesińska, D. Antón-García, I. Szabó, E. Rosta, M. R. Wasielewski and O. A. Scherman, Chem. Sci., 2020, 11, 812–825 RSC.
- P. Madaan and V. K. Tyagi, J. Oleo Sci., 2008, 57, 197–215 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2000466. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/D0PY01374A |
| This journal is © The Royal Society of Chemistry 2021 |



