Pentacyclic spermidine alkaloids with radioprotective and anti-inflammatory activities from Orychophragmus violaceus†
Abstract
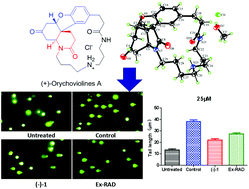
Two pairs of novel pentacyclic spermidine alkaloid enantiomers, (±)-orychoviolines A and B ((±)-1 and (±)-2), were isolated from the seeds of Orychophragmus violaceus and represented the first example of a 2-piperidinone-fused hydrodibenzofuran skeleton, constructed from a 6/5/6/6 tetracyclic system and an 18 atomic ring. The most unexpected novelty was the formation of one more piperidinone ring by a connection between C-6 and N-7. Their structures and absolute configurations were determined by spectroscopic analyses, X-ray crystallography, and ECD analysis. Compared to Ex-RAD (sodium salt of 4-carboxystyryl-4-chlorobenzylsulfone), (−)-1 exhibited a significant radioprotective effect on cell survival and DNA damage. (−)-1 also exhibited remarkable anti-inflammatory activity by inhibiting the production of NO in RAW 264.7 cells activated by lipopolysaccharide with an IC50 value of 20.3 ± 1.58 μM, which was equivalent to that of dexamethasone.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...