Visible-light- and bromide-mediated photoredox Minisci alkylation of N-heteroarenes with ester acetates†
Abstract
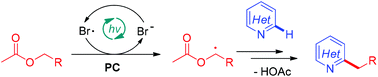
A visible-light-induced photoredox Minisci alkylation reaction of N-heteroarenes with ethyl acetate has been reported. The low-toxic ethyl acetate was used for the first time as an alkylation reagent. Hence, 4-quinazolinones, quinolines and pyridines reacted smoothly in the current reaction system. Mechanistic studies indicate that LiBr plays a key role to dramatically improve the efficiency of the reaction by the mediation of hydrogen atom transfer.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...