An environmentally benign regioselective synthesis of 2-benzyl-4-arylquinoline derivatives using aryl amines, styrene oxides and aryl acetylenes†
Abstract
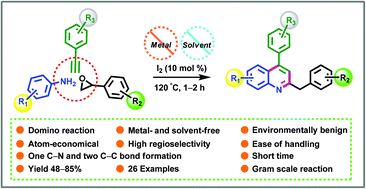
A novel and an expedient metal- and solvent-free synthesis of a wide variety of 2-benzyl-4-arylquinoline derivatives is described from readily available aryl amines, styrene oxides and aryl acetylenes in the presence of 10 mol% molecular iodine. This domino reaction occurs under metal- and solvent-free conditions at 120 °C, which avoids the usage of metal catalyst and as a consequence generation of metal waste. The salient features of this methodology are the use of simple starting materials, ease of handling, high regioselectivity, shorter reaction time, atom-economical, step-economical, the formation of one C–N and two C–C bonds and a wide range of functional groups tolerance.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...