Open Access Article
Open Access ArticleThree-component synthesis, utilization and biological activity of phosphinoyl-functionalized isoindolinones†
Nóra
Popovics-Tóth
a,
Bettina
Rávai
a,
Ádám
Tajti
a,
Bence
Varga
 a,
Péter
Bagi
a,
Péter
Bagi
 a,
Franc
Perdih
a,
Franc
Perdih
 b,
Pál Tamás
Szabó
b,
Pál Tamás
Szabó
 c,
László
Hackler
Jr.
d,
László G.
Puskás
d and
Erika
Bálint
c,
László
Hackler
Jr.
d,
László G.
Puskás
d and
Erika
Bálint
 *a
*a
aDepartment of Organic Chemistry and Technology, Budapest University of Technology and Economics, Budafoki út 8, 1111 Budapest, Hungary. E-mail: balint.erika@vbk.bme.hu
bFaculty of Chemistry and Chemical Technology, University of Ljubljana, Večna pot 113, 1000 Ljubljana, Slovenia
cMS Metabolomics Research Group, Centre for Structural Study, Research Centre for Natural Sciences, Eötvös Loránd Research Network, Magyar tudósok krt. 2, 1117 Budapest, Hungary
dAvidin Ltd Alsó kikötő sor 11/D, 6726 Szeged, Hungary
First published on 20th September 2021
Abstract
A new method for the synthesis of 3-oxoisoindolin-1-ylphosphine oxides bearing same or different substituents on the phosphorus atom is described. The one-pot three-component reaction of 2-formylbenzoic acid, primary amines and achiral or P-stereogenic secondary phosphine oxides provided the target compounds under catalyst-free, mild conditions and for short reaction times. The deoxygenation of a 3-oxoisoindolin-1-ylphosphine oxide was also studied, and the phosphine obtained could be converted to a sulphide and to a platinum complex. The crystal structures of a selected phosphine oxide and the corresponding platinum species were investigated by X-ray diffraction analysis. The biological activity, such as in vitro cytotoxicity on different cell lines and antibacterial activity of the 3-oxoisoindolin-1-ylphosphine oxides was also investigated. Based on the IC50 values obtained, several derivatives showed moderate activity against the HL-60 cell line and two compounds containing 3,5-dimethylphenyl groups on the phosphorus atom showed promising activity against Bacillus subtilis bacteria.
Introduction
Nitrogen-heterocycles as one of the major classes of organic compounds play an important role in scientific research. Several papers reported on their synthesis, emphasizing their biological properties as pharmaceuticals or agrochemicals and their utilization as dyes.1Isoindolin-1-ones as naturally occurring and pharmacologically relevant N-heterocycles have attracted considerable attention.2 They may possess a variety of biological activities, including antiviral,3 anti-inflammatory and antipsychotic4 properties. A few derivatives have also been reported to be effective for treating cancer,5 arrhythmia6 and diabetes.7
Compounds containing both an isoindolinone scaffold and a phosphonate moiety, such as 3-oxoisoindolin-1-ylphosphonates, can act as bioisosteres of natural α-amino acids, and may often show biological effects,8 such as they may be used as pesticides.9
Multicomponent reactions continuously attract great attention as one of the most useful and efficient tools for the synthesis of versatile heterocyclic compounds.10 The following advantages can be highlighted from the numerous benefits of this synthetic strategy. Products are usually formed in a single step from simple starting materials in high atom efficient reactions. The possibility of applying diverse reagents makes them ideal for creating new molecular libraries. Moreover, in most cases, the principles of green chemistry also prevail to save time and energy.
In recent years, many efforts have been made to synthesize isoindolin-1-ones.2,11 However, only a few methods have been reported for the preparation of 3-oxoisoindolin-1-ylphosphonates. Ordóñez and his research group described a microwave (MW)-assisted special Kabachnik–Fields reaction of 2-formylbenzoic acid, dimethyl phosphite and as the third component, aromatic amines,12 aminoacetaldehyde dimethyl acetal13 or amino alcohols.14 They also studied the condensation with optically active amines under conventional heating.15 Others reported syntheses in the presence of a special catalyst or additive, such as NaH,16 T3P®17 or OSU-618 in MeOH, EtOAc or EtOH, respectively. In our previous study, we have described an efficient catalyst-free method for the batch and continuous flow synthesis of 3-oxoisoindolin-1-ylphosphonates (1) containing alkyl substituents on the nitrogen atom by the three-component reaction of 2-formylbenzoic acid, aliphatic primary amines and dialkyl phosphites or ethyl phenyl-H-phosphinate19 (Scheme 1).
3-Oxoisoindolin-1-ylphosphine oxides are much less studied; they have only been mentioned in the literature as intermediates. Couture and co-workers prepared α-amidophosphine oxides 3 by three different methods (method A, B or C), and carried out their ring closure reaction in the presence of potassium bis(trimethylsilyl)amide (KHMDS) and 18-crown-6 (Scheme 2).20
Deniau and co-workers developed an asymmetric synthesis of diarylphosphine oxide-substituted isoindolinones bearing an (S)-2-alkoxymethyl-pyrrolidin-1-yl type auxiliary (6) by a three-step reaction starting from phthalic anhydride and (S)-1-amino-2-alkyloxymethylpyrrolidine (5) (Scheme 3).21
Both approaches applied multistep syntheses using complex and/or expensive reagents and required special treatments.
To the best of our knowledge, there is no example in the literature for the synthesis of 3-oxoisoindolin-1-ylphosphine oxides by a multicomponent reaction, and their utilization has not been investigated yet.
In this paper, we describe the first multicomponent synthetic method for the preparation of 3-oxoisoindolin-1-ylphosphine oxides containing the same or different substituents on the phosphorus atom. Our approach is based on the three-component reaction of 2-formylbenzoic acid, primary amines and achiral or P-chiral secondary phosphine oxides, and this method required neither catalyst/additive nor special conditions. We have also investigated the utilization of a 3-oxoisoindolin-1-ylphosphine oxide as a phosphine ligand precursor. After deoxygenation, the 3-oxoisoindolin-1-ylphosphine obtained was applied as a ligand in the synthesis of a monodentate platinum(II) complex. In addition, the in vitro cytotoxicity and antibacterial activity of the title compounds were also studied.
Results and discussion
At first, the special Kabachnik–Fields reaction of 2-formylbenzoic acid, primary amines (butyl-, cyclohexyl-, benzylamine or aniline) and diphenylphosphine oxide was investigated without any catalyst (Table 1). Carrying out the condensation with butylamine in the absence of any solvent at room temperature for 5 min, 58% of diphenyl (2-butyl-3-oxo-2,3-dihydro-2H-isoindol-1-yl)phosphine oxide (7a) was formed (Table 1, entry 1). To improve the conversion, a small amount of solvent was added to overcome the heterogeneity of the reaction mixture. Among tested solvents (ethanol, toluene and acetonitrile), acetonitrile was the most efficient, since a conversion of 88% was achieved (Table 1, entries 2–4). Increasing the reaction time to 10 min, the reaction was complete (Table 1, entry 5). The acetonitrile and the water formed were eliminated in vacuum, and the crude product was passed through short plug of silica to obtain product 7a in a yield of 98%. Starting from cyclohexylamine, the condensation was not complete after 10 min, and the conversion was only 83% (Table 1, entry 6). Applying longer reaction time of 15 or 20 min, led to a proportion of 91% and 100% of the desired product (7b), respectively (Table 1, entries 7 and 8). The decrease in the reaction rate may be caused by the larger steric hindrance of the cyclohexyl ring. The diphenyl (2-cyclohexyl-3-oxo-2,3-dihydro-2H-isoindol-1-yl)phosphine oxide (7b) was isolated in a yield of 94%. In case of benzylamine or aniline, a reaction time of 10 min was enough for a complete conversion similarly to the condensation of butylamine (Table 1, entries 5, 9 and 10). The corresponding 3-oxoisoindolin-1-ylphosphine oxides (7c and 7d) were obtained in yields of 97% and 96%, respectively.| Entry | Solvent | R | t [min] | Conversionb [%] | Yieldc [%] |
|---|---|---|---|---|---|
| a The reactions were carried out with 1.0 mmol of 2-formylbenzoic acid, 1.0 mmol of primary amine and 1.0 mmol of diphenylphosphine oxide in the absence of any solvent or in 1 mL of solvent. b Determined by HPLC (222 nm). c Isolated yield. | |||||
| 1 | — | Bu | 5 | 58 | — |
| 2 | EtOH | Bu | 5 | 79 | — |
| 3 | PhMe | Bu | 5 | 82 | — |
| 4 | MeCN | Bu | 5 | 88 | — |
| 5 | MeCN | Bu | 10 | 100 | 98 (7a) |
| 6 | MeCN | c Hex | 10 | 83 | — |
| 7 | MeCN | c Hex | 15 | 91 | — |
| 8 | MeCN | c Hex | 20 | 100 | 94 (7b) |
| 9 | MeCN | Bn | 10 | 100 | 97 (7c) |
| 10 | MeCN | Ph | 10 | 100 | 96 (7d) |
Next, the three-component reaction of 2-formylbenzoic acid with a wide range of primary amines and secondary phosphine oxides was investigated using the optimized conditions (25 °C, 10–20 min) (Scheme 4). Performing the condensation of 2-formylbenzoic acid, butylamine and bis(p-tolyl)-, bis(3,5-dimethylphenyl)- or bis(2-naphthyl)phosphine oxide, the corresponding 3-oxoisoindolin-1-ylphosphine oxides (8a–10a) were prepared in yields of 96–99%. Dibenzylphosphine oxide was also used as the P-reagent. In this case, a slightly increased reaction time of 15 min was necessary to reach full conversion, and the dibenzyl (2-butyl-3-oxo-2,3-dihydro-2H-isoindol-1-yl)phosphine oxide (11a) was isolated in a yield of 96%. Carrying out the reaction of 2-formylbenzoic with cyclohexylamine and the secondary phosphine oxides mentioned above, the condensations were slightly slower (20 or 25 min). The desired 3-oxoisoindolin-1-ylphosphine oxides (8b–11b) were also obtained in high yields (94–97%). Applying benzylamine or aniline as the amine component, the reactions took place similarly to that with butylamine, and the corresponding products (8c–11c, 8d and 9d) were synthesized in yields of 96–98%.
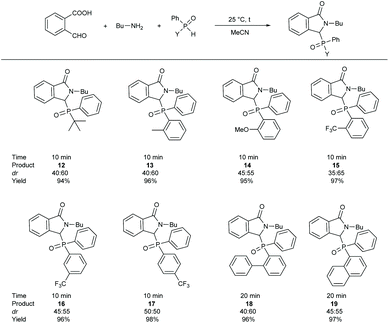
In the next series of experiments, the special Kabachnik–Fields reaction of 2-formylbenzoic acid and butylamine was extended using P-stereogenic phosphine oxides, such as tert-butyl(phenyl)phosphine oxide, 2-methylphenyl(phenyl)-phosphine oxide, 2-methoxyphenyl(phenyl)phosphine oxide, 2-, 3- or 4-trifluoromethylphenyl(phenyl)phosphine oxide, as well as biphenyl(phenyl)phosphine oxide or 1-naphthyl(phenyl)phosphine oxide (Scheme 5). The condensations were performed at 25 °C, for 10 or 20 min without any catalyst in acetonitrile, according to the method described above. Altogether eight 3-oxoisoindolin-1-ylphosphine oxides (12–19) having different substituents on the phosphorus atom were synthesized in excellent yields (94–98%). Due to the P-stereogenic centre in the P-functionality, all products (12–19) were formed as a mixture of two diastereomers, and both diastereomers were racemates. Therefore, two signals were observed in the 31P NMR spectra, and two signals were visible in the 13C and 1H NMR spectra. However, the diastereomeric ratio (dr) of the compounds synthesized was different. Most of the 3-oxoisoindolin-1-ylphosphine oxides (12–14, 16, 18 and 19) were obtained as a 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 or 45
60 or 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 mixture of the diastereomers based on the 31P NMR spectra. Compound 17 incorporating a 4-trifluormethyl group on the phosphorus atom was formed as an equal (50
55 mixture of the diastereomers based on the 31P NMR spectra. Compound 17 incorporating a 4-trifluormethyl group on the phosphorus atom was formed as an equal (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) mixture of diastereomers. The condensation was more diastereoselective, when 2-trifluormethylphenyl-(phenyl)phosphine oxide was used as the P-reagent, in this case, the diastereomeric ratio was 35
50) mixture of diastereomers. The condensation was more diastereoselective, when 2-trifluormethylphenyl-(phenyl)phosphine oxide was used as the P-reagent, in this case, the diastereomeric ratio was 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65. Due to the bigger difference of the functional groups on the phosphorus atom of 1-naphthyl(phenyl) (2-butyl-3-oxo-2,3-dihydro-2H-isoindol-1-yl)phosphine oxide (19), the diastereomers were successfully separated by column chromatography.
65. Due to the bigger difference of the functional groups on the phosphorus atom of 1-naphthyl(phenyl) (2-butyl-3-oxo-2,3-dihydro-2H-isoindol-1-yl)phosphine oxide (19), the diastereomers were successfully separated by column chromatography.
Altogether, 26 3-oxoisoindolin-1-ylphosphine oxides (7–19) were prepared in high yields at ambient temperature for short reaction times (10–25 min), and fully characterized by 31P, 13C and 1H NMR spectroscopy, as well as by HRMS.
As the next step, the diphenyl (2-butyl-3-oxo-2,3-dihydro-2H-isoindol-1-yl)phosphine oxide (7a) was utilized as a precursor for a monodentate P-ligand in the synthesis of a platinum complex. First, the deoxygenation of 7a was studied applying phenylsilane as a reducing agent under microwave (MW) irradiation in the absence of any catalyst and solvent (Table 2). The phosphine (20) obtained was immediately converted to a sulphide (21), and the mixture was analyzed by HPLC-MS and 31P NMR. Performing the reaction at 100 °C for 2 h, the reduction was not complete (Table 2, entry 1). Applying a higher temperature of 140 °C for 4 h, the conversion significantly increased to 60% (Table 2, entry 2). After an irradiation of 6 h, the reduction was complete, and the sulphide (21) was isolated in a yield of 81% after column chromatography (Table 2, entry 3).
| Entry | T [°C] | t [h] | Conversionb [%] | Yieldc [%] |
|---|---|---|---|---|
| a First step of the reaction was performed with 1.0 mmol of 7a and 3.0 mmol of phenylsilane without any solvent under N2 atmosphere in a microwave reactor. In the second step, 1.2 mmol of sulphur in 10 mL of degassed DCM was added to 20. b Determined by 31P NMR. c After column chromatography. | ||||
| 1 | 100 | 2 | 17 | — |
| 2 | 140 | 4 | 60 | — |
| 3 | 140 | 6 | 100 | 81 (21) |
Finally, the diphenyl (2-butyl-3-oxo-2,3-dihydro-2H-isoindol-1-yl)phosphine (20) obtained after deoxygenation was converted to a monodentate platinum(II) complex (22) by the reaction with 0.5 equiv. of dichlorodibenzonitrile platinum at 25 °C in dichloromethane (Scheme 6). The platinum(II) complex (22) could be isolated in a yield of 80% by column chromatography, and it was characterized by 31P, 13C, 1H NMR and HRMS, as well as by single crystal X-ray diffraction analysis.
The relative spatial orientation (cis or trans) of platinum-phosphine coordination compounds can be inferred from the magnitude of the stereospecific platinum-phosphorus coupling constant (1JPt–P) in the 31P NMR spectrum. It is known from the literature that the 1JPt–P coupling constant is between 3400 to 3600 Hz for cis arrangements, while trans complexes display typical 1JPt–P coupling constants of 2500–3000 Hz.22
The 1JPt–P coupling constant was 2519 Hz, which means that the trans platinum complex ((trans)-22) was formed. Moreover, in the 31P NMR spectrum of the platinum(II) complex ((trans)-22), two very close peaks in a ratio of ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and their satellites could be observed. As the isoindolinone ring contains a stereogenic centre, the platinum complex ((trans)-22) was obtained as a mixture of a homochiral and heterochiral diastereomer.
1 and their satellites could be observed. As the isoindolinone ring contains a stereogenic centre, the platinum complex ((trans)-22) was obtained as a mixture of a homochiral and heterochiral diastereomer.
Single-crystal XRD analysis was used to reveal the molecular structures of 7a and 22 (Fig. 1). In the crystal lattice, 7a, molecules are connected into a hydrogen-bonded chain through C–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P interactions along the c-axis, and these chains are further connected into 3D network via C–H⋯O2 interactions with the isoindolin-1-one oxygen atom as well as C–H⋯π interactions (Fig. S1 and Table S2†). The XRD analysis also confirmed the trans geometry in platinum complex 22. A hydrogen-bound layer is formed through the C–H⋯O1 interactions, and a 3D network is achieved via C–H⋯π interactions (Fig. S2 and Table S2†).
P interactions along the c-axis, and these chains are further connected into 3D network via C–H⋯O2 interactions with the isoindolin-1-one oxygen atom as well as C–H⋯π interactions (Fig. S1 and Table S2†). The XRD analysis also confirmed the trans geometry in platinum complex 22. A hydrogen-bound layer is formed through the C–H⋯O1 interactions, and a 3D network is achieved via C–H⋯π interactions (Fig. S2 and Table S2†).
The biological activity, such as in vitro cytotoxicity and antibacterial activity of 3-oxoisoindolin-1-ylphosphine oxides bearing the same substituents on the phosphorus atom (7–11) were also studied. Cytotoxicity assays used the human lung adenocarcinoma A549 cell line, the mouse fibroblast NIH/3T3 as a healthy cell line and the human promyelocytic leukemia HL-60 cell line. During the measurements, the fluorescent Resazurin assay as described previously was applied.23 For the A549 and NIH/3T3 cell lines, doxorubicin was the positive control (IC50 = 0.31 ± 0.24 μM and 5.65 ± 0.81 μM, respectively), while for HL60, it was bortezomib (IC50 = 7.42 ± 2.60 nM). The antibacterial activity of the compounds was investigated on green fluorescent protein (GFP) producing Bacillus subtilis (Gram–positive) and Escherichia coli (Gram–negative) bacterial cells. The GFP producing bacteria are efficient tools for screening the antibacterial activity, since the GFP signal measured by fluorimetry is proportional to the number of the bacterial cells. Active compounds kill bacterial cells, which decreases the GFP fluorescence signal, therefore it is convenient for evaluating the antimicrobial effect of different agents. Positive controls were doxycycline and gentamicin for Bacillus subtilis (IC50 = 0.126 ± 0.029 μM and 0.115 ± 0.001 μM) and for Escherichia coli (IC50 = 0.10 ± 0.02 μM and 4.23 ± 0.99 μM) bacterial cells. The IC50 values (50% inhibiting concentration) obtained are shown in Table 3.
| Compound | R | In vitro cytotoxicity [IC50, μM] | Antibacterial activity [IC50, μM] | |||
|---|---|---|---|---|---|---|
| A549 | NIH/3T3 | HL-60 | B. subtilis | E. coli | ||
| a Data were expressed as mean ± standard deviation. | ||||||

|
Bu (8a) | >30 | >30 | 25.03 ± 2.07 | >10 | >10 |
| c Hex (8b) | >30 | >30 | >30 | >10 | >10 | |
| Bn (8c) | >30 | >30 | >30 | >10 | >10 | |

|
Bu (9a) | >30 | >30 | 17.55 ± 1.70 | 4.60 ± 1.13 | >10 |
| c Hex (9b) | >30 | >30 | >30 | >10 | >10 | |
| Bn (9c) | >30 | >30 | 18.31 ± 1.33 | 3.61 ± 1.25 | >10 | |

|
Bu (10a) | 28.2 ± 1.05 | 25.94 ± 1.06 | 12.26 ± 1.02 | >10 | >10 |
| c Hex (10b) | >30 | >30 | 28.81 ± 1.17 | >10 | >10 | |
| Bn (10c) | >30 | >30 | 25.61 ± 1.12 | >10 | >10 | |
| Doxorubicin | 0.31 ± 0.24 | 5.65 ± 0.81 | — | — | — | |
| Bortezomib | — | — | 7.42 × 10−3 ± 2.60 × 10−3 | — | — | |
| Doxycycline | — | — | — | 0.126 ± 0.029 | 0.10 ± 0.02 | |
| Gentamicin | — | — | — | 0.115 ± 0.001 | 4.23 ± 0.99 | |
According to the results, those (3-oxo-2,3-dihydro-2H-isoindol-1-yl)phosphine oxides (8–10) showed activity to some extent, which contain substituted phenyl groups (p-tolyl or 3,5-dimethylphenyl) or naphthyl rings on the phosphorus atom. Among the bis(p-tolyl) (3-oxo-2,3-dihydro-2H-isoindol-1-yl)phosphine oxides, only the N-butyl substituted derivative (8a) showed modest activity against HL-60 cell line (IC50 = 25.03 ± 2.07 μM). In case of bis(3,5-dimethylphenyl) (3-oxo-2,3-dihydro-2H-isoindol-1-yl)phosphine oxides, compounds containing butyl (9a) or benzyl (9c) group on the nitrogen atom were slightly effective against HL-60 cells. Furthermore, these 3-oxoisoindolin-1-ylphosphine oxides (9a and 9c) also showed promising antibacterial activity, since the growth of Bacillus subtilis bacteria was reduced by them, and the IC50 values obtained (4.60 ± 1.13 μM and 3.61 ± 1.25 μM) were slightly close to the value of doxycycline and gentamicin, respectively. Among the derivatives containing 2-naphthyl groups on the phosphorus atom, compounds 10b and 10c were rather active against HL-60 cells. In contrast, the bis(2-naphthyl) (2-butyl-3-oxo-2,3-dihydro-2H-isoindol-1-yl)-phosphine oxide (10a) showed cytotoxicity against all the three cell lines, and the best activity was showed against HL-60 cells (12.26 ± 1.02).
The most active compounds were the bis(3,5-dimethylphenyl) (2-butyl-3-oxo-2,3-dihydro-2H-isoindol-1-yl)phosphine oxide (9a) and the bis(3,5-dimethylphenyl) (2-benzyl-3-oxo-2,3-dihydro-2H-isoindol-1-yl)phosphine oxide (9c), since they showed activity in the 3–4 μM range against Gram positive bacteria.
Conclusions
In conclusion, we have developed a new and practical catalyst-free method to novel phosphinoyl-functionalized isoindolin-1-ones (7–19) by the special Kabachnik–Fields reaction of 2-formylbenzoic acid, primary amines and achiral or P-chiral secondary phosphine oxides at ambient temperature for short reaction times (10–25 min). This procedure means a promising approach to attain these new heterocycles, since it applies mild and easily operational conditions (no special reagents, catalysts or additives, no heating). Altogether 26 new 3-oxoisoindolin-1-ylphosphine oxides (7–19) were synthesized in high to excellent yields and these derivatives were fully characterized. One of the 3-oxoisoindolin-1-ylphosphine oxides (7a) has been utilized as P-ligand for the synthesis of a monodentate platinum complex (trans-22). The crystal structure of compound 7a and platinum(II) complex 22 was studied by single-crystal XRD analysis. The biological activity of the (3-oxo-2,3-dihydro-2H-isoindol-1-yl)phosphine oxides (7–11) was also tested in in vitro cytotoxicity and antibacterial assays. Several 3-oxoisoindolin-1-ylphosphine oxides showed modest activity against HL-60 cell line, furthermore, two derivatives 9a and 9c incorporating 3,5-dimethylphenyl groups on the phosphorus atom were also active against selected Gram-positive bacteria.Author contributions
E. B. and N. P.-T. planned the experiments, N. P.-T. and B. R. carried out the experiments, B. V. and P. B. synthesized the P-stereogenic secondary phosphine oxides, F. P. performed the crystal structure analysis, P. T. Sz. carried out the high-resolution mass spectrometric measurements, L. H. performed the biological evaluation (screening), E. B. and L. G. P. contributed reagents/materials/analysis tools, E. B., B. R. and N. P.-T. wrote the paper. Á. T., B. V., P. B., L. H. and L. G. P. reviewed the paper. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The project was supported by the Hungarian Research Development and Innovation Office (FK123961) and by the bilateral Hungarian-Slovenian Science and Technology Cooperation project (2018-2.1.11-TÉT-SI-2018-00008) as well as by the Slovenian Research Agency (P1-0230-0175). N. P.-T. was supported by the Servier-Beregi PhD Research Fellowship. E. B. was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (BO/00278/17/7) and by the ÚNKP-20-5-BME-288 New National Excellence Program of the Ministry of Human Capacities. B. V. acknowledges the financial support of the New National Excellence Program of the Ministry of Human Capacities (ÚNKP-21-3-II-BME-299). F. P. thanks EN-FIST Centre of Excellence, Slovenia, for the use of the Supernova diffractometer.Notes and references
- (a) N. Kerru, L. Gummidi, S. Maddila, K. K. Gangu and S. B. Jonnalagadda, Molecules, 2020, 25, 1909 CrossRef CAS PubMed; (b) A. P. Taylor, R. P. Robinson, Y. M. Fobian, D. C. Blakemore, L. H. Jones and O. Fadeyi, Org. Biomol. Chem., 2016, 14, 6611 RSC; (c) E. G. Brown, Ring Nitrogen and Key Biomolecules, The Biochemistry of N-heterocycles, Springer Netherlands, 1998 CrossRef.
- (a) F. Peytam, M. Adib, S. Mahernia, M. Rahmanian-Jazi, M. Jahani, B. Masoudi, M. Mahdavi and M. Amanlou, Bioorg. Chem., 2019, 87, 1 CrossRef CAS PubMed; (b) M. Jiang, Z. Wu, L. Liu and S. Chen, Org. Biomol. Chem., 2021, 19, 1644 RSC.
- C. Maugeri, M. A. Alisi, C. Apicella, L. Cellai, P. Dragone, E. Fioravanzo, S. Florio, G. Furlotti, G. Mangano, R. Ombrato, R. Luisi, R. Pompei, V. Rincicotti, V. Russo, M. Vitiello and N. Cazzolla, Bioorg. Med. Chem., 2008, 16, 3091 CrossRef CAS PubMed.
- N. P. Muddala, B. Nammalwar and R. A. Bunce, RSC Adv., 2015, 5, 28389 RSC.
- C. P. Gordon, N. Byrne and A. McCluskey, Green Chem., 2010, 12, 1000 RSC.
- A. Bjore, J. Bostrom, O. Davidsson, H. Emtenas, U. Gran, T. Iliefski, J. Kajanus, R. Olsson, G. Strandlund, J. Sundell, Z.-Q. Yuan and L. Sandberg, United States Patent, 0015237, 2008 Search PubMed.
- K. R. Guertin, United States Patent, 0082260, 2002 Search PubMed.
- V. P. Kukhar and H. R. Hudson, Aminophosphonic and Aminophosphinic acids: Chemistry and Biological Activity, Wiley, Chichester, 2000 Search PubMed.
- W. G. Phillips, United States Patent, 4164406, 1979 Search PubMed.
- (a) B. Jiang, T. Rajale, W. Wever, S.-J. Tu and G. Li, Chem. – Asian J., 2010, 5, 2318 CrossRef CAS PubMed; (b) A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083 CrossRef PubMed; (c) T. J. J. Müller, Multicomponent Reactions 1, Science of Synthesis, Thieme, Stuttgart, Germany, 2014 Search PubMed; (d) E. R. Baral, K. Sharma, M. S. Akhtar and Y. R. Lee, Org. Biomol. Chem., 2016, 14, 10285 RSC.
- (a) F. Peytam, M. Adib, S. Mahernia, M. Rahmanian-Jazi, M. Jahani, B. Masoudi, M. Mahdavi and M. Amanlou, Bioorg. Chem., 2019, 87, 1 CrossRef CAS PubMed; (b) J. C. Breytenbach, S. van Dyk, I. van den Heever, S. M. Allin, C. C. Hodkinson, C. J. Northfield and M. I. Page, Bioorg. Med. Chem. Lett., 2000, 10, 1629 CrossRef CAS PubMed; (c) K. Smith, G. A. El-Hiti, A. S. Hegazy and B. Kariuki, Beilstein J. Org. Chem., 2011, 7, 1219 CrossRef CAS PubMed.
- M. Ordóñez, G. D. Tibhe, A. Zamudio-Medina and J. L. Viveros-Ceballos, Synthesis, 2012, 569 CrossRef.
- M. A. Reyes-Gonzalez, A. Zamudio-Medina and M. Ordóñez, Tetrahedron Lett., 2012, 53, 5756 CrossRef CAS.
- M. A. Reyes-Gonzalez, A. Zamudio-Medina, O. A. Ramirez-Marroquin and M. Ordóñez, Monatsh. Chem., 2014, 145, 1001 CrossRef CAS.
- J. L. Viveros-Ceballos, C. Cativiela and M. Ordóñez, Tetrahedron: Asymmetry, 2011, 22, 1479 CrossRef CAS.
- S. Failla and P. Finocchiaro, Phosphorus, Sulfur Silicon Relat. Elem., 1995, 105, 195 CrossRef CAS.
- M. Milen, A. Dancsó, T. Földesi, P. Slégel and B. Volk, Tetrahedron, 2016, 72, 5091 CrossRef CAS.
- N. P. Muddala, B. Nammalwar and R. A. Bunce, RSC Adv., 2015, 5, 28389 RSC.
- Á. Tajti, N. Tóth, B. Rávai, I. Csontos, P. T. Szabó and E. Bálint, Molecules, 2020, 25, 3307 CrossRef PubMed.
- (a) A. Couture, E. Deniau, P. Grandclaudon, H. Rybalko-Rosen, S. Léonce, B. Pfeiffer and P. Renard, Bioorg. Med. Chem. Lett., 2002, 12, 3557 CrossRef CAS PubMed; (b) A. Moreau, A. Couture, E. Deniau and P. Grandclaudon, Synthesis, 2004, 1664 CAS; (c) A. Couture, E. Deniau and P. Grandclaudon, Tetrahedron, 1997, 53, 10313 CrossRef CAS; (d) A. Couture, E. Deniau, P. Grandclaudon and S. Lebrun, Synlett, 1997, 1475 CAS.
- E. Deniau, D. Enders, A. Couture and P. Grandclaudon, Tetrahedron: Asymmetry, 2005, 16, 875 CrossRef CAS.
- L. Kollár and G. Szalontai, J. Organomet. Chem., 1991, 421, 341 CrossRef.
- G. J. Szebeni, A. Balázs, I. Madarász, G. Pocz, F. Ayaydin, I. Kanizsai, R. Fajka-Boja, R. Alföldi, L. Hackler and L. G. Puskás, Int. J. Mol. Sci., 2017, 18, 2105 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental procedures, characterization data and copies of 31P, 1H and 13C NMR spectra. CCDC 2100126 and 2100127. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ob01610e |
| This journal is © The Royal Society of Chemistry 2021 |