QM/MM modeling of class A β-lactamases reveals distinct acylation pathways for ampicillin and cefalexin†
Abstract
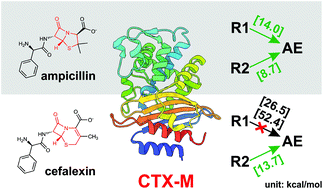
Efficient mechanism-based design of antibiotics that are not susceptible to β-lactamases is hindered by the lack of comprehensive knowledge on the energetic landscapes for the hydrolysis of various β-lactams. Herein, we adopted efficient quantum mechanics/molecular mechanics simulations to explore the acylation reaction catalyzed by CTX-M-44 (Toho-1) β-lactamase. We show that the catalytic pathways for β-lactam hydrolysis are correlated to substrate scaffolds: using Glu166 as the only general base for acylation is viable for ampicillin but prohibitive for cefalexin. The present computational workflow provides quantitative insights to facilitate the optimization of future β-lactam antibiotics.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...