Regio- and stereoselective (3 + 2)-cycloaddition reactions of nitrones with cyclic allenes†
Abstract
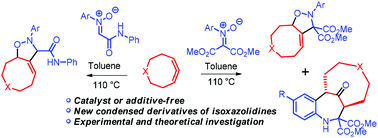
An effective approach to access functionalized 2H-cyclonona(deca)[d]isoxazoles and 15-oxo-3,10-methanobenzo[b][1]azacyclododecines has been developed by the reaction of N-aryl-C,C-bis(methoxycarbonyl)nitrones with cyclonona(deca)-1,2-dienes in a one-pot fashion. The reaction of N-aryl-C-(phenylcarbamoyl)nitrones with these allenes proceeds strictly regioselectively giving the mixtures of diastereomeric isoxazolidines containing a double bond at the C4-position of the isoxazolidine ring. The quantum chemical calculations show that the regioselectivity of these reactions is in good agreement with the reactivity indices of the considered compounds.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...