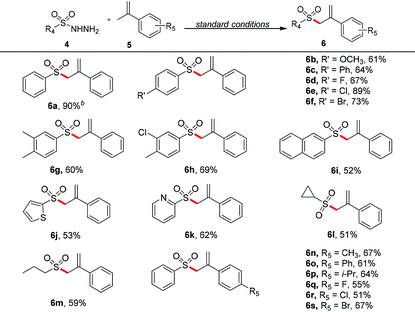
Photoredox/cobaloxime co-catalyzed allylation of amines and sulfonyl hydrazines with olefins to access α-allylic amines and allylic sulfones†
Hui
Xu‡
,
Hong
Zhang‡
,
Qing-Xiao
Tong
 and
Jian-Ji
Zhong
and
Jian-Ji
Zhong
 *
*
Department of Chemistry and Key Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, and Chemistry and Chemical Engineering Guangdong Laboratory, Shantou, Guangdong 515063, P. R. China. E-mail: jjzhong@stu.edu.cn
First published on 22nd July 2021
Abstract
Herein, we reported a dual-catalytic platform for the allylation of amines and sulfonyl hydrazines with olefins to selectively access α-allylic amines and allylic sulfones in good yields by combining photoredox catalysis and cobaloxime catalysis. This strategy avoided the use of a stoichiometric amount of terminal oxidant and the use of pre-functionalized allylic precursors, representing a green and ideal atom- & step-economical process. Good substrate scope and gram-scale synthesis demonstrated the utility of this protocol. Mechanistic studies revealed that a radical process is probably involved in this reaction.
Dehydrogenative cross-couplings of C–H and C–H or X–H have emerged as an attractive strategy1–4 to construct C–C and C–X bonds in synthetic chemistry due to their step- and atom-economy. Traditionally, a stoichiometric amount of sacrificial oxidants such as peroxides, high-valence metal salts or iodine(III) oxidants is always required, which would result in a stoichiometric amount of chemical waste and make the process less desirable from the view point of green chemistry.5–8 In recent years, an elegant dual catalytic strategy by combining visible-light photoredox catalysis9–25 and cobaloxime catalysis26–28 for cross-coupling hydrogen evolution reactions,29–34 which was first reported by Wu and coworkers,35,36 has received much attention. Therein, a photocatalyst was used to absorb light energy as a driving force for cross-coupling under mild reaction conditions, and a catalytic amount of a cobaloxime complex instead of a stoichiometric amount of external oxidants was employed as the proton reduction catalyst to capture the electrons and protons from the substrates or reaction intermediates to produce molecular hydrogen (H2). This photoredox/cobaloxime co-catalysis strategy avoids the use of a stoichiometric amount of terminal oxidant and generates H2 as the sole byproduct, which make it a green and ideal atom-economical process. Notably, this mild dual catalytic system is particularly useful for reactions that involve species sensitive to terminal oxidants,37 and provides a potential synthetic strategy for achieving acceptorless dehydrogenation. In the past decade, this photoredox/cobaloxime co-catalysis strategy has been demonstrated to be a powerful synthetic tool by many research groups38–40 for a variety of organic transformations. For example, Wu and coworkers41–46 have proved it to be useful for a diversity of C–C, C–O, C–P and C–N bond-forming reactions. The Lei group47,48 has exploited this strategy to realize the anti-Markovnikov oxidation of styrenes and the dehydrogenative cross-coupling of alkenes with alcohols or azoles. The Li group49 has employed this strategy to accomplish the acceptorless dehydrogenation of saturated N-heterocycles with the extrusion of H2 to achieve various heteroaromatic products. The Wu group50 has disclosed its application in the decarboxylative Heck-Type coupling of unactivated aliphatic acids and terminal alkenes. The Ritter group51 has utilized this strategy to enable the dehydrogenative decarboxyolefination of carboxylic acids to access alkenes. Our group52 has also reported a selective photoredox/cobaloxime co-catalyzed allylation reaction53–55 of α-ketoacids and methacrylates to obtain allylic ketones.
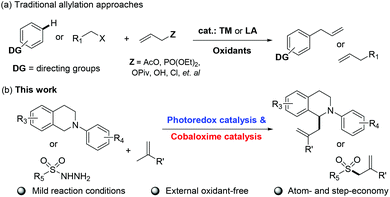
In light of the fact that allylic compounds are important and versatile building blocks for the synthesis of structurally complex natural products and pharmaceuticals, the methodologies for their synthesis have attracted much attention, including the nucleophilic reactions of allylic organometallics with acyl halides,56 or the transition-metal catalyzed reactions of pre-functionalized allylic precursors with acyl precursors57 (Scheme 1a). Although considerable progress has been achieved, the pre-functionalization of allylic precursors limited the application of these traditional methods. Owing to the important value of allylic compounds and inspired by our previous findings52 of accessing allylic ketones by direct addition of acyl radicals to olefins, we continued to further explore the reactions of other radical precursors with olefins for constructing various allylic compounds by combining photoredox catalysis and cobaloxime catalysis. In the present work, we would like to report the successful application of photoredox/cobaloxime co-catalysis for the allylation of amines and sulfonyl hydrazines with olefins to access α-allylic amines and allylic sulfones under mild and external oxidant-free conditions (Scheme 1b).
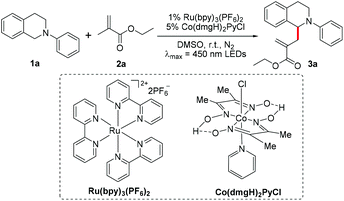
The functionalization of N-phenyltetrahydroisoquinoline by photoredox catalysis has been extensively investigated. Therein the carbon-centered radical is generated after single-electron photo-oxidation followed by one proton elimination. Inspired by our previous work,52 we wondered if the generated carbon-centered radical could react with olefins to undergo α-allylation of N-phenyltetrahydroisoquinoline using the photoredox/cobaloxime co-catalysis strategy. To demonstrate the possibility of our hypothesis, the cross-coupling of N-phenyltetrahydroisoquinoline 1a with ethyl methacrylate 2a was initially selected as the model reaction. To our delight, the irradiation of 1a with 2a in the presence of 1 mol% Ru(bpy)3(PF6)2 and 5 mol% Co(dmgH)2PyCl in deaerated dimethyl sulfoxide (DMSO) using 450 nm LEDs at room temperature indeed resulted in the α-allylic amine product 3a in 84% yield (Table 1, entry 1). Other photocatalysts such as 4-CzIPN and Acr+-MesClO4− were also examined, and the reaction yield sharply decreased (Table 1, entries 2 and 3). The solvent test proved that DMSO is the best choice in this reaction (Table 1, entries 4–6). When other cobaloximes such as Co(dmgH)2(4-AcO-Py)Cl, Co(dmgBF2)2(H2O)2 and Co(dmgH)2(4-Me-Py)Cl replacing Co(dmgH)2PyCl were employed, the yields of 38%, 31% and 26% were achieved, respectively (Table 1, entries 7–9). Furthermore, we anticipated that the addition of a base such as t-BuOK and 2,6-lutidine could promote the reaction efficiency; however, it just resulted in a low reaction efficiency (Table 1, entries 10 and 11). When the reaction was conducted under air, only a trace amount of the desired product was observed (Table 1, entry 12). Control experiments indicated that the photocatalyst, cobaloxime catalyst and light were essential parameters for this photoredox/cobaloxime co-catalyzed α-allylation reaction (Table 1, entries 13–15).
| Entry | Variation from the “standard conditions” | 3a yieldb (%) |
|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.4 mmol), Ru(bpy)3(PF6)2 (1 mol%), Co(dmgH)2PyCl (5 mol%), DMSO (2 mL), under N2, room temperature, 450 nm LED irradiation for 14 h. b Isolated yields. | ||
| 1 | None | 84 |
| 2 | 4-CzIPN as the photocatalyst | 29 |
| 3 | Acr+-MesClO4− as the photocatalyst | 19 |
| 4 | CH3CN as the solvent | 39 |
| 5 | THF as the solvent | 21 |
| 6 | CH3OH as the solvent | Trace |
| 7 | Co(dmgH)2(4-AcO-Py)Cl as the co-catalyst | 38 |
| 8 | Co(dmgBF2)2(H2O)2 as the co-catalyst | 31 |
| 9 | Co(dmgH)2(4-Me-Py)Cl as the co-catalyst | 26 |
| 10 | t-BuOK as an additive | 48 |
| 11 | 2,6-Lutidine as an additive | 25 |
| 12 | Under air | Trace |
| 13 | No photocatalyst | 0 |
| 14 | No cobaloxime catalyst | 0 |
| 15 | No light | 0 |
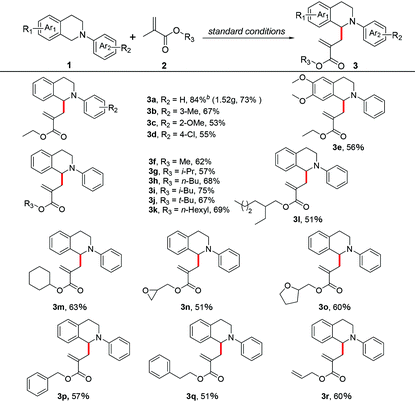
After optimizing the reaction conditions, we proceeded to explore the substrate scope of this photoredox/cobaloxime co-catalyzed α-allylation reaction. We first examined a variety of N-phenyltetrahydroisoquinolines. As shown in Table 2, no matter the Ar2 ring of N-phenyltetrahydroisoquinolines was substituted by electron-donating groups or electron-withdrawing groups, the corresponding α-allylic amine products were obtained in moderate to good yields (3a–3d). When the Ar1 ring of N-phenyltetrahydroisoquinoline was di-substituted by the methoxy group, the desired product 3e was afforded in 56% yield. Next, the scope of methacrylate derivatives in this reaction was investigated. To our satisfaction, methacrylates bearing various groups were tolerated well with good yields in this transformation (3f–3r), including different linear or branched aliphatic chain groups (3f–3l), a cyclohexyl group (3m), oxygen-containing heterocyclic groups (3n, 3o) and benzyl groups (3p, 3q). It is noteworthy that when an allyl methacrylate was examined in this reaction, the carbon-centered radical selectively reacted with the double bond of the methylallyl group rather than the allyl group to form the corresponding α-allylic amine 3r in 60% yield. To demonstrate the utility and practicality of this α-allylation reaction, a gram-scale reaction of 1a (1.36 g, 6.5 mmol) with 2a (1.48 g, 13 mmol) in DMSO under a N2 atmosphere upon 450 nm LED irradiation for 20 h was performed, and the desired product 3a was obtained in 73% yield (1.52 g, Scheme S1†).
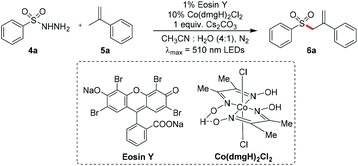
Encouraged by the above results, we further attempted to explore the reaction of other radical precursors with olefins to synthesize allylic compounds using the photoredox/cobaloxime co-catalysis strategy. The sulfonyl radical could be generated after hydrogen atom abstraction from sulfonyl hydrazide with the release of molecular nitrogen.58 Hence, we focused on the reaction of benzenesulfonyl hydrazide 4a and α-methyl styrene 5a. As shown in Table 3, after examining a series of reaction parameters, including the photocatalyst (entries 2–4), solvent (entries 5–7), cobaloxime co-catalyst (entries 8 and 9) and base additive (entries 10 and 11), we identified the standard reaction conditions. The acetonitrile/water mixed-solution of 4a with 5a in the presence of 1 mol% eosinY, 10 mol% Co(dmgH)2Cl2 and 1.0 equivalent of Cs2CO3 was irradiated with 510 nm LEDs at room temperature under a nitrogen atmosphere, and the desired allylic sulfone 6a could be obtained in 90% yield (entry 1). When the reaction was conducted under air, only a trace amount of the desired product was observed (entry 12). Note that the base additive played a key role in improving the reaction efficiency in this reaction; just 29% yield of 6a was obtained without any base addition (entry 13). Control experiments revealed that if any one parameter among the photocatalyst, cobaloxime catalyst and light was absent, the reaction could not occur (entries 14–16).
| Entry | Variation from the “standard conditions” | 6a yieldb (%) |
|---|---|---|
a Reaction conditions: 4a (0.1 mmol), 5a (0.1 mmol), eosin Y (1 mol%), Co(dmgH)2Cl2 (10 mol%), Cs2CO3 (1.0 equiv.), CH3CN/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), under N2, room temperature, 510 nm LED irradiation for 20 h.
b Isolated yields. 1), under N2, room temperature, 510 nm LED irradiation for 20 h.
b Isolated yields.
|
||
| 1 | None | 90 |
| 2 | 4-CzIPN as the photocatalyst | 53 |
| 3 | Ru(bpy)3(PF6)2 as the photocatalyst | 61 |
| 4 | Ir(ppy)3 as the photocatalyst | 45 |
| 5 | CH3CN as the solvent | 49 |
| 6 | CH3OH as the solvent | 62 |
| 7 | CH2Cl2 as the solvent | Trace |
| 8 | Co(dmgBF2)2(H2O)2 as the co-catalyst | 60 |
| 9 | Co(dmgH)2(4-Me-Py)Cl as the co-catalyst | 37 |
| 10 | Pyridine as an additive | Trace |
| 11 | Li2CO3 as an additive | 75 |
| 12 | Under air | Trace |
| 13 | No base | 29 |
| 14 | No photocatalyst | 0 |
| 15 | No cobaloxime catalyst | 0 |
| 16 | No light | 0 |
With the optimized reaction conditions in hand, we next investigated the generality of this approach. As shown in Table 4, various benzenesulfonyl hydrazides with electron-donating groups or electron-withdrawing groups on the phenyl ring were found to be compatible substrates with good yields in this reaction (6a–6f). When the phenyl ring was di-substituted (6g and 6h), the corresponding allylic sulfone products could also be obtained in moderate yields. Naphthyl and heterocyclic sulfonyl hydrazides (including thienyl and pyridyl) could also undergo this reaction to give the yields of 52%, 53% and 62%, respectively (6i–6k). Moreover, alkyl sulfonyl hydrazides were also found to smoothly react with α-methyl styrene to produce the corresponding allylic sulfones with moderate yields (6l and 6m). In addition, a variety of α-methyl styrenes were examined, regardless of the electron-donating groups or electron-withdrawing groups on the phenyl ring of substrates, and moderate to good yields of the desired products could be achieved (6n–6s).
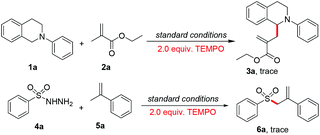
Several experiments were carried out to gain more insight into the mechanism of this photoredox/cobaloxime co-catalytic allylation reaction. First, luminescence quenching experiments showed that 1a could efficiently quench the emission of Ru(bpy)3(PF6)2, indicating that the electron transfer from 1a to Ru(bpy)3(PF6)2 is feasible (Fig. S1†). Furthermore, we found that when a radical inhibitor (TEMPO) was added to the optimal reaction system, the reaction was completely suppressed (Scheme 2), revealing that a radical process is probably involved in this reaction. These results are in accordance with our previous findings.52
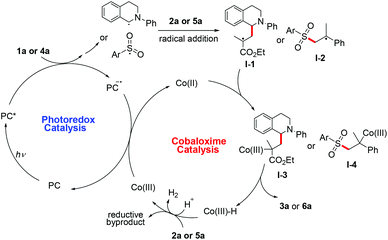
Based on the above experiments and previous reports, a general plausible reaction pathway is proposed for this photoredox/cobaloxime co-catalytic allylation reaction (Scheme 3). The excited photocatalyst (*PC) is reductively quenched by substrate 1a or 4a to generate the corresponding radical intermediate and the reductive species PC−. Then the radical addition to olefins generates a new carbon-centered radical I-1 or I-2, which could be captured by Co(II) to form transient Co(III)–C species I-3 or I-4. The cleavage of Co(III)–C and subsequent β-elimination result in the desired final allylic compound 3a or 6a and Co(III)–H species. According to the previous findings and reports, a plausible pathway of Co(III)–H species reacting with a proton or the olefin could regenerate the cobaloxime catalytic cycle and the subsequent single electron transfer from the reductive PC− to Co(III) could regenerate the photoredox catalytic cycle.
Conclusions
In summary, we have described the successful allylation reactions of amines and sulfonyl hydrazines with olefins to access α-allylic amines and allylic sulfones by combining photoredox catalysis and cobaloxime catalysis. Olefins as allylic precursors avoided the pre-functionalization process. Such a protocol features mild reaction conditions, external oxidant-free conditions and atom- & step-economy. Encouraged by these results, we believe that the photoredox/cobaloxime co-catalysis strategy will provide more opportunities for other substrates, which could be photo-reductively quenched to generate a reactive radical intermediate, which upon the allylation reaction could provide more valuable allylic compounds.Author contributions
Hui Xu: software, formal analysis, investigation, and data curation. Hong Zhang: software, conceptualization, formal analysis, investigation, and data curation. Qing-Xiao Tong: writing–reviewing and editing, and supervision. Jian-Ji Zhong: conceptualization, methodology, writing–reviewing and editing, project administration, and funding acquisition. All the authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (21801163), the STU Scientific Research Foundation for Talents (NTF18003), the Chemistry and Chemical Engineering Guangdong Laboratory (1922003), the 2020 Li Ka Shing Foundation Cross-Disciplinary Research Grant (2020LKSFG05A) and the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme 2019 (GDUPS2019).Notes and references
- C.-J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS PubMed.
- C. Jia, T. Kitamura and Y. Fujiwara, Acc. Chem. Res., 2001, 34, 633 CrossRef CAS PubMed.
- S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068 RSC.
- S. A. Girard, T. Knauber and C.-J. Li, Angew. Chem., Int. Ed., 2014, 53, 74 CrossRef CAS PubMed.
- B. M. Trost, Science, 1991, 254, 1471 CrossRef CAS PubMed.
- B. M. Trost, Angew. Chem., Int. Ed. Engl., 1995, 34, 259 CrossRef CAS.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301 RSC.
- R. A. Sheldon, Chem. Soc. Rev., 2012, 41, 1437 RSC.
- K. Zeitler, Angew. Chem., Int. Ed., 2009, 48, 9785 CrossRef CAS PubMed.
- T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527 CrossRef CAS PubMed.
- J. M. Narayanam and C. R. Stephenson, Chem. Soc. Rev., 2011, 40, 102 RSC.
- S. E. Creutz, K. J. Lotito, G. C. Fu and J. C. Peters, Science, 2012, 338, 647 CrossRef CAS PubMed.
- L. Shi and W. Xia, Chem. Soc. Rev., 2012, 41, 7687 RSC.
- C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed.
- D. Ravelli, D. Dondi, M. Fagnoni and A. Albini, Chem. Soc. Rev., 2009, 38, 1999 RSC.
- J. Xuan and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828 CrossRef CAS PubMed.
- N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075 CrossRef CAS PubMed.
- Q. Liu and L.-Z. Wu, Natl. Sci. Rev., 2017, 4, 359 CrossRef CAS.
- Y. Chen, L.-Q. Lu, D.-G. Yu, C.-J. Zhu and W.-J. Xiao, Sci. China: Chem., 2019, 62, 24 CrossRef CAS.
- O. Reiser, Acc. Chem. Res., 2016, 49, 1990 CrossRef CAS PubMed.
- I. Ghosh, L. Marzo, A. Das, R. Shaikh and B. König, Acc. Chem. Res., 2016, 49, 1566 CrossRef CAS PubMed.
- D. C. Fabry and M. Rueping, Acc. Chem. Res., 2016, 49, 1969 CrossRef CAS PubMed.
- X.-Y. Yu, Q.-Q. Zhao, J. Chen, W.-J. Xiao and J.-R. Chen, Acc. Chem. Res., 2020, 53, 1066 CrossRef CAS PubMed.
- J.-J. Zhong, W.-P. To, Y. Liu, W. Lu and C.-M. Che, Chem. Sci., 2019, 10, 4883 RSC.
- X.-K. Qi, H. Zhang, Z.-T. Pan, R.-B. Liang, C.-M. Zhu, J.-H. Li, Q.-X. Tong, X.-W. Gao, L.-Z. Wu and J.-J. Zhong, Chem. Commun., 2019, 55, 10848 RSC.
- J. L. Dempsey, B. S. Brunschwig, J. R. Winkler and H. B. Gray, Acc. Chem. Res., 2009, 42, 1995 CrossRef CAS PubMed.
- T. Lazarides, T. McCormick, P. W. Du, G. G. Luo, B. Lindley and R. Eisenberg, J. Am. Chem. Soc., 2009, 131, 9192 CrossRef CAS PubMed.
- V. Artero, C. M. Kerlidou and M. Fontecave, Angew. Chem., Int. Ed., 2011, 50, 7238 CrossRef CAS PubMed.
- B. Chen, L.-Z. Wu and C.-H. Tung, Acc. Chem. Res., 2018, 51, 2512 CrossRef CAS PubMed.
- J.-J. Zhong, Q.-Y. Meng, B. Chen, C.-H. Tung and L.-Z. Wu, Acta Chim. Sin., 2017, 75, 34 CrossRef CAS.
- K. Dong, L. Qiang and L.-Z. Wu, Acta Chim. Sin., 2020, 78, 299 CrossRef.
- H. Wang, X. Gao, Z. Lv, T. Abdelilah and A. Lei, Chem. Rev., 2019, 119, 6769 CrossRef CAS PubMed.
- K.-H. He and Y. Li, ChemSusChem, 2014, 7, 2788 CrossRef CAS PubMed.
- K. C. Cartwright, A. M. Davies and J. A. Tunge, Eur. J. Org. Chem., 2020, 1245 CrossRef CAS.
- Q.-Y. Meng, J.-J. Zhong, Q. Liu, X.-W. Gao, H.-H. Zhang, T. Lei, Z.-J. Li, K. Feng, B. Chen, C.-H. Tung and L.-Z. Wu, J. Am. Chem. Soc., 2013, 135, 19052 CrossRef CAS PubMed.
- J.-J. Zhong, Q.-Y. Meng, B. Liu, X.-B. Li, X.-W. Gao, T. Lei, C.-J. Wu, Z.-J. Li, C.-H. Tung and L.-Z. Wu, Org. Lett., 2014, 16, 1988 CrossRef CAS PubMed.
- Q. Xiao, H. Zhang, J.-H. Li, J.-X. Jian, Q.-X. Tong and J.-J. Zhong, Org. Lett., 2021, 23, 3604 CrossRef CAS PubMed.
- W.-L. Yu, Y.-C. Luo, L. Yan, D. Liu, Z.-Y. Wang and P.-F. Xu, Angew. Chem., Int. Ed., 2019, 58, 10941 CrossRef CAS PubMed.
- K. Zhang, L.-Q. Lu, Y. Jia, Y. Wang, F.-D. Lu, F. Pan and W.-J. Xiao, Angew. Chem., Int. Ed., 2019, 58, 13375 CrossRef CAS PubMed.
- S. U. Dighe, F. Juliá, A. Luridiana, J. J. Douglas and D. Leonori, Nature, 2020, 584, 75 CrossRef PubMed.
- X.-W. Gao, Q.-Y. Meng, J.-X. Li, J.-J. Zhong, T. Lei, X.-B. Li, C.-H. Tung and L.-Z. Wu, ACS Catal., 2015, 5, 2391 CrossRef CAS.
- M. Xiang, Q.-Y. Meng, J.-X. Li, Y.-W. Zheng, C. Ye, Z.-J. Li, B. Chen, C.-H. Tung and L.-Z. Wu, Chem. – Eur. J., 2015, 21, 18080 CrossRef CAS PubMed.
- C.-J. Wu, Q.-Y. Meng, T. Lei, J.-J. Zhong, W.-Q. Liu, L.-M. Zhao, Z.-J. Li, B. Chen, C.-H. Tung and L.-Z. Wu, ACS Catal., 2016, 6, 4635 CrossRef CAS.
- Y.-W. Zheng, B. Chen, P. Ye, K. Feng, W. Wang, Q.-Y. Meng, L.-Z. Wu and C.-H. Tung, J. Am. Chem. Soc., 2016, 138, 10080 CrossRef CAS PubMed.
- Q. Yang, L. Zhang, C. Ye, S. Luo, L.-Z. Wu and C.-H. Tung, Angew. Chem., Int. Ed., 2017, 56, 3694 CrossRef CAS PubMed.
- W.-Q. Liu, T. Lei, S. Zhou, X.-L. Yang, J. Li, B. Chen, J. Sivaguru, C.-H. Tung and L.-Z. Wu, J. Am. Chem. Soc., 2019, 141, 13941 CrossRef CAS PubMed.
- H. Yi, L. Niu, C. Song, Y. Li, B. Dou, A. K. Singh and A. Lei, Angew. Chem., Int. Ed., 2017, 56, 1120 CrossRef CAS PubMed.
- G. Zhang, X. Hu, C.-W. Chiang, H. Yi, P. Pei, A. K. Singh and A. Lei, J. Am. Chem. Soc., 2016, 138, 12037 CrossRef CAS PubMed.
- K.-H. He, F.-F. Tan, C.-Z. Zhou, G.-J. Zhou, X.-L. Yang and Y. Li, Angew. Chem., Int. Ed., 2017, 56, 3080 CrossRef CAS PubMed.
- H. Cao, H. Jiang, H. Feng, J. M. C. Kwan, X. Liu and J. Wu, J. Am. Chem. Soc., 2018, 140, 16360 CrossRef CAS PubMed.
- X. Sun, J. Chen and T. Ritter, Nat. Chem., 2018, 10, 1229 CrossRef CAS PubMed.
- H. Zhang, Q. Xiao, X.-K. Qi, X.-W. Gao, Q.-X. Tong and J.-J. Zhong, Chem. Commun., 2020, 56, 12530 RSC.
- K. Takizawa, T. Sekino, S. Sato, T. Yoshino, M. Kojima and S. Matsunaga, Angew. Chem., Int. Ed., 2019, 58, 9199 CrossRef CAS PubMed.
- J.-L. Liu, J.-L. Tu and F. Liu, Org. Lett., 2020, 22, 7369 CrossRef CAS PubMed.
- G. Zhang, L. Zhang, H. Yi, Y. Luo, X. Qi, C.-H. Tung, L.-Z. Wu and A. Lei, Chem. Commun., 2016, 52, 10407 RSC.
- L. Zhang, Y. Huang, H. Jiang, D. Jun and Y. Liao, J. Org. Chem., 1992, 57, 774 CrossRef CAS.
- Y. Obora, Y. Ogawa, Y. Imai, T. Kawamura and Y. Tsuji, J. Am. Chem. Soc., 2001, 123, 10489 CrossRef CAS PubMed.
- X. Li, X. Xu and C. Zhou, Chem. Commun., 2012, 48, 12240 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ob01307f |
| ‡ The two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |