Enantioselective construction of substituted pyridine and a seven-membered carbocyclic skeleton: biomimetic synthesis of (−)-rupestine D, (−)-guaipyridine, (−)-epiguaipyridine, and (−)-cananodine and their stereoisomers†
Abstract
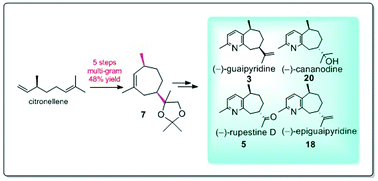
Guaipyridine alkaloids (−)-rupestine D, (−)-guaipyridine, (−)-epiguaipyridine, and (−)-cananodine together with two stereoisomers 8-epi-rupestine D and 5-epi-cananodine were synthesized enantioselectively from readily available citronellol. The key steps in this synthesis are (i) intermolecular opening of a trisubstituted epoxide for the formation of a chiral center at C-8; (ii) ring-closing metathesis for the construction of a seven-membered carbocyclic ring; and (iii) biomimetic cyclization of a 1,5-dicarbonyl compound for the construction of a pyridine-fused bicyclic skeleton.
- This article is part of the themed collection: Total synthesis in OBC

 Please wait while we load your content...
Please wait while we load your content...